Abstract
All species of animals display aggression in order to obtain resources such as territories, mates, or food. Appropriate displays of aggression rely on the correct identification of a potential competitor, an evaluation of the environmental signals, and the physiological state of the animal. With a hard-wired circuitry involving fixed numbers of neurons, neuromodulators like serotonin offer adaptive flexibility in behavioral responses without changing the “hard-wiring”. In a recent report, we combined intersectional genetics, quantitative behavioral assays and morphological analyses to identify single serotonergic neurons that modulate the escalation of aggression. We found anatomical target areas within the brain where these neurons appear to form synaptic contacts with 5HT1A receptor-expressing neurons, and then confirmed the likelihood of those connections on a functional level. In this Extra View article, we offer an extended discussion of these recent findings and elaborate on how they can link a cellular and functional mapping of an aggression-regulating circuit at a single-cell resolution level.
Keywords: 5HT1A receptor, aggression, behavior, PLP neurons, serotonin, ventrolateral protocerebrum
Serotonin (5HT) has long been implicated in the regulation of aggression in a wide variety of animal species up to and including humans 1-4. From relatively small populations of central nervous system neurons, 5HT ends up widely distributed throughout the nervous systems of most animal species, where it plays key roles in many physiological processes and essential behaviors like learning and memory, sleep, behavioral arousal and locomotion. Much progress has been made towards identifying the neuronal hard-wired circuitries concerned with such behaviors, but how and where serotonergic neurons fit into that circuitry remains relatively unknown. Serotonin actions are mediated by distinct types of receptors expressed on the surface of target neurons, where they commonly modulate the firing properties of neurons and/or change the effects of excitatory (glutamate) and inhibitory (γ-amino-butyric acid, GABA) signals to and from the cells.5 In mammalian systems, at least 14 subtypes of serotonin receptors have been found. 6 To add to the complexity, in some brain regions 5HT receptors have been localized on neurons that have no direct serotonergic innervation, suggesting that 5HT can be diffusely released as well. In the simpler Drosophila brain only about 100 serotonergic neurons and 5 subtypes of 5HT receptors are found. As in other species, serotonergic neurons in the fly nervous system display arbors of processes that ramify widely in multiple neuropil areas. Earlier studies from several laboratories, including ours, showed that manipulating total populations of 5HT neurons in Drosophila influence a wide variety of different behaviors 7-12. How do serotonergic neurons function to exert such a wide spectrum of behavioral actions? Do single serotonergic neurons exert generalized actions on multiple behaviors, selective actions on specific behaviors, or both? In either case, can we find the subsets of 5HT neurons involved in aggression?
In the Drosophila model of aggression, 13 the acute shut down of the entire population of serotonergic neurons with Shi(ts1) produced male flies that still engaged in fights but with a reduced ability to escalate aggression. 11 Similar results were reported using a less direct technique in an earlier study. 12 Induced dTrpA1-mediated activation of serotonergic neurons, in contrast, resulted in males that escalated fights faster and fought at higher intensities. 11 In our most recent study14 we used an intersectional genetics approach to restrict the population of serotonergic neurons that can be reproducibly manipulated in an attempt to identify those that selectively modulate aggression. We found that two serotonergic neurons per hemisphere from the posterior lateral protocerebrum (PLP) cluster are sufficient to enhance male aggression. Silencing these 5HT-PLP neurons with tetanus toxin light chain reduced, and activating them with dTrpA1 increased the display of high-intensity aggression in male flies. By manipulating either the entire serotonergic system11 or this particular pair of individual 5HT neurons, 14 we observed an approximate two-fold change in the aggression display. This suggests that a substantial part of the serotonergic modulation of aggression comes from the proper functioning of just these specific pairs of 5HT-PLP neurons.
