Abstract
Drosophila melanogaster is an ideal model organism for developmental studies. This study tests the potential of semolina-jaggery (SJ) diet as a new formulation for bulk rearing of flies. Semolina and jaggery are organic products obtained from wheat endosperm and cane sugar, respectively. Semolina is a rich source of carbohydrates and protein. Jaggery has a high content of dietary sugars. Moreover, preparation of semolina jaggery diet is cost-effective and easy. Thus, the current study aimed to compare survival and developmental parameters of flies fed the SJ diet to flies fed the standard cornmeal-sugar-yeast (CSY) diet. SJ diet enhanced survival of flies without affecting fecundity; male flies showed increased resistance to starvation. A higher number of flies emerged at F2 and F3 generation when fed the SJ diet than when fed the control CSY diet. SJ diet did not increase fly body weight and lipid percentage. Therefore, SJ diet can be used for bulk rearing of healthy flies at par with the standard cornmeal-sugar-yeast diet.
Keywords: bulk rearing, Drosophila melanogaster, longevity, semolina-jaggery diet
Introduction
The fruit fly Drosophila melanogaster is the most studied and well-established model organism in biological research.1 Flies exhibit a short generation time and high fecundity and have a well-differentiated central nervous system, cardiac anatomy, malphigian tubules (mammalian kidney analogs), and fat body (mammalian adipose tissue analogs).2-7 Also, the ease of inducing gene knockouts and mutagenesis has rendered D. melanogaster as a powerful model to pursue demographic studies on various metabolic disorders.8
Although rearing flies is a relatively simple process, it requires knowledge of the appropriate individual components in media formulation. Originally, Thomas Hunt Morgan used banana medium to rear flies under laboratory conditions.9 Thereafter, various modifications have led to the development of simple and economic fly rearing media. Prior rearing of flies in an appropriate food medium is an imperative in any given study. Most pharmacological, gerontological, and demographic investigations require an abundance of flies for individual treatment.10-14 Thus, the maintenance of a bulk population of flies in such studies is of prime significance.
Semolina is an organic resource obtained from coarsely ground wheat endosperm.7 Production of semolina is widely distributed and used around the globe for the preparation of cereals, pasta, spaghetti, etc.7,15,16 Semolina is nutritious with high carbohydrate and protein content.15 Semolina contains 70.9% carbohydrate, 12.3% protein, 4.4% fat, and 11.6% moisture, with a caloric content of ˜372 kcal/100 g.15 Moreover, semolina has a low glycemic response, which makes it a healthy palatable choice.17 Jaggery is a non-centrifugal organic product of cane sugar and is a good source of dietary sugars.18 It is composed of up to 50% sucrose, 20% invert sugars, and 20% moisture, with ash, protein, and bagasse fines making up the remainder.18 Production of jaggery is vastly spread, with a world annual production of 11.05 million tons.10 Moreover, jaggery has been shown to possess various medicinal properties that include antioxidant, anti-oxygenic, and anti-carcinogenic.10,19,20 Prices range from approximately $0.25 to $1.00 per kilogram for semolina and $1.00 to $3.00 per kilogram of jaggery, thereby making them an cost-effective choice. Besides, quick and hassle free preparation of semolina-jaggery (SJ) diet might prompt students and novice fly-handlers, along with professional investigators, to use the SJ diet with ease. Thus, it is worthwhile to investigate the potential of the SJ diet to be incorporated as an optimal medium for bulk rearing of flies, at par with regularly used cornmeal-sugar-yeast diet.
The main objective of the study was to evaluate survival and development of flies reared on SJ diet. Different parameters were analyzed: fly longevity, fecundity, eclosion time, filial generation, lipid percentage, and weight. In addition, flies were analyzed for their resistance to starvation upon being fed with SJ diet.
