Abstract
Objective:
Pityriasis rosea (PR) is an acute self-limiting disease. Despite vigorous efforts by generations of researchers since nearly 150 years, present treatment modalities for PR are not very gratifying. Ultraviolet radiation has been recommended in PR, although only a few studies validate this proposal. This study was conducted to explore the therapeutic effect of NBUVB on the symptoms, course, and severity of PR.
Materials and Methods:
This study involved a hundred patients who were randomly divided into two groups, using computer-generated randomization chart. Group A underwent treatment with fixed dose NBUVB of 250 mJ/cm2 three times (nonconsecutive) a week for 4 weeks. Group B formed the placebo group who did not receive any treatment. The two groups were compared with each other for the intensity of pruritis, course and duration of disease, and PR severity score (PRSS).
Results:
The t values of improvement in PRSS score in Group A (t = 12.796) were higher as compared with that in Group B (t = 10.066). Similarly, the t value of the pruritus scale in Group A (t = 7.758) was higher than Group B (t = 5.754) indicating the symptomatic improvement in itching.
Conclusion:
Fixed-dose NBUVB phototherapy resulted in marked improvement in the severity and symptoms of the disease as quantitatively assessed by PRSS.
Keywords: Narrowband ultraviolet B phototherapy, pityriasis rosea, PR severity score
INTRODUCTION
Pityriasis rosea (PR) also known as PR of Gibert is a common, self-limiting, papulosquamous disorder. It was first described by the Edinburg dermatologist Robert Willan in 1860.[1] PR is a disease of multifactorial (infectious and noninfectious) etiology. Only anecdotal reports documenting a viral or bacterial etiology for the disease exist at present date. In view of the unclear etiology and its self-limiting nature, definitive therapeutics are lacking in PR.[2,3] However, sometimes active interventions are needed in patients with an extensive eruption and bothersome pruritus. Sunlight or artificial UV radiation has been stated to be useful in PR.[4] In 1974, Merchant and Hammond first enunciated the therapeutic effect of ultraviolet (UVB) rays in PR. Phototherapy has however not gained importance in the treatment of PR as few reports documenting its efficacy have appeared since;[4] nonetheless, interest has risen again lately.[5,6,7] The present study was conducted to explore the therapeutic effect of NBUVB on the symptoms, course, and severity of PR.
MATERIALS AND METHODS
We conducted a prospective, randomized, double-blinded, placebo-controlled study on new and relapsing cases of PR attending the skin outpatient department of a tertiary care hospital. Patients with photosensitivity, eye disorders, history of mood swings, mania, pregnancy, lactation, positive serology for syphilis, and age less than five years were excluded from the study. Clinically suspected cases of PR were confirmed histopathologically after taking informed consent for biopsy. Hundred patients who satisfied the above-mentioned criteria were allocated into two groups by a computer-generated randomization chart. Patients in Group A (n = 50; males 23, females = 27) underwent treatment with fixed dose narrowband UVB phototherapy of 250mJ/cm2 three times (nonconsecutive) a week for four weeks. These patients were asked minimise exposure to ambient sunlight during the study. Patients in Group B (n = 50; males = 29, females = 21) were administered a topical emollient only. The final outcome was assessed subjectively using the PRSS score, which was calculated at initiation of treatment and at four weeks following the intervention.
Clinical grading of severity of PR
Severity of the disease was calculated using the Pityriasis Rosea Severity Score (PRSS).[3] Two areas were evaluated for determining PRSS: (1) Head and trunk (t) and (2) Upper and lower extremities (e). Extent of the disease (N) was assessed on a scale of 0 to 3 (0 = absence of lesions, 1 = 1 to 9 lesions, 2 = 10 to 19 lesions, 3 ≥ 20 lesions). To estimate the severity of the lesions, the three features of erythema (E), infiltration (I), and scaling (S) were graded on a scale of 0–3, in which 0 meant a complete lack of cutaneous involvement and 3 represented the most severe involvement. To calculate the PRSS, sum of the severity grading of these three features were multiplied with the numeric value (N) of the extent of the disease. The formula used was: PRSS = Nt (Et + It + St) + Ne (Ee + Ie + Se). The subscript “t” indicated one side of the trunk and head, and the subscript “e” indicated one side of the extremities.[4] Clearance of PR was defined as PRSS score of 2 or less.
