Abstract
In clinical practice, evaluation of clinical efficacy of treatment planning stems from the radiation oncologist's experience in accurately targeting tumors, while keeping minimal toxicity to various organs at risk (OAR) involved. A more objective, quantitative method may be raised by using radiobiological models. The purpose of this work is to evaluate the potential correlation of OAR-related toxicities to its radiobiologically estimated parameters in simultaneously integrated boost (SIB) intensity modulated radiation therapy (IMRT) plans of patients with head and neck tumors at two institutions. Lyman model for normal tissue complication probability (NTCP) and the Poisson model for tumor control probability (TCP) models were used in the Histogram Analysis in Radiation Therapy (HART) analysis. In this study, 33 patients with oropharyngeal primaries in the head and neck region were used to establish the correlation between NTCP values of (a) bilateral parotids with clinically observed rates of xerostomia, (b) esophagus with dysphagia, and (c) larynx with dysphagia. The results of the study indicated a strong correlation between the severity of xerostomia and dysphagia with Lyman NTCP of bilateral parotids and esophagus, respectively, but not with the larynx. In patients without complications, NTCP values of these organs were negligible. Using appropriate radiobiological models, the presence of a moderate to strong correlation between the severities of complications with NTCP of selected OARs suggested that the clinical outcome could be estimated prior to treatment.
Keywords: Dysphagia, head and neck cancers, normal tissue complication probability, radiobiology, xerostomia
Introduction
Treatment of head and neck cancers using intensity modulated radiation therapy (IMRT) is a promising technique due to its ability to conform high doses to irregularly shaped treatment volumes, and also the clever use of inverse planning techniques to steer radiation doses away from multiple critical normal organs. Two of the most common complications associated with IMRT of head and neck tumor treatments are xerostomia (inadequate and even lack of salivary production) and dysphagia (increased swallowing difficulty of the esophagus). While the former is reported to be due to irradiation of parotid tissues,[1] the latter is due to irradiation of pharyngeal constrictors, esophagus, and larynx;[2] both processes are biologically and physiologically complex.
Lyman normal tissue complication probability (NTCP) model is one of the most popular radiobiological models typically used in modern dose-effect calculations.[3] This model is based on calculated dose volume histograms (DVH) of the organs at risk (OAR);[4,5,6] this work was detailed by Luxton et al. who outlined the key values of the main parameters, namely TD50,5, slope parameter (m), and the volume parameter (n).[7] In this project, we introduced and evaluated the tumor control probability (TCP) and NTCP values of multiple targets and OARs, from their respective DVH statistics using the histogram analysis in radiation therapy (HART) software.[8] The treatment plans were clinically produced and strictly followed in regard to organ tolerances as established by the quantitative analyses of normal tissue effects in the clinic (QUANTEC) guidelines.[9]
Materials and Methods
Patient population
In this study, we focused on the toxicities observed in parotids, esophagus, and larynx. IMRT plans of the head and neck cancers were retrospectively reviewed in this study, which was approved by the Institutional Review Board at both institutions (Institutions 1, 2). Between 2009 and 2011, 95 consecutive patients with oropharyngeal primaries were identified. Accessibility of the treatment plan and the follow-up data limited the available pool to 61 patients. Among these patients who received IMRT due to head and neck cancers, 33 (54%) cases whom developed clinically significant xerostomia (N = 23), dysphagia (N = 22), or both complications (N = 12) in the long-term were studied. All patients were treated using simultaneously integrated boost (SIB) technique; these 33 cases formed the cohort of this study. The mean age was 68 years (range: 55–82 years). The follow-up time ranged from 1.5 to 3.0 years from the end of treatment, with a mean of 2.1 years. Free-breathing computed tomography (CTs) were acquired on a GE-LightSpeed 16-slice CT scanner (GE Healthcare, Waukesha, WI), and immobilization was generally acquired by the use of a thermoplastic mask. The patients were treated using a 6MV photon beam from 23EX linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) with a multi-leaf collimator having 60 pairs of leaves. Nine (9) patients were treated using volumetric modulated arc therapy (VMAT) with two opposing arcs at a maximum dose rate of 600 MU/min. The remaining 24 cases were treated using 7–9 step and shoot IMRT beams in coplanar or noncoplanar configuration. The 33 patients were treated with the prescription dose (PD) ranging between 63.0 and 70.2 Gy (average, 69.0 Gy) over 29–39 fractions (average, 34 fractions). Up to 3 SIB-based target volumes (PTV1, PTV2, and PTV3) were delineated [Figure 1]. The mean value ± standard deviation of PTV1, PTV2, and PTV3 were 317.0 ± 252.4 cc, 524.7 ± 359.7 cc, and 282.6 ± 121.8 cc, respectively. The Pinnacle TPS (ver. 7.6c, Philips Healthcare, The Netherlands) and XiO TPS (ver. 4.50, CMS, St Louis, USA) were used for treatment planning. The OARs included the parotid glands, nasopharynx, larynx, mandibles, optic structures, brainstem, spinal cord, brachial plexus, and whole brain. The treatment plan objectives were based on the QUANTEC guidelines. The contours, field arrangement, dose plan, and target conformity were approved by the respective institutional prescribing radiation oncologists.