Results and Discussion
Effect of semolina- jaggery diet on survival and fecundity in flies
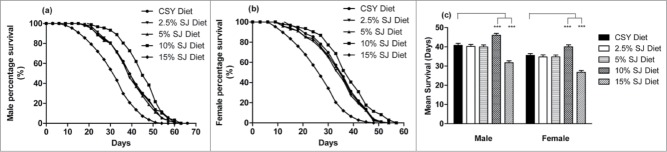
Longevity and fecundity are excellent indicators of good health in any organism. Flies were reared on SJ diet to assess whether the diet could elicit beneficial survival and development traits for bulk production. A pilot study was performed with varying range of semolina and jaggery (2.5%, 5%, 10%, and 15%) to screen for the optimum formulation for maintenance of a healthy fly population. Fig. 1a and 1b depict the effect of different concentrations of SJ diet (2.5%, 5%, 10%, and 15%) on fly longevity. Among the diets, only 10% SJ diet was able to significantly extend lifespan of both male and female flies. Male flies reared on 10% SJ diet had a mean lifespan of 46 ± 0.98 days (P < 0.001) as compared to 40.78 ± 1.08 days on CSY diet (Fig. 1c). Female flies reared on 10% SJ diet had mean lifespan of 40 ± 1.03 days (P < 0.001) as compared to 35.53 ± 0.95 days on CSY diet (Fig. 1c). Organic products are associated with higher amounts of vitamins, essential amino acids, and polyphenols. A recent study has highlighted the health benefits to Drosophila melanogaster provided by organic foods.21 Semolina and jaggery are organic by-products of plants with high nutritive value.15-18,22 This might partly explain the positive benefits of the SJ diet on flies.
Figure 1.
Effect of different concentrations of semolina-jaggery diet on fly longevity. (A) Survivorship of adult male flies. (B) Survivorship of adult female flies. Data presented as percentage survival of flies as function of time (in days). Percentage survival was calculated using Kaplan-Meier survival analysis using OASIS software. (C) Mean lifespan of male and female flies. Interaction between diet and sex was P < 0.001, 2-way ANOVA. **P < 0.01, ***P < 0.001, 2-way ANOVA, Bonferroni post-test (control CSY vs. different concentration of SJ diet). For each diet and sex: replicates = 10, n = 30 flies in each replicate.
Diminished reproductive output is a frequent trade-off associated with prolonged lifespan.22-26 As such, female fecundity was analyzed in flies reared on 10% SJ diet to warrant for the extended longevity. Fecundity was unaffected in female flies reared on 10% SJ diet as compared to CSY diet (Fig. 2). This observation is crucial since bulk production requires healthy maintenance of flies with unaltered life-traits. Thus, it could be safely inferred that enhanced survival of flies reared on SJ diet was not at the expense of reduced fecundity .
Figure 2.
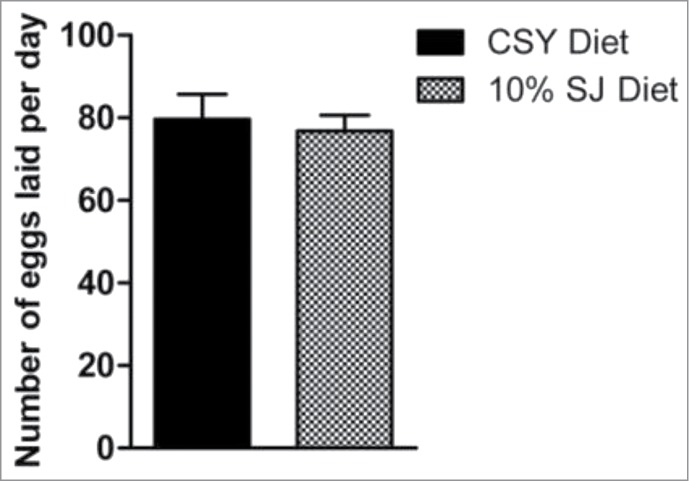
Effect of semolina-jaggery diet on female fecundity. Data presented as mean number of eggs laid by a female per day with error bars denoting standard deviation, unpaired t-test, non-parametric (control CSY vs. 10% SJ diet). Ten replicate vials were set up for each diet, with 2 males and 2 females in each replicate.
