Abstract
Arthropods employ a large family of up to 100 putative taste or gustatory receptors (Grs) for the recognition of a wide range of non-volatile chemicals. In Drosophila melanogaster, a small subfamily of 8 Gr genes is thought to mediate the detection of sugars, the fly's major nutritional source. However, the specific roles for most sugar Gr genes are not known. Here, we report the generation of a series of mutant sugar Gr knock-in alleles and several composite sugar Gr mutant strains, including a sugar blind strain, which will facilitate the characterization of this gene family. Using Ca2+ imaging experiments, we show that most gustatory receptor neurons (GRNs) of sugar blind flies (lacking all 8 sugar Gr genes) fail to respond to any sugar tested. Moreover, expression of single sugar Gr genes in most sweet GRNs of sugar-blind flies does not restore sugar responses. However, when pair-wise combinations of sugar Gr genes are introduced to sweet GRNs, responses to select sugars are restored. We also examined the cellular phenotype of flies homozygous mutant for Gr64a, a Gr gene previously reported to be a major contributor for the detection of many sugars. In contrast to these claims, we find that sweet GRNs of Gr64a homozygous mutant flies show normal responses to most sugars, and only modestly reduced responses to maltose and maltotriose. Thus, the precisely engineered genetic mutations of single Gr genes and construction of a sugar-blind strain provide powerful analytical tools for examining the roles of Drosophila and other insect sugar Gr genes in sweet taste.
Keywords: behavior, Drosophila, GAL4, gene knock out, Gr genes, labial palp, neurobiology, sugar blind, sugar receptors, taste
Introduction
Detection of sugars and other calorie-containing compounds and their discrimination from other chemicals are critical behavioral tasks that enable animals to feed from nutritious food sources. These processes are embedded in the gustatory system, a hallmark of which is the cellular segregation of receptor proteins that detect different groups of chemicals, such as sugars, proteins, and bitter-tasting compounds. In all characterized animal model systems, food chemicals stimulate different types of taste receptor cells from those stimulated by chemicals with no nutritional value or harmful and toxic compounds by virtue of cell-specific expression of cognate receptors.1
In Drosophila, taste sensilla constitute the sensory structures for the detection of all soluble chemicals. Taste sensilla, which are the functional equivalents of mammalian taste buds, are found in several major body parts, especially the labellum and the legs. Most taste sensilla contain 4 gustatory neurons (GRNs; some sensilla contain only 2 GRNs), as well as a mechanosensory neuron2; the four neurons are thought to be dedicated to different taste modalities, which have been associated with 3 appetitive promoting (sweet, modestly salty, and water) and one aversive (bitter/highly salty) modalities. Additionally, flies are also known to respond with acceptance behavior when provided with amino acids or fatty acids,3-5 the cellular mechanism of which is not well understood.
Most Drosophila species are frugivores, and taste plays a central role in flies' feeding behavior. Sweet sensation is mediated by the sweet GRNs present in most if not all sensilla of the 2 main taste organs, the labial palps and the distal most segments of the tarsi. Each of these sweet GRNs is thought to express members of a Gr gene subfamily composed of 8 sugar Gr genes (Gr5a, Gr61a, and Gr64a to Gr64f).6-10 A ninth Gr gene, Gr43a, was recently shown to be critical for sensing internal (brain hemolymph) fructose.11 In the taste system, Gr43a is expressed in only a pair of tarsal taste sensilla, and its contribution to sucrose and fructose sensing is secondary to receptors formed by sugar Gr proteins.11
While Gr5a and Gr61a have been characterized in some detail and shown to play a critical role in trehalose and glucose sensing, respectively,8,12-14 the specific functions of each of the Gr64 genes are less defined, other than the fact that as a whole, this gene cluster is essential for sugar responses both at the behavioral and cellular level.6,15 This paucity is due to lack of specific mutations in single Gr64 genes, a consequence of the densely clustered organization of the Gr64 locus (see Fig. 1). Regardless, specific roles have been assigned for some of these genes based on phenotypes of deletion and insertion mutations. However, these types of mutations are likely to alter expression of structurally unaffected genes within the locus, which is exemplified by the more severe phenotypes ascribed to the Gr64ab mutation (deleting Gr64a, and Gr64b) than the more subtle phenotype observed in Gr64a2 (deleting Gr64a, Gr64b, and Gr64c).7,8 Thus, lacking defined mutations and comprehensive expression profiles, it is not possible to determine the specific roles of the 6 Gr64 genes in sweet taste or the composition and tuning profiles of receptor complexes to specific sugars.