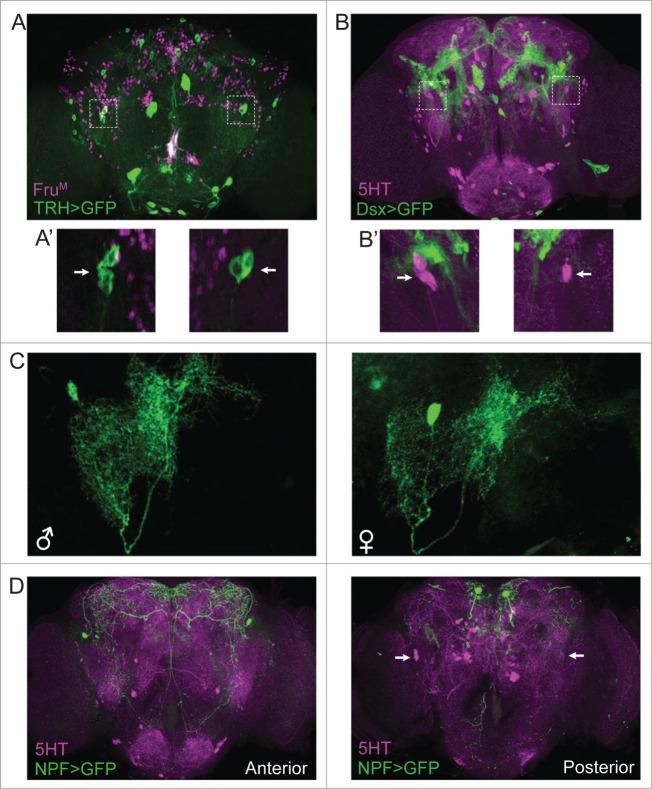
Examination of the anatomical profiles of these aggression-modulating PLP neurons revealed that they course through the entire brain, forming several dense innervation fields along the way. The cell bodies of the identified 5HT-PLP neurons and their axons are located close to the posterior surface of the brain, while their projections are directed anteriorly to form three distinct arborization zones: one in the ventrolateral protocerebrum; a second in close proximity to the fan-shaped body of the central complex; and a third around the peduncles of the mushroom body near the ellipsoid body of the central complex (Figure 1A). The densest arborization is in the ventrolateral protocerebrum, a region previously characterized as an integrative center for auditory, 15 visual, 16 and olfactory information processing. 17 More recently, this region also has been found to be important for courtship. 18 Aggression is a complex adaptable innate behavior that is likely to be regulated by multiple neuronal modulatory networks and influenced by multiple sensory cues. Thus, it may not be surprising that the branches of the 5HT-PLP neurons intermingle with other neuronal processes in well known integrative brain centers, where they likely form numerous input and output connections. To date, the neuronal pathways in fruit flies that trigger aggression remain largely unknown although manipulations of certain gustatory receptors do influence its display19,20. Both males and females show aggressive behavior towards individuals of their own sex, with behavioral patterns that are sexually dimorphic. 21 Only males display the high-intensity aggression pattern called “lunge” that is required to win the fight and only males establish dominance relationships. 13 These male-specific behavioral patterns are controlled by a subgroups of neurons expressing male forms of fruitless proteins (FruM). 22 A recent study reported that a group of male-specific FruM-positive tachykinin (Tk) neurons has been found that promote aggression. 23 The aggression-enhancing serotonergic PLP neurons that we have found do not co-express FruM (Figure 2A) or Doublesex (Figure 2B) and have similar morphological profiles in males and females (Figure 2C). Moreover, the arbors of processes of the 5HT-PLP neurons do not appear to overlap with the processes of FruM-Tk neurons, suggesting that alternative modulatory pathways exist that influence the display of male-specific high-intensity aggression. Additionally, in an earlier study, neuropeptide F (NPF), the invertebrate homolog of neuropeptide Y, has also been reported to influence aggression in male flies, 12 in a direction opposite to the FruM-Tk and 5HT-PLP neurons. The aggression-related part of NPF circuitry too is reported to be male-specific. The 5HT-PLP neurons do not co-express NPF (Figure 2D, posterior). However, NPF neurons have been reported to innervate the fan-shaped body of the central complex24,25 and to partly surround the mushroom body neuropil regions26 as well (also see Figure 2D, anterior). Both of these brain regions are nearby arborization fields of the 5HT-PLP neurons. In earlier studies, we identified aggression-modulating individual dopaminergic neurons from the PPM3 cluster and showed that they too send axonal terminal fields to the fan-shaped body of the central complex.27 Thus, although several potential entry points into aggression-related modulatory networks have been reported recently, downstream anatomical targets of the neurons that make up such aggression-influencing elements remain poorly understood.