Semolina- jaggery diet improved fly output at F2 and F3 generation
Even though ample nutrition is required during larval growth and development, there is a threshold nutritional requirement beyond which excess nutrition might prove detrimental. Since the SJ diet extended lifespan in flies, it was imperative to note any probable changes in developmental parameters of larvae reared on this diet. Data from eclosion time were comparable with those observed for the control CSY diet, with no significant changes in larvae eclosion time when reared on 10% SJ diet (Fig. 3). An appropriate fly-rearing diet should sustain multiple filial generations without a decline in fly output. As such, the number of flies emerging during F1 to F3 filial generations was compared between 10% SJ diet and control CSY diet. As shown in Figure 4, 10% SJ diet exhibited no significant change in number of emerged flies at F1 generation. However, a significantly higher number of flies emerged in 10% SJ diet, with 208 flies in the F2 generation (P < 0.001) and 209 flies in the F3 generation (P < 0.01), as compared to CSY diet, with 187 flies in the F2 generation and 188 flies in the F3 generation. Thus, the SJ diet is nutritionally favorable for the sustenance of multiple generations of flies.
Figure 3.
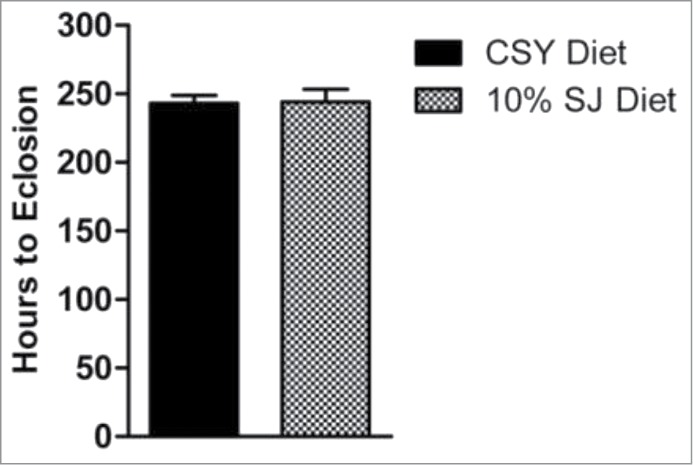
Time required for emergence of flies from pupal cases in different diets. Data presented as mean eclosion time (in hours) with error bars denoting standard deviation, unpaired t-test, non-parametric (control CSY vs. 10% SJ diet). First instar larvae were allowed to rear on respective diets till pupation. The pupae were marked on sides of vials and time taken by individual pupae to eclose as a fly was noted down. For each diet, replicates = 5, n = 50 flies in each replicate.
Figure 4.
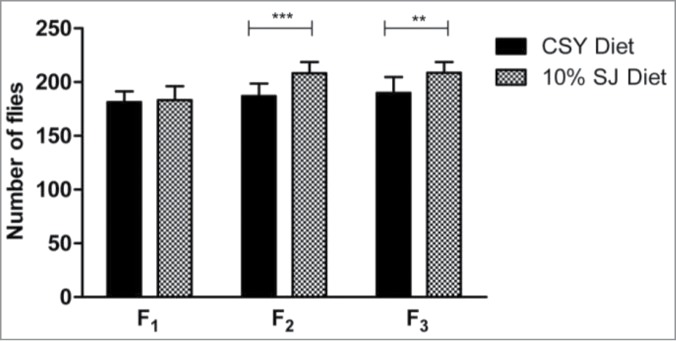
Effect of semolina-jaggery diet on multiple filial generations. Data presented as number of flies emerging at F1, F2 and F3 filial generations with error bars denoting standard deviation. Interaction between diet and filial generation was P < 0.01, 2-way ANOVA. **P < 0.01, ***P < 0.001, 2-way ANOVA, Bonferroni post-test (control CSY vs. 10% SJ diet). For each diet, replicates = 10, with 3 pairs of male and female in each replicate.