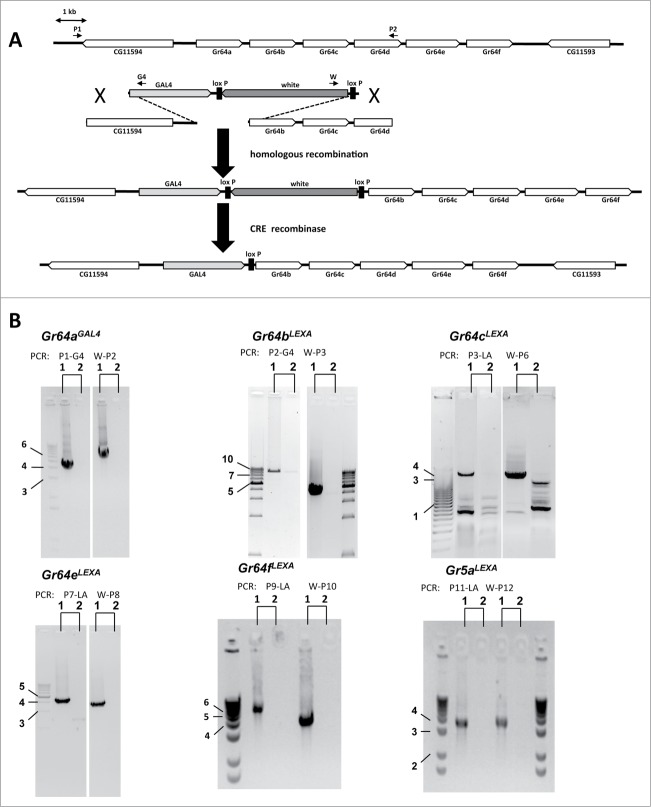
Figure 1 (See previous page).
GAL4/LEXA knock-in strategy for sugar Gr genes using homologous recombination. (A) Genomic region of the Gr64 locus and the targeting construct for Gr64aGAL4 are shown in the 2 diagrams at the top. Homologous recombination replaces Gr64a with GAL4 and the w+ minigene, which is removed via CRE mediated recombination (bottom diagrams). Positions of primers used for PCR analysis are indicated by short arrows. Note that the coding sequences of all other sugar Gr genes were replaced by LEXA. (B) PCR analysis of genomic DNA isolated from successfully targeted homozygous lines (1) and respective donor lines (2). Location of primes P1, P9 and P2, P10 (see A) anneal to genomic DNA upstream and downstream of, but not within, the donor construct; Expected DNA fragment sizes for the 5′ and 3′ products are 4.2 kb and 5.9 kb for Gr64aGAL4 (Gr64a), 4.6 kb and 4.6 kb (Gr64b), 6.3 kb and 4.6 kb (Gr64c) and 4.6 kb and 4.6 kb (Gr64e), 5.7 kb and 4.6 kb (Gr64fLEXA) and 3.7 kb and 3.8 kb (Gr5a). Primers G4, LA and w (see Experimental Procedures) are specific for GAL4, LEXA and white gene. All PCR fragments had the expected size, except for the 5′ fragment of Gr64b. Additional PCR analysis revealed a ˜4kb DNA insertion upstream of the Gr64a gene. Numbers refer to location of molecular weight marker bands and indicate fragment size (in kb). Primer sequences are shown in Figure S1.
Here, we report the generation of powerful genetic tools that allow us to address these and several additional questions about insect sugar receptors. We constructed a number of Gr mutations via homologous recombination that revealed detailed expression of 5 of the 6 Gr64a genes.16 Moreover, we created a sugar-blind strain in which all 8 sugar Gr genes were deleted. We use this strain to show, contrary to a recent report,17 that functional sugar receptors are composed of at least 2 sugar Gr protein subunit. Lastly, we identify 2 functional receptor complexes for recognition of the sugars maltose and sucrose, as well as glycerol.