The pruritus was evaluated as per a score of 0–3 as follows: 0 = absence of pruritus; 1 = mild pruritus (if it occurred only intermittently and it did not interfere with work or rest), 2 = moderate pruritus (if it was present for much of the day, but at a more tolerable level) and 3 = severe pruritus (if it interfered with daytime activities or sleep).[4]
The PRSS and pruritus scores were measured at presentation and at four weeks within each group and compared for improvement. Student's t test was used for comparing the mean, whereas the Chi-square test was used for comparing differences in proportions wherever necessary. P < 0.05 was considered significant.
RESULTS
Out of 100 patients, the most common variant was papulosquamous PR (80% of patients) followed by papular (14%) and purpuric PR (6%). No cases of pustular and vesicular PR were recorded. None of our patients were lost to follow up. The characteristics of the patients in two groups are depicted in [Table 1].
Table 1.
Patient characteristics
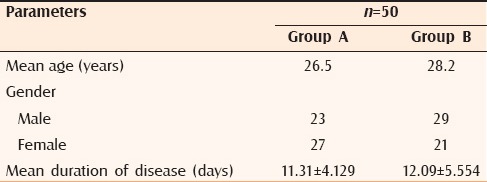
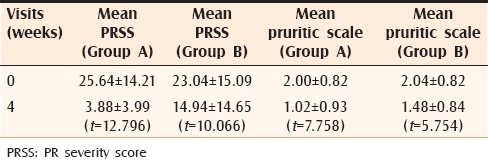
The mean age of the patients in Group A was 26.5 years. In Group A, the pruritus score improved from 2.00 ± 0.82 before treatment to 1.02 ± 0.93 at 4 weeks. The mean PRSS before commencing therapy was 25.64 ± 14.21, which improved to 3.88 ± 3.99 at 4 weeks. Student's t test revealed the improvement to be statistically significant (P < 0.001) for both the parameters [Figures 1 and 2]. The mean age of the patients in Group B was 28.2 years. Patients in Group B also noted improvement in pruritus scores with the values dropping from 2.04 ± 0.82 at 0 weeks to 1.48 ± 0.84 at 4 weeks. This decrease was found to be statistically significant (P < 0.001). The mean PRSS score improved from 23.04 ± 15.09 at 0 weeks to 14.94 ± 14.65 at 4 weeks, which was statistically significant. However, when the t value of improvement in PRSS score in Group A (t = 12.796) was compared with that of Group B (t = 10.066), the t value was higher in Group A as compared with Group B, signifying improvement of patients in Group A was markedly more than their counterparts in Group B. Likewise, the t value of pruritus score in Group A (t = 7.758) was higher than in Group B (t = 5.754) indicating the symptomatic improvement of pruritus in Group A as compared with Group B [Table 2]. Over 4 weeks, 21 patients in Group A reported complete healing of lesions as compared to 9 patients in Group B; 16 patients were relieved of itching in Group A as compared with 6 in Group B. Although all patients in Group A were satisfied with the treatment and reported marked improvement of their symptoms, majority of the patients in Group B were symptomatic at 4 weeks, intensity of disease activity showing a falling trend. The average time for healing of lesions in Group A was 3.8 weeks as compared with 7.4 weeks in Group B. As far as side effects are concerned, in Group A, lesions healed with hyperpigmentation in 62% (31) patients and hypopigmentation in 16% (8) patients and 6% (3) patients complained of a burning sensation.
Figure 1.
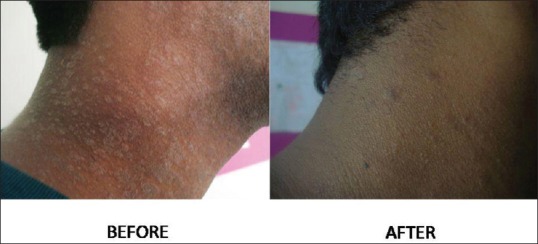
A 23-year-old male patient with papulosquamous PR showing marked improvement in the severity of disease after NBUVB radiation
Figure 2.