Male flies exhibited enhanced starvation resistance upon rearing on semolina- jaggery diet
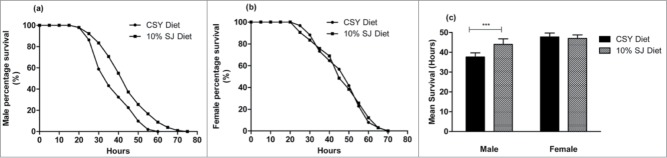
Improved longevity and developmental traits in flies grown in the SJ diet prompted us to hypothesize that the SJ diet could enhance stress resistance in flies. As such, flies reared on SJ diet were subjected to starvation stress. However, obtained data were only partly in favor of our hypothesis as only male flies exhibited significant resistance to starvation when reared on 10% SJ diet (Fig. 5a and 5b). As shown in Figure 5c, male flies reared on 10% SJ diet showed a mean lifespan of 44 ± 2.82 hours (P < 0.001), as compared to 37 ± 2.08 hours on CSY diet, while female flies did not exhibit significant differences between the respective diets.
Figure 5.
Effect of semolina-jaggery diet on starvation stress in flies. (A) Percentage survival of adult male flies fed during starvation stress. (B) Percentage survival of adult female flies during starvation stress. Data presented as percentage survival of flies during starvation stress as function of time (in hours). Percentage survival was calculated by Kaplan-Meier survival using OASIS software. (C) Mean survival of male and female flies during starvation stress. Error bars denote standard deviation. Interaction between diet and sex was P < 0.001, 2-way ANOVA. ***P < 0.001, 2-way ANOVA, Bonferroni post-test (control CSY vs. 10% SJ diet). For each diet and sex, replicates = 10, n = 30 flies in each replicate.
Body weight and lipid percentage of flies remained unchanged with semolina- jaggery diet
Diets rich in carbohydrate content have been associated with obesity and insulin resistance in flies. 27,28 Semolina and jaggery are good sources of carbohydrates and proteins.15-18,22 Thus, flies reared on the SJ diet were assessed for any changes in body weight and lipid percentage and compared to flies reared on the CSY diet. There was no significant increase in body weight in male and female flies when reared on 10% SJ diet (Fig. 6). Lipid percentages in male and female flies reared on 10% SJ diet were comparable to those of flies reared on control CSY diet (Fig. 7).
Figure 6.
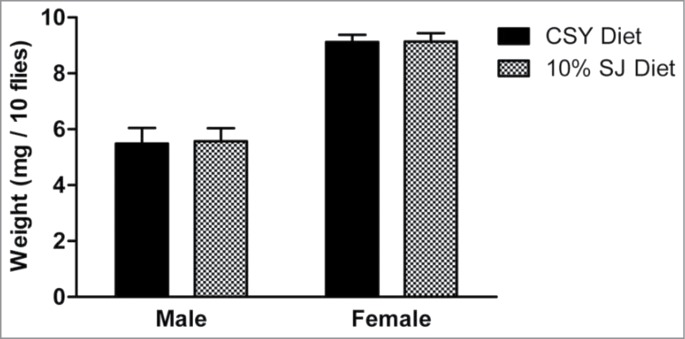
Effect of semolina-jaggery diet on body weight of male and female flies. Data represented as mean weight (mg) per 10 flies with error bars representing standard deviation. Interaction between diet and sex was P < 0.001, 2-way ANOVA, Bonferroni post-test (control CSY vs. 10% SJ diet). For each diet and sex, replicates = 10, n = 10 flies in each replicate.
Figure 7.