Figure 1.
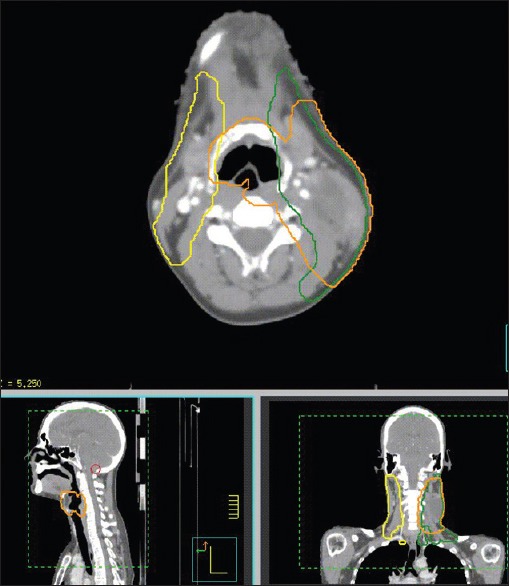
Transverse, sagittal, and coronal slices of computed tomography images of a patient with head and neck cancer treated by simultaneously integrated boost intensity modulated radiation therapy. In this particular example, three (3) PTV levels in yellow, green and orange receiving 5445, 5940 and 6996 cGy were shown, respectively
Complications
The severity of complications was graded using the Common Terminology Criteria for Adverse Events guidelines (CTCAE ver. 4.0).[10] National Cancer Institute- based CTCAE has been widely accepted across the oncology community as the standard for adverse event grading. While grades 1, 2, and 3 stands for mild, moderate and severe symptoms, grades 4 and 5 are typically related to life-threatening consequences and death from the inciting adverse events, respectively. There was no grade 5 toxicity recorded in this study.
Radiobiological modeling
The TCP model predicts that cell killing is based on Poisson statistics. For a given heterogeneous dose distribution defined by discrete DVH (dDVH) values {Di, vi}, TCP is given by the following expression:[11]
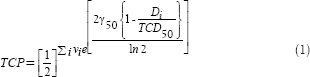
Where, TCD50 is the dose producing 50% TCP and γ50 is the normalized slope at the 50% probability level. The PD considered in this study was the largest dosage prescribed to the PTVs. In TCP computation, the range of the reference values of Di used to estimate normalized volume (vi) was 20%PD, 40% PD, 60% PD, 80% PD, 100% PD, 110% PD, and 120% PD.
The Lyman NTCP model for normal tissue irradiation was based on the sigmoidal dose-response relationship and the equivalent uniform dose (EUD) concept:
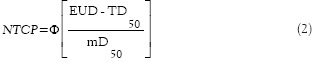
where 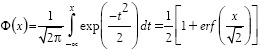
The reference values of Di used to estimate vi in Lyman NTCP estimation were 25% PD, 50% PD, 75% PD, 100% PD. The parameter m indicated the slope of the dose-response curve, and TD50 determined the position of a dose-response curve at the 50% probability of complication. EUD, or generalized equivalent uniform dose (gEUD), represented the uniform dose that would produce the same radiobiological effect as the given heterogeneous dose specified by the DVH.[12]

Whereas, {Di, vi} were the dose-volume values in the dDVH and n determined the dose-volume dependence of a tissue which was deterministic based on tissue architecture differences.