Figure 1.
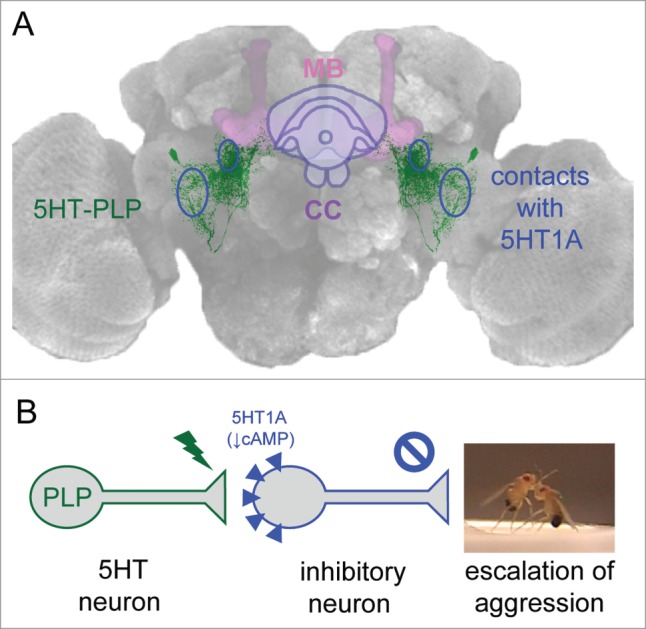
A symmetrical pair of serotonergic PLP neurons promotes the escalation of aggression in Drosophila males. (A) 5HT-PLP neurons and their anatomical target areas, where synaptic contacts with 5HT1A receptor-bearing neurons are formed (blue ovals). CC-central complex, MB-mushroom bodies. (B) Model of the inhibitory control of aggression. An activation of the 5HT-PLP neurons releases the inhibitory input from 5HT1A receptor-bearing neurons, which leads to the escalation of aggression.
Figure 2.
Characterization of the aggression-modulating serotonergic PLP neurons. (A) 5HT-PLP neurons do not co-express FruM. Anti-FruM staining is shown in magenta, serotonergic neurons are visualized by TRH-Gal4/UAS-CD8:GFP (green). The full z stack frontal projection is shown. A' shows the optical section through the 5HT-PLP cell bodies (white arrows). (B) 5HT-PLP neurons do not co-express Dsx. Anti-5HT staining is shown in magenta, Doublesex-positive neurons are visualized by Dsx-Gal4/UAS-CD8:GFP. The full z stack frontal projection is shown. B' shows the optical section through the 5HT-PLP cell bodies (white arrows). (C) 5HT-PLP neurons have similar morphology in males and females. Individual 5HT-PLP neurons are visualized by TRH-Gal4/UAS>stop>CD8:GFP in combination with 417-FLP. (D) 5HT-PLP neurons do not co-express NPF. Anti-5HT staining is shown in magenta, Neuropeptide F-positive neurons are visualized by NPF-Gal4/UAS-CD8:GFP. The anterior and posterior z stack frontal projections are shown separately. White arrows point to the 5HT-PLP cell bodies (posterior view).