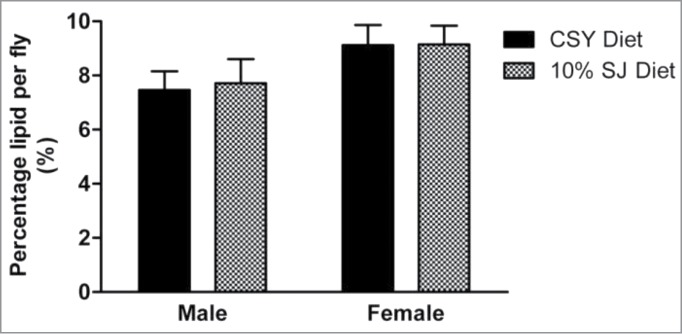
Effect of semolina-jaggery diet on lipid percentage in male and female flies. Data represented as mean lipid percentage (%) with error bars representing standard deviation. For individual diet and sex, lipid percentage per fly was calculated by dividing total lipid concentration by body weight per fly. Interaction between diet and sex was P < 0.05, 2-way ANOVA, Bonferroni post-test (control CSY vs. 10% SJ diets). For each diet and sex, replicates = 10, n = 10 in each replicate.
Semolina-jaggery diet can be used as an optimal diet for fly rearing and maintenance
Different formulations of SJ diets were used to select an optimum diet formulation. Among 2.5%, 5%, 10%, and 15% SJ diets, 10% SJ diet enhanced survival in male and female flies without compromising female fecundity. With SJ diet, more flies emerged at F2 and F3 filial generations. The SJ diet did not have detrimental effects on body weight and lipid percentage in flies. Male flies fed with the SJ diet exhibited increased resistance to starvation. Therefore, these results emphasize that the semolina-jaggery diet can be optimally incorporated for bulk rearing of flies at par with the standard cornmeal-sugar-yeast diet.
Materials and Methods
Diet preparation
Semolina-jaggery (SJ) diet was prepared at 2.5%, 5%, 10%, and 15% concentrations (Table 1). To prepare one liter SJ diet, jaggery was completely dissolved in 500 ml of lukewarm distilled water followed by addition of semolina. Quantities of all ingredients are given in Table 1. The mix was boiled for 10 minutes with continuous stirring to obtain a viscous consistency. Agar was dissolved in 100 ml of warm distilled water and added to the mix. Final volume was made up by addition of distilled water and the mix cooked for another 10 minutes with continuous stirring. Care should be taken to stir continuously during preparation, as uncooked semolina tends to clump at the bottom. When the temperature reached 70°C, propionic acid and methyl paraben were added and mix was immediately dispensed into vials. The cornmeal-sugar yeast (CSY) diet [5.2% cornmeal, 11% sugar, 2.4% autolyzed yeast extract, 2% agar, 0.3% propionic acid, 3% methyl paraben] was used as control due to its rigorous use as a standard diet in many studies.29,30
Table 1.
Composition of semolina-jaggery diet
| 2.5% SJ | 5% SJ | 10% SJ | 15% SJ | |
|---|---|---|---|---|
| Semolina (g, w/v) | 25 | 50 | 100 | 150 |
| Jaggery (g, w/v) | 25 | 50 | 100 | 150 |
| Agar (g, w/v) | 20 | |||
| 0.3% Propionic acid (ml, v/v) | 5 | |||
| 3% Methyl paraben (ml, v/v) | 30 | |||
| Final volume (ml) | 1000 | |||
Fly husbandry
Experiments were performed with wild type Canton-S (CS) flies reared at 25°C ± 1°C on 12:12 hours light:dark cycle under standard fly rearing conditions. Prior to the assays, flies were bulk reared in 300 ml polypropylene bottles containing 30 ml of standard cornmeal diet. Each bottle housed 50 flies to avoid overcrowding. Eggs were carefully collected over 24 hours and transferred to new bottles containing SJ diet (30 ml). Eggs were allowed to hatch followed by larval pupariation. For all assays, newly eclosed flies were collected and allowed to mate for 48 hours. Flies were then segregated according to their sex and used for subsequent assays. For evaluation of eclosion time and filial generation, flies were reared in 300 ml bottles, each with 30 ml of SJ diet. For the remaining assays, flies were reared in 50 ml polypropylene vials, each with 7 ml of SJ diet. The same procedures were followed for control CSY diet.
Longevity assay
To measure fly longevity, single-sex flies were segregated into vials containing the respective diet. Flies were transferred to vials with fresh food every 2 days; during this time, the number of dead flies was recorded. Assay was continued until all flies were dead. For diet and sex, 10 replicate vials were set up with 30 flies in each vial.