The values that were used in this study for calculation of TCP and NTCP were obtained from Luxton et al.[7] For tumor control, TCD50 =63.8 Gy, and α/β=10 Gy. For bilateral parotids, TD50,5 = 28.4 Gy, m = 0.18, and n = 1 were applied. For esophagus, TD50,5 =47 Gy, m = 0.36, and n = 0.69. Finally, for larynx, TD50,5 =70 Gy, m = 0.17 and n = 0.08. The HART computational platform was used for calculating the TCP and NTCP values from DVH. The NTCP values of the three OARs reported above (calculated from HART program) were used to correlate toxicity levels which led to complications.[8]
As a baseline measurement, the NTCP values of the parotids, esophagus, and larynx were estimated for the remainder of the patient population without any reported long-term xerostomia or dysphagia complications.
Results
In the 33 IMRT head and neck cancer cases, the TCP and NTCP values were derived from the DVH statistics using HART program. CT slice of a representative patient with the highest PD of 69.96 Gy which was treated using VMAT is shown in Figure 1. The reference values of dose and fractional volumes used in computation of NTCP value of parotid glands of the representative patient were: (1749 cGy, 0.745), (3498 cGy, 0.517), (5247 cGy, 0.37), and (6996 cGy, 0.156). The patient had a NTCP value of 0.71 of parotid glands, and xerostomia severity of 2 by grade. Values were quoted as mean ± standard error at 95% confidence level (SE). For the patient population studied (N = 33), the mean ± SE TCP value was estimated to be 0.8 ± 0.03; while the NTCP values of the parotids, esophagus and larynx were 0.4 ± 0.1, 0.2 ± 0.1, and 0.1 ± 0.1, respectively. The PD ranged between 63.0 and 70.2 Gy (mean dose prescribed was 69.0 Gy).
The severity of xerostomia was found to correlate well with the NTCP value of parotids, with a correlative strength of 0.63 (Pearson correlation coefficient value, R2), as shown in Figure 2a. Using a paired two-tailed Student's t-test, statistical significant difference was observed with P < 0.01. The correlation between the severity of dysphagia and NTCP value of esophagus was also found to be statistically significant, with P < 0.01 and R2 = 0.74 indicating strong correlation, as shown in Figure 2b. The solid line shows the best fit of the severity of complications with the NTCP values based on the available data; the line of best fit equations were measured at y = 1.38x + 0.89 for xerostomia, and y = 5.75x + 0.89 for dysphagia, where y represents the severity of complication by grade, and x being the calculated NTCP values. However, the Lyman model provided a very poor correlation between dysphagia severity and NTCP of the larynx (R2 = 0.01, which indicated a poor level of correlation). In addition, no correlation was found between the complication severity and the PD or DVH values for any of the three organs.
Figure 2.
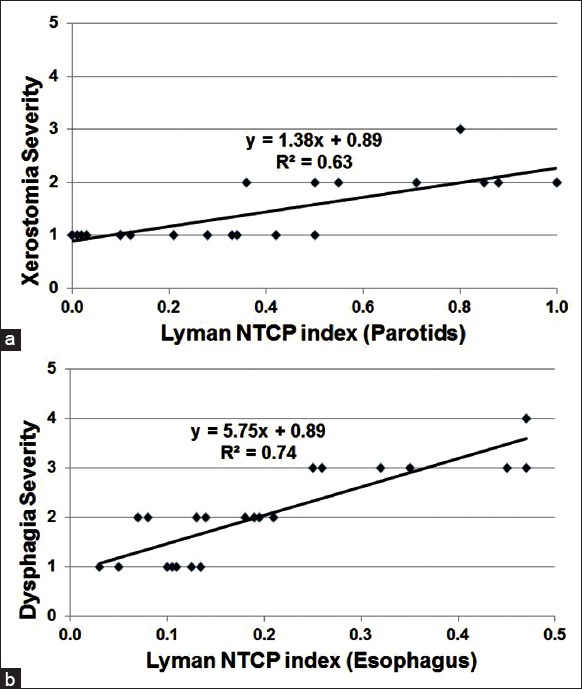
Correlation between the severity of (a) xerostomia and (b) dysphagia with the Lyman normal tissue complication probability indices of bilateral parotid glands for TD50,5 = 28.4 Gy (R2 = 0.63, P < 0.01) and esophagus for TD50, 5 = 47Gy (R2 = 0.74, P < 0.01), respectively
In a sub-group study of 15 patients treated at higher PDs of 70 ± 0.3 Gy, grade 2 + xerostomia, and 3 + dysphagia toxicities were observed in 9 and 7 patients, respectively. In this sub-group, the corresponding mean ± SE NTCP values were 0.7 ± 0.1 (N = 15) for parotids and 0.4 ± 0.04 (N = 15) for esophagus, respectively.