In an effort to identify downstream synaptic partners and possible targets of the 5HT-PLP neurons, we first examined the distribution and functioning of the five known subtypes of 5HT receptors within Drosophila brain neuropil regions. The 5HT1B receptor type is known to regulate circadian activity 8 and is highly expressed in the β and γ lobes of the mushroom bodies, but it is absent from the ventrolateral protocerebrum or the central complex. Not surprisingly, therefore, silencing of 5HT1B receptor-expressing neurons with Kir 2.128 or activating them with the dTrpA1 channel (data not shown) had no effects on male aggression. Members of the 5HT2 category (5HT2A and the recently discovered close homologue 5HT2B29) reportedly regulate larval heart rate30, and larval feeding behavior also is regulated by 5HT2A receptors29. The brain distribution of 5HT2B receptors has not been determined, but 5HT2A31, 5HT1A32 and 5HT733 receptor subtypes are expressed in many neuropil regions where they mediate various behaviors in Drosophila. These three subtypes of receptors therefore are potential downstream anatomical targets of the processes of the 5HT-PLP neurons.
A variety of Gal4 driver lines presumably target 5HT receptor-bearing neurons, but none have been fully characterized since antibodies to specific subtypes of Drosophila 5HT receptors are not yet available. We attempted to utilize the GFP reconstitution across synaptic partners (GRASP) method 34 to search for putative synaptic contacts between 5HT neurons and 5HT receptor-expressing neurons. Four of eight candidate receptor Gal4 lines (one 5HT1A- and three 5HT2-Gal4 lines) showed no GRASP signal when examined for putative contacts with 5HT neurons, even though strong and broad Gal4 expression was found. This suggests that those GAL4 lines are either not reliable tools for studying expression patterns of 5HT receptors, or that some receptor neurons have no direct serotonergic innervation since 5HT can be diffusely released as well. Notably, the GRASP analyses did show putative synaptic connections with serotonergic neurons within the terminal arborization areas of the aggression-modulating 5HT-PLP neurons for three of the tested Gal4 lines. Two of these were 5HT1A- and one was a 5HT7-receptor Gal4 driver line. It was of interest that the two anatomical regions where serotonergic neurons formed putative contacts with 5HT1A-expressing neurons – the ventrolateral protocerebrum and the area surrounding the peduncles of the mushroom bodies - were targeted by two different 5HT1A-Gal4 driver lines with non-overlapping expression patterns. A possible explanation for this result might be that the promoter region of the Drosophila 5HT1A gene has a complex structure in parallel with the repressor- and polymorphism-enriched transcriptional regulation of the mammalian Htr1a gene. 35 In that case different promoter regions chosen to generate the Gal4 lines might result in targeting different non-overlapping groups of neurons.
Further functional analysis revealed that dTrpA1-induced activation of neurons targeted by both 5HT1A-Gal4 drivers, but not by the 5HT7-Gal4 driver, resulted in reductions of aggression. This suggests that the 5HT1A-bearing neurons might themselves serve an inhibitory role in aggression pathways or might serve as direct links to a descending inhibitory control pathway. The former suggestion fits well with a model proposing that in vertebrate systems, activation of 5HT1A receptors, located postsynaptically on GABAergic interneurons, mediates hyperpolarizing responses to released 5HT, thereby reducing postsynaptic neuronal excitability and firing rates. 36 The authors of this study suggest that mechanisms of that type might be important links in releasing emotion-related behaviors. Mammalian 5HT1A receptors are implicated in the regulation of mood, emotions and stress responses and are candidate targets in the management of various neuropsychiatric disorders. 37 In Drosophila, 38 as in mammalian systems, 39 activation of 5HT1A receptors inhibits cAMP production, hyperpolarizes neurons, and reduces neuronal excitability. If the 5HT-PLP neurons do target inhibitory, possibly GABAergic, interneurons expressing 5HT1A hetero receptors, a similar “inhibition of inhibition” control mechanism might be operating to release the escalation of aggression in Drosophila upon activation of the 5HT-PLP neurons (Figure 1B).