Measurement of fly fecundity
Newly eclosed flies were kept in groups of 2 males and 2 virgin females on the respective diets. Flies were transferred to fresh diet every day and the number of eggs laid was counted every day for 15 days. For each diet, 10 replicates were set up, with 2 males and 2 females in each replicate.
Eclosion time
Freshly hatched, age matched 1st instar larvae were collected from bulk fly stock cultures and carefully transferred to bottles with individual diet. Larvae were allowed to rear and pupariate. Each pupa was numerically marked on the side of bottles and observed further. Time required by individual larvae to pupariate and eclose as a fly was monitored and noted down. For each diet, 5 replicates were set up with 50 flies in each bottle.
Filial generation
The assay was initiated by placing 3 pairs of newly eclosed, adult male and female flies in bottles having respective diets. This generation of flies, designated as parental (P) generation, gave rise to the first filial (F1) generation. Parental flies were removed after 48 hours to ensure synchronous larval growth. Larvae obtained from P generation were allowed to rear, pupariate and emerge as F1 flies. The number of F1 generation flies was counted and noted down. From F1 generation flies, 3 pairs of randomly selected male and female flies were transferred to bottles with fresh diets, which ultimately gave rise to second filial (F2) generation. The procedure was repeated to obtain F3 generation. For each diet, 10 replicates were set up.
Starvation resistance
Newly eclosed adult young flies were allowed to feed on respective diet for 15 days. In the post-treatment, groups of single sex flies were kept in vials containing 1% agar and transferred to fresh vials for every 5 hours. Number of dead flies was recorded during each transfer. For each diet and sex, 10 replicate vials were set up, with 30 flies in each vial.
Body weight
Newly eclosed, single sex flies were allowed to feed on respective diets for 2 weeks. Flies were then subjected to mild etherization followed by immediate measurement of body weight. For each diet, 10 replicate vials were set up, with 10 flies in each vial.
Estimation of total lipid
Lipid content in flies was estimated according to the van Handel's protocol, with slight modifications. 31 Newly eclosed single flies were fed on respective diet for 15 days. To prepare fly extract, 3 single sex flies were pooled from the respective medium and homogenized in 2% sodium sulfate followed by addition of double volume of chloroform/methanol mix (1:1 v/v). Centrifugation was performed at 4000 rpm for 8 minutes at 4°C. The supernatant was made up with double distilled water and centrifuged again. The lower fraction of the supernatant was collected for estimation of total lipid content. The lower fraction of fly extract was diluted 5 times with chloroform and boiled until complete evaporation. Concentrated sulphuric acid was mixed with the solution and boiled for 10 minutes. After cooling, freshly prepared vanillin reagent was added and thoroughly mixed. Absorbance was measured at 525 nm. For each diet and sex, 10 replicate vials were set up, with 10 flies in each vial.
Statistical analysis
Survival curves were prepared by Kaplan-Meier survival analysis and analyzed using the OASIS software. 32 Fecundity and eclosion time were interpreted by nonparametric t-tests using Graph-Pad Prism software. Remaining experiments were analyzed by ANOVA followed by post-tests using Graph-Pad Prism software. For all assays, P-values for levels of significance are represented as * < 0.05, ** < 0.01, and *** < 0.001.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Acknowledgments
We thank Dr. NB Ramachandra at Mysore University, National Drosophila Stock Center, India, for providing the wild-type Canton-S fly strain.