From the baseline study on patients without any report of xerostomia or dysphagia complications, the mean ± SE values of NTCP of parotids, esophagus and larynx were found to be 0.06 ± 0.03, 0.05 ± 0.02, and 0.04 ± 0.04, respectively. The mean ± SE TCP value was estimated to be 0.75 ± 0.05.
Discussion
The IMRT dosimetric planning objectives of head and neck tumors were similar across patients and followed the guidelines outlined in QUANTEC.[9] However, the radiation-related complications which occurred in patients may depend on various dosimetric and radiobiological factors including the spatial distance of OAR's from the tumor, beam weighting, and OAR classification based on functional subunits; it is certainly multifactorial across a number of different domains in the realms of clinical and biological radiation oncology.
Our study looked at a few complications that were prevalent among the head and neck tumor patients who received IMRT. A pattern of correlation was identified between the severity of complications and the NTCP of three organs of interest. Among the three possible correlations, only 2 (xerostomia and dysphagia for parotid and esophageal toxicities, respectively) were considered significant on the two-tailed Student's t-test and the Pearson correlation coefficients; however, the number of cases in this study were limited. Among the patients with neither of the two complications, NTCP of the three OARs were found to be negligible. The correlation between the corresponding toxicities and NTCPs of the examined OARs depends on the availability of the proper dosimetric information and the accuracy of clinical follow-up data on the reported toxicity levels.
A multicenter study showed that among many factors, the mean parotid dose was the most important predictor of moderate to severe xerostomia at 6 months.[13] However, no long term toxicity data were mentioned in their work. Roesnik et al. modeled the reduction of individual parotid flow rates using the LKB model.[14] Many studies demonstrated that the salivary flow could be reduced exponentially with a mean parotid gland threshold dose above 26 Gy.[15,16] Quantitative models describing the reduction of the parotid function with the dose distribution over the major and minor salivary glands were previously reported.[17,18,19]
Increased dose to a large volume of swallowing structures is reported to result in higher levels of dysphagia in a number of studies.[20,21,22] In a study on 82 head and neck tumor patients treated with SIB-IMRT, long term dysphagia was related to radiation dose delivered to the swallowing structures.[23] Taking into account the complex sequence of swallowing event, it is highly unlikely a single structure is important. Even though xerostomia can be reduced with parotid-sparing IMRT, the comparable advances in successful reduction of dysphagia toxicities have been largely limited.