It is probably an oversimplification to assume that the 5HT-PLP neurons, just as any other single aminergic or peptidergic neuron type, act as sole control element in the regulation of aggression in Drosophila. In our studies14 we note small effects on locomotion and sleep when we reduce activity of the 5HT-PLP neurons, suggesting that these neurons likely serve roles in multiple behaviors including aggression. Also, little is known about the downstream 5HT1A receptor-bearing target neurons reported here. 5HT1A receptor mutant males have been shown to display significant sleep deficits, which can be rescued by reintroducing this receptor subtype into mushroom bodies7. We demonstrated that the 5HT-PLP neurons display three prominent sets of processes, one of them near the peduncles of the mushroom bodies where they form putative contacts with 5HT1A receptor-expressing neurons. It may be possible that via separated sets of processes and nerve endings, the 5HT-PLP together with the 5HT1A receptor neurons may influence two distinct behaviors - aggression and sleep. Another neuropil region associated with the 5HT-PLP neurons near the central complex could be the site where locomotion is influenced, as the central complex is a well-know locomotion integration center. 40,41 Future studies in which different arborization regions of the same serotonergic neurons might be separately activated, perhaps by optogenetic means, would help in unraveling the functioning of these fascinating modulatory neurons.
In summary, the identification of bilateral pairs of aggression-promoting serotonergic neurons revealed the brain regions where likely anatomical connections are formed between these 5HT neurons and a subpopulation of 5HT1A receptor-expressing neurons, establishing key elements of an aggression-regulating circuit. Further experiments are needed to precisely identify the neurons specifically concerned with high-intensity aggression within the group of 5HT1A receptor expressing neurons and to reveal their neurochemical identity. These studies will be crucial next steps towards understanding at least one mechanism through which the high-intensity aggression necessary for the formation of dominance relationships is triggered in fruit flies.
Experimental procedures
The following fly lines were used: NPF-Gal4, UAS-CD8:GFP and UAS> stop>CD8::GFP from the Bloomington Stock Center (Bloomington, IN), Dsx-Gal4 was a gift from Stephen Goodwin (University of Oxford, Oxford, UK), TRH-Gal4 and 417-FLP were described previously.11,14 Adult male and female brains were dissected, fixed, treated with primary and secondary antibodies, and prepared for confocal imaging as described previously.14 The following primary antibodies were used: mouse anti-GFP (1:500) (Invitrogen, Carlsbad, CA), rabbit anti-5HT (1:1000) (Sigma-Aldrich, St. Louis, Missouri), mouse nc82 (1:20) (Developmental Studies Hybridoma Bank, Iowa City, IA) and rabbit anti-Fru (1:5000) (a gift from Barry Dickson, Janelia Research Campus, Ashburn, VA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by grants from the National Institute of General Medical Sciences (GM099883 and GM074675) to EAK and by a departmental NIH training grant to OVA. We thank the Neurobiology Department and the Neurobiology Imaging Facility for consultation and instrument availability that supported this work. This facility is supported in part by the Neural Imaging Center as part of an NINDS P30 Core Center grant #NS072030. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol 2005; 526:51–64. [DOI] [PubMed] [Google Scholar]
- 2.Coccaro EF. Central serotonin and impulsive aggression. Br J Psychiatry Suppl 1989:52-62. [PubMed] [Google Scholar]
- 3.Miczek KA, Weerts E, Haney M, Tidey J. Neurobiological mechanisms controlling aggression: preclinical developments for pharmacotherapeutic interventions. Neurosci Biobehav Rev 1994; 18:97-110. [DOI] [PubMed] [Google Scholar]
- 4.Oliver B. Serotonin and Aggression. Ann NY Acad Sci 2004; 1036:382-92. [DOI] [PubMed] [Google Scholar]
- 5.Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol 2006; 4:101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 2002; 71:533-54. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol 2006; 16:1051-62. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron 2005; 47:115-27. [DOI] [PubMed] [Google Scholar]
- 9.Neckameyer WS, Coleman CM, Eadie S, Goodwin SF. Compartmentalization of neuronal and peripheral serotonin synthesis in Drosophila melanogaster. Genes Brain Behav 2007; 6:756-69. [DOI] [PubMed] [Google Scholar]