References
- 1.Avanesian A, Semnani S, Jafari M. Can Drosophila melanogaster represent a model system for the detection of reproductive adverse drug reactions? Drug Discov Today 2009; 14:761-6; PMID:19482095; http://dx.doi.org/ 10.1016/j.drudis.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 2.Demerec M, Kaufmann BP. Drosophila guide: introduction to the genetics and cytology of Drosophila melanogaster. Carnegie Institution of Washington; Washington, DC, 1965 [Google Scholar]
- 3.Dwivedi V, Anandan EM, Mony RS, Muraleedharan TS, Valiathan MS, Mutsuddi M, Lakhotia SC. In vivo effects of traditional Ayurvedic formulations in Drosophila melanogaster model relate with therapeutic applications. PLoS One 2012; 7:e37113; PMID:22606337; http://dx.doi.org/ 10.1371/journal.pone.0037113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markow TA, O'Grady P. Drosophila: a guide to species identification and use. Academic Press, 2005 [Google Scholar]
- 5.Jones MA, Grotewiel M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Experimental Gerontol 2011; 46:320-5; http://dx.doi.org/ 10.1016/j.exger.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou S, Sinclair J, Wilson MA, Carey JR, Liedo P, Oropeza A, Kalra A, de Cabo R, Ingram DK, Longo DL, et al.. Comparative approaches to facilitate the discovery of prolongevity interventions: effects of tocopherols on lifespan of three invertebrate species. Mech Ageing Dev 2007; 128:222-6; PMID:17169403; http://dx.doi.org/ 10.1016/j.mad.2006.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mani U, Pradhan S, Mehta N, Thakur D, Iyer U, Mani I. Glycaemic index of conventional carbohydrate meals. British J Nutrition 1992; 68:445-50; http://dx.doi.org/ 10.1079/BJN19920102 [DOI] [PubMed] [Google Scholar]
- 8.Partridge L, Alic N, Bjedov I, Piper MD. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp Gerontol 2011; 46:376-81; PMID:20849947; http://dx.doi.org/ 10.1016/j.exger.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearl R. A Synthetic Food Medium for the Cultivation of Drosophila : Preliminary Note. J Gen Physiol 1926; 9:513-9; PMID:19872271; http://dx.doi.org/ 10.1085/jgp.9.4.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra MJ, Mujica MV. Physical and chemical properties of granulated cane sugar” panelas. Food Science Technol 2010; 30:250-7; http://dx.doi.org/ 10.1590/S0101-20612010005000012 [DOI] [Google Scholar]
- 11.Jafari M, Zarban A, Pham S, Wang T. Rosa damascena decreased mortality in adult Drosophila. J Medicinal Food 2008; 11:9-13; http://dx.doi.org/ 10.1089/jmf.2007.546 [DOI] [PubMed] [Google Scholar]
- 12.Avanesian A, Khodayari B, Felgner JS, Jafari M. Lamotrigine extends lifespan but compromises health span in Drosophila melanogaster. Biogerontol 2010; 11:45-52; http://dx.doi.org/ 10.1007/s10522-009-9227-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jafari M, Khodayari B, Felgner J, Bussel II, Rose MR, Mueller LD. Pioglitazone: an anti-diabetic compound with anti-aging properties. Biogerontol 2007; 8:639-51; http://dx.doi.org/ 10.1007/s10522-007-9105-7 [DOI] [PubMed] [Google Scholar]
- 14.Gospodaryov DV, Yurkevych IS, Jafari M, Lushchak VI, Lushchak OV. Lifespan extension and delay of age-related functional decline caused by Rhodiola rosea depends on dietary macronutrient balance. Longev Healthspan 2013; 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oladunmoye OO, Aworh OC, Maziya‐Dixon B, Erukainure OL, Elemo GN. Chemical and functional properties of cassava starch, durum wheat semolina flour, and their blends. Food Sci Nutri 2014; 2:132-8; http://dx.doi.org/ 10.1002/fsn3.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur G, Sharma S, Nagi H, Dar BN. Functional properties of pasta enriched with variable cereal brans. J Food Sci Technol 2012; 49:467-74; PMID:23904655; http://dx.doi.org/ 10.1007/s13197-011-0294-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoyama WH, Hudson CA, Knuckles BE, Chiu M-CM, Sayre RN, Turnlund JR, Schneeman BO. Effect of barley β-glucan in durum wheat pasta on human glycemic response. Cereal Chemistry 1997; 74:293-6; http://dx.doi.org/ 10.1094/CCHEM.1997.74.3.293 [DOI] [Google Scholar]