Conclusion
This study suggested that a moderate to strong correlation may exist between the severities of xerostomia and also dysphagia, with the calculated NTCP values of bilateral parotids and esophagus as OARs, respectively. The existence of a reasonable degree of correlation between the observed complication rates and NTCP values demonstrates that clinically apparent complications or side effects may be further improved by using selective radiobiological parameters in radiotherapy planning that are directly derived from appropriate models.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58–63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang YC, Chen SY, Lui LT, Wang TG, Wang TC, Hsiao TY, et al. Dysphagia in patients with nasopharyngeal cancer after radiation therapy: A videofluoroscopic swallowing study. Dysphagia. 2003;18:135–43. doi: 10.1007/s00455-002-0096-x. [DOI] [PubMed] [Google Scholar]
- 3.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–9. [PubMed] [Google Scholar]
- 4.Kutcher GJ, Burman C, Brewster L, Goitein M, Mohan R. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys. 1991;21:137–46. doi: 10.1016/0360-3016(91)90173-2. [DOI] [PubMed] [Google Scholar]
- 5.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 6.Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–35. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 7.Luxton G, Keall PJ, King CR. A new formula for normal tissue complication probability (NTCP) as a function of equivalent uniform dose (EUD) Phys Med Biol. 2008;53:23–36. doi: 10.1088/0031-9155/53/1/002. [DOI] [PubMed] [Google Scholar]
- 8.Pyakuryal A, Myint WK, Gopalakrishnan M, Jang S, Logemann JA, Mittal BB. A computational tool for the efficient analysis of dose-volume histograms from radiation therapy treatment plans. J Appl Clin Med Phys. 2010;11:3013. doi: 10.1120/jacmp.v11i1.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks LB, Ten Haken RK, Martel MK. Guest editor's introduction to QUANTEC: A users guide. Int J Radiat Oncol Biol Phys. 2010;76:S1–2. doi: 10.1016/j.ijrobp.2009.08.075. [DOI] [PubMed] [Google Scholar]
- 10. [Last accessed on 2015 Jan 09]. Available from: http://www.evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010.06.14_QuickReference_8.5x11.pdf .
- 11.Warkentin B, Stavrev P, Stavreva N, Field C, Fallone BG. A TCP-NTCP estimation module using DVHs and known radiobiological models and parameter sets. J Appl Clin Med Phys. 2004;5:50–63. doi: 10.1120/jacmp.v5i1.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method. Int J Radiat Oncol Biol Phys. 1989;16:1623–30. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 13.Beetz I, Schilstra C, Burlage FR, Koken PW, Doornaert P, Bijl HP, et al. Development of NTCP models for head and neck cancer patients treated with three-dimensional conformal radiotherapy for xerostomia and sticky saliva: The role of dosimetric and clinical factors. Radiother Oncol. 2012;105:86–93. doi: 10.1016/j.radonc.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Roesink JM, Moerland MA, Battermann JJ, Hordijk GJ, Terhaard CH. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2001;51:938–46. doi: 10.1016/s0360-3016(01)01717-5. [DOI] [PubMed] [Google Scholar]
- 15.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–87. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 16.Blanco AI, Chao CK, Deasy JO, Low DA. Recovery kinetics of salivary function in patients with head and neck cancers receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2002;54:166. [Google Scholar]
- 17.Deasy JO, Fowler JF. Radiobiology of IMRT. In: Mundt AJ, Roeske JC, editors. Intensity Modulated Radiation Therapy – A Clinical Perspective. Hamilton, London: BC Decker Inc; 2005. [Google Scholar]
- 18.Chao KS, Deasy JO, Markman J, Haynie J, Perez CA, Purdy JA, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: Initial results. Int J Radiat Oncol Biol Phys. 2001;49:907–16. doi: 10.1016/s0360-3016(00)01441-3. [DOI] [PubMed] [Google Scholar]
- 19.Blanco AI, Chao KS, El Naqa I, Franklin GE, Zakarian K, Vicic M, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055–69. doi: 10.1016/j.ijrobp.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 20.Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: Frequency, intensity and correlation with dose and volume parameters. Radiother Oncol. 2007;85:74–82. doi: 10.1016/j.radonc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Dirix P, Abbeel S, Vanstraelen B, Hermans R, Nuyts S. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: Dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009;75:385–92. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Christianen ME, Schilstra C, Beetz I, Muijs CT, Chouvalova O, Burlage FR, et al. Predictive modelling for swallowing dysfunction after primary (chemo) radiation: Results of a prospective observational study. Radiother Oncol. 2012;105:107–14. doi: 10.1016/j.radonc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Peponi E, Glanzmann C, Willi B, Huber G, Studer G. Dysphagia in head and neck cancer patients following intensity modulated radiotherapy (IMRT) Radiat Oncol. 2011;6:1. doi: 10.1186/1748-717X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