- 10.Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, Rottinghaus GE, Zars T. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci U S A 2008; 105:5579-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One 2010; 5:e10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet 2007; 39:678-82. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A 2002; 99:5664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alekseyenko OV, Chan YB, Fernandez MP, Bülow T, Pankratz MJ, Kravitz EA. Single Serotonergic Neurons that Modulate Aggression in Drosophila. Curr Biol 2014; 24:2700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai JS, Lo SJ, Dickson BJ, Chiang AS. Auditory circuit in the Drosophila brain. Proc Natl Acad Sci U S A 2012; 109:2607-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuna H, Ito K. Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J Comp Neurol 2006; 497:928-58. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka NK, Endo K, Ito K. Organization of antennal lobe-associated neurons in adult Drosophila melanogaster brain. J Comp Neurol 2012; 520:4067-130. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H, Lkhagva A, Daubnerova I, Chae HS, Simo L, Jung SH, Yoon YK, Lee NR, Seong JY, Zitnan D, et al.. Natalisin, a tachykinin-like signaling system, regulates sexual activity and fecundity in insects. Proc Natl Acad Sci U S A 2013; 110:E3526-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci 2011; 14:757-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews JC, Fernandez MP, Yu Q, Leary GP, Leung AK, Kavanaugh MP, Kravitz EA, Certel SJ. Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males. PLoS Genet 2014; 10:e1004356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A 2004; 101:12342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan YB, Kravitz EA. Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A 2007; 104:19577-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, Gonzalez CR, Eyjolfsdottir EA, Perona P, Anderson DJ. Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell 2014; 156:221-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahsai L, Winther AM. Chemical neuroanatomy of the Drosophila central complex: distribution of multiple neuropeptides in relation to neurotransmitters. J Comp Neurol 2011; 519:290-315. [DOI] [PubMed] [Google Scholar]
- 25.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 2009; 139:416-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassel DR, Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling? Peptides 2011; 32:1335-55. [DOI] [PubMed] [Google Scholar]
- 27.Alekseyenko OV, Chan YB, Li R, Kravitz EA. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci U S A 2013; 110:6151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan Q, Song Y, Yang CH, Jan LY, Jan YN. Female contact modulates male aggression via a sexually dimorphic GABAergic circuit in Drosophila. Nat Neurosci 2014; 17:81-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasque G, Conway S, Huang J, Rao Y, Vosshall LB. Small molecule drug screening in Drosophila identifies the 5HT2A receptor as a feeding modulation target. Sci Rep 2013; 3:srep02120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majeed ZR, Stacy A, Cooper RL. Pharmacological and genetic identification of serotonin receptor subtypes on Drosophila larval heart and aorta. J Comp Physiol B 2014; 184:205-19. [DOI] [PubMed] [Google Scholar]
- 31.Nichols CD. 5-HT2 receptors in Drosophila are expressed in the brain and modulate aspects of circadian behaviors. Dev Neurobiol 2007; 67:752-63. [DOI] [PubMed] [Google Scholar]
- 32.Luo J, Becnel J, Nichols CD, Nassel DR. Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT(1A) receptor. Cell Mol Life Sci 2012; 69:471-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becnel J, Johnson O, Luo J, Nassel DR, Nichols CD. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS One 2011; 6:e20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 2008; 57:353-63. [DOI] [PubMed] [Google Scholar]
- 35.Albert PR. Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos Trans R Soc Lond B Biol Sci 2012; 367:2402-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altieri SC, Garcia-Garcia AL, Leonardo ED, Andrews AM. Rethinking 5-HT1A receptors: emerging modes of inhibitory feedback of relevance to emotion-related behavior. ACS Chem Neurosci 2013; 4:72-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. P5-HT1A receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl) 2014; 231:623-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J 1992; 11:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polter AM, Li X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal 2010; 22:1406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci 1993; 13:1852-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol 2002; 12:633-8. [DOI] [PubMed] [Google Scholar]