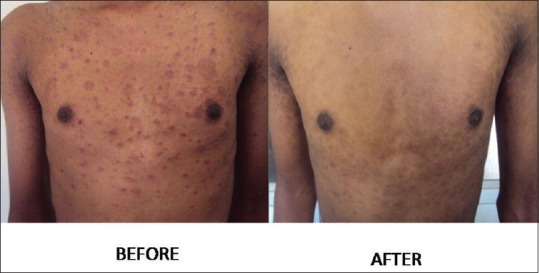
A 17-year-old male patient with erythematous scaly eruption of PR before NBUVB and healing of lesions with hyperpigmentation after treatment
Table 2.
Comparison of PRSS and Pruritic scale in groups A and B
DISCUSSION
PR is considered to be an acute exanthem of vague etiology that accounts for 0.3%–3% of new patients attending dermatology clinics.[1] The cause of PR still remains a dilemma, as is the mechanism of the therapeutic effect of NBUVB irradiation. Some authors consider that a cell-mediated immune mechanism with increased numbers of Langerhans cells may be operative in PR pathogenesis. Recent advances in immunopathology have thrown light on the role of both humoral and cell-mediated immune mechanisms in the pathogenesis of PR.
Antibodies against the cytoplasm of normal human epidermal cells and deposition of IgM in the epidermal cells was demonstrated by Takaki et al.[8] They suggested that anticytoplasmic antibodies produced by some unknown cause may induce the development of secondary eruption of the disease.
The likely rationale of NBUVB in the treatment of PR is suppression of the cell-mediated immune response in PR, facilitating improvement in the patients' symptoms. The variation in the number and function of Langerhans cells in the skin following UV irradiation could be indicative of the mechanism of action of NBUVB light in PR.[6,7]
Itching is the commonest presenting feature in various studies conducted on PR.[3,4,9] Only a few studies discuss the role of NBUVB radiation in ameliorating PR-associated pruritus. Valkova et al. in their study on the role of UVB in 101 cases of PR, did not observe any significant improvement in itching or reduction of symptoms after UVB phototherapy.[10] On the contrary, we observed a significant improvement in the intensity of pruritus. Our observations are in concordance with Leenutaphong and Jiamton, who in their study of 17 patients with PR witnessed a marked reduction in severity of pruritus with UVB phototherapy as compared to UVA radiation.[4]
Various dose regimes of NBUVB have been described in the literature. Most of the studies on UVB in PR have used escalating doses. Leenutaphong and Jiamton compared UVA and UVB radiation for PR and verified that 10 daily erythemogenic exposures of UVB resulted in a significant decrease in disease severity as compared with the control site in 15 of 17 patients.[4] Escalating erythmogenic doses can predispose the individual to dose-related side effects.[11] The above known fact prompted us to use a fixed dose regimen (250 J/cm2) of NBUVB. We noted a substantial improvement in the intensity of pruritus and disease severity with respect to erythema, induration, and scaling.
Our results were in concordance with a previous study by Arndt et al. in which five consecutive erythemogenic NBUVB phototherapy sessions were administered to one half of the body in 20 patients, and it was observed that the extent of disease and pruritus improved more on the treated side than on the untreated side.[12] Similar results have been observed by other authors.[4,13]
Few studies have emphasized on the healing of PR lesions.[4,5,13,14] We observed that the treatment of PR patients with fixed dose NBUVB of 250 mJ/cm2 for 4 weeks facilitates the reduction of disease symptomology (mainly pruritus) and morphological parameters in terms of erythema, scaling, and induration. We also observed that in Group A, there was a slightly increased score in the healing of lesions over the extremities (Ne) when compared to lesions over the head and trunk (Nt). This phenomenon may be attributed to the probability of healing of PR lesions in a caudocephalic manner, a feature that warrants further research.
The drawback of our study was the small sample size. A lager sample could have enabled us to compare NBUVB with other treatment modalities, as well as its effectiveness in other morphological variants of PR.