- 18.Kaplinsky R. Sugar processing. The development of a third-world technology. Intermediate Technol Publications 1983; Delhi: Oxford University Press. [Google Scholar]
- 19.Sahu A, Paul B. P3A12-The role of dietary whole sugar-jaggery in prevention of respiratory toxicity of air toxics and in lung cancer. Toxicology Letters 1998; 95:154; http://dx.doi.org/ 10.1016/S0378-4274(98)80615-2 [DOI] [Google Scholar]
- 20.Nayaka MH, Sathisha U, Manohar M, Chandrashekar K, Dharmesh SM. Cytoprotective and antioxidant activity studies of jaggery sugar. Food Chemistry 2009; 115:113-8; http://dx.doi.org/ 10.1016/j.foodchem.2008.11.067 [DOI] [Google Scholar]
- 21.Huber M, Rembiałkowska E, Średnicka D, Bügel S, Van De Vijver L. Organic food and impact on human health: Assessing the status quo and prospects of research. NJAS-Wageningen J Life Sci 2011; 58:103-9; http://dx.doi.org/ 10.1016/j.njas.2011.01.004 [DOI] [Google Scholar]
- 22.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci 2008; 105:2498-503; PMID:18268352; http://dx.doi.org/ 10.1073/pnas.0710787105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piper MD, Bartke A. Diet and aging. Cell Metabolism 2008; 8:99-104; PMID:18680711; http://dx.doi.org/ 10.1016/j.cmet.2008.06.012 [DOI] [PubMed] [Google Scholar]
- 24.Flatt T. Survival costs of reproduction in Drosophila. Experimental Gerontol 2011; 46:369-75; http://dx.doi.org/ 10.1016/j.exger.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 25.Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell 2005; 120:461-72; PMID:15734679; http://dx.doi.org/ 10.1016/j.cell.2005.01.026 [DOI] [PubMed] [Google Scholar]
- 26.Jafari M, Rose MR. Rules for the use of model organisms in antiaging pharmacology. Aging Cell 2006; 5:17-22; PMID:16441839; http://dx.doi.org/ 10.1111/j.1474-9726.2006.00195.x [DOI] [PubMed] [Google Scholar]
- 27.Jumbo-Lucioni P, Ayroles JF, Chambers MM, Jordan KW, Leips J, Mackay TF, De Luca M. Systems genetics analysis of body weight and energy metabolism traits in Drosophila melanogaster. BMC Genomics 2010; 11:297; PMID:20459830; http://dx.doi.org/ 10.1186/1471-2164-11-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Wheeler CT, Alberico T, Sun X, Seeberger J, Laslo M, Spangler E, Kern B, de Cabo R, Zou S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age 2013; 35:69-81; PMID:22083438; http://dx.doi.org/ 10.1007/s11357-011-9332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner M. Drosophila. A laboratory handbook. Cold Spring Harbor Laboratory Press, 1989 [Google Scholar]
- 30.Lee SH, An HS, Jung YW, Lee EJ, Lee HY, Choi ES, An SW, Son H, Lee SJ, Kim JB, et al.. Korean mistletoe (Viscum album coloratum) extract extends the lifespan of nematodes and fruit flies. Biogerontology 2014; 15:153-64; PMID:24337961; http://dx.doi.org/ 10.1007/s10522-013-9487-7 [DOI] [PubMed] [Google Scholar]
- 31.Van Handel E. Rapid determination of total lipids in mosquitoes. J Am Mosq Control Assoc 1985; 1:302-4; PMID:2906672 [PubMed] [Google Scholar]
- 32.Yang J-S, Nam H-J, Seo M, Han SK, Choi Y, Nam HG, Lee S-J, Kim S. OASIS: online application for the survival analysis of lifespan assays performed in aging research. PLoS One 2011; 6:e23525; PMID:21858155; http://dx.doi.org/ 10.1371/journal.pone.0023525 [DOI] [PMC free article] [PubMed] [Google Scholar]