Before concluding the study, we tried answering the question: What is the need for treating a self-limiting disease such as PR? To let the disease resolve on its own would mean added psychological stress to the patient, with possible worsening of pruritus and spread of lesions. To the best of our knowledge, no randomized double-blind controlled trials of NBUVB in PR have been done so far. Treatment of PR with narrowband UVB considerably decreased the severity of disease and hastened recovery. Our findings should help in formulating future treatment guidelines in the management of PR, although we recommend a larger multicentric trial to exclude sampling bias on account of ethnicity or climatic conditions.
CONCLUSION
The treatment strategy usually pursued in PR is to reassure the patient and to let the disease resolve on its own. However, the long course of the disease of 6–8 weeks and patients' anxiety often compel the physician to administer some treatment. Although diverse treatment modalities are available, NBUVB is a safe, cost effective, and easily available treatment that could be adopted in management of PR, as corroborated by our study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bjornberg A, Tegner E. Pityriasis rosea. In: Fitzpatrick TB, Eisen AZ, editors. Fitzpatrick's Dermatology in General Medicine. 5th ed. New York: McGraw Hill Book Co; pp. 445–9. [Google Scholar]
- 2.Rajpara SN, Ormerod AD, Gallaway L. Adalimumab-induced pityriasis rosea. J Eur Acad Dermatol Venereol. 2007;21:1294–6. doi: 10.1111/j.1468-3083.2007.02197.x. [DOI] [PubMed] [Google Scholar]
- 3.Chuh A, Lee A, Zawar V, Sciallis G, Kempf W. Pityriasis rosea—An update. Indian J Dermatol Venereol Leprol. 2005;71:311–5. doi: 10.4103/0378-6323.16779. [DOI] [PubMed] [Google Scholar]
- 4.Leenutaphong V, Jiamton S. UVB phototherapy for pityriasis rosea: A bilateral comparison study. J Am Acad Dermatol. 1995;33:996–9. doi: 10.1016/0190-9622(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 5.Merchant M, Hammond R. Controlled study of ultraviolet light for Pityriasis rosea. Cutis. 1974;14:548–9. [Google Scholar]
- 6.Bos JD, Huisman PM, Krieg SR, Faber WR. Pityriasis rosea (Gibert): Abnormal distribution pattern of antigen presenting cells in situ. Acta Derm Venereol. 1985;65:132–37. [PubMed] [Google Scholar]
- 7.Richmond VA, Parsons JM. Pityriasis rosea update. J Am Acad Dermatol. 1986;15:159–67. doi: 10.1016/s0190-9622(86)70151-5. [DOI] [PubMed] [Google Scholar]
- 8.Takaki Y, Miyanzaki H. Cytological degerneration of keratinocytes adjacent to Langerhans cells in Pityriasis rosea (Gibert) Acta Derm Venereol. 1976;56:99–103. [PubMed] [Google Scholar]
- 9.Hellgren L. Pityriasis rosea: Die Pravalenz in Geschlechts. Alters and Berfsagruppen in den gernzen Beziol kerungsg ruppen. Hautarzt. 1972;23:492–4. [PubMed] [Google Scholar]
- 10.Valkova S, Trashlieva M, Christova P. UVB phototherapy for Pityriasis rosea. J Eur Acad Dermatol Venereol. 2004;18:111–2. doi: 10.1111/j.1468-3083.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 11.Blauvelt A. Pityriasis rosea. In: Wolff K, Goldsmith L, Paller A, Katz S, Gilchrest BA, Gilchrest B, et al., editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. Vol. 1. New York: McGraw-Hill; 2008. pp. 362–6. [Google Scholar]
- 12.Arndt KA, Paul BS, Stern RS, Parrish JA. Treatment of pityriasis rosea with UV radiation. Arch Dermatol. 1983;119:381–2. [PubMed] [Google Scholar]
- 13.An appraisal of narrow band (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996) Br J Dermatol. 1997;137:327–30. [PubMed] [Google Scholar]
- 14.Cohen EL. Pityriasis rosea. Br J Dermatol. 1967;79:533–7. doi: 10.1111/j.1365-2133.1967.tb11408.x. [DOI] [PubMed] [Google Scholar]