Results
The genetic tools presented in this paper will overcome 3 major impediments that have slowed progress in our understanding of sweet taste in Drosophila. First, there is a lack of precise and useful mutations for the 6 densely clustered genes in the Gr64 locus. Second, expression for many sugar Gr genes has not been established and, hence, the role of such genes in sweet taste remains speculative. Third, we currently lack a tool that unequivocally associates specific sugar chemicals with Gr proteins. The tools presented here will help overcome these obstacles, and they not only provide a path to a clear understanding of the role of each sugar Gr gene in sweet taste, but also will aid in elucidating the composition of specific insect sugar taste receptors.
Gene targeting of Gr5a and Gr64a-f loci
The GAL4/UAS expression system has been successfully employed in many studies for the analysis of many Gr genes.9,10,18,19 However, the success rate for Gr64-GAL4 transgenes has been poor, and for half of the genes, no cellular expression profile has ever been reported with this system. Therefore, we generated a series of sugar Gr knock-in alleles through homologous recombination.20,21 We generated 7 transgenic fly strains containing a LEXA or GAL4 targeting construct on the second chromosome, consisting of 5′ and 3′ non-translated sequences of all 6 Gr64 genes and Gr5a (Fig. 1, Fig. S1). While null alleles for both Gr5a and Gr61a are available,8,13,14 the former, but not the latter, was included in this study because 2 independently generated Gr5a transgenes were found to be expressed not only in sweet GRNs, but also in additional taste neurons.16,19 With the exception of the Gr64d construct, all transgenes were successfully recombined into their target site, replacing the Gr coding sequence with LEXA or GAL4 and producing 6 new knock-in/null alleles: Gr5aLEXA, Gr64aGAL4, Gr64bLEXA, Gr64cLEXA, Gr64eLEXA, and Gr64fLEXA (for details, see Experimental Procedures). When these alleles are combined in a fly with specific reporter genes containing transcription factor binding sites for GAL4 or LEXA (UAS-RFP or lexAop-GFP), they should replicate endogenous Gr gene activity. Indeed, all new knock-in alleles revealed expression either only in GRNs or in GRNs and additional chemosensory cells of the olfactory system or nutrient sensing brain neurons. A detailed expression analysis of these alleles is described in a separate study.16
Effects of individual knock-in mutations on cellular response
The utility of the single gene mutations in assessing the effect on gustatory receptor neuron responses was tested for the Gr64aGAL4 mutation. Gr64a was chosen because it has been proposed to be essential for proper sensing of many sugars, including fructose, maltose, maltotriose, stachyose, raffinose, and others7,8 by labellar taste neurons. Yet, lack of expression of Gr64aGAL4 in labellar neurons, as well as absence of a PER phenotype,16 is not consistent with a major role for this Gr gene in sugar sensing. Thus, we determined the cellular responses of homozygous and heterozygous Gr64aGAL4 mutant flies in tarsal neurons, where Gr64aGAL4 is expressed, using Ca2+ imaging (Fig. 2). We focused these imaging experiments on GRNs of the 5b sensilla, as opposed to the 5v sensilla (albeit both produced similar responses; see Fig. S2), because the neurons of the latter also express the Gr43 fructose receptor,16 which alone is sufficient to mediate response to sucrose and fructose.11 Heterozygous control flies showed robust neuronal responses to all sugars tested (Fig. 2B). Consistent with the relatively mild behavioral deficits of Gr64aGAL4 mutant flies,16 GRNs of such flies produced robust responses upon stimulation with most sugars, and reduction, but not a complete loss, of Ca2+ responses to maltose and maltotriose only (Fig. 2B). Thus, the precise gene knock-in mutations are likely to provide a more accurate assessment for the contribution of individual sugar Gr genes than gene deletions used in previous studies, many of which also included regulatory sequences.7,8
Figure 2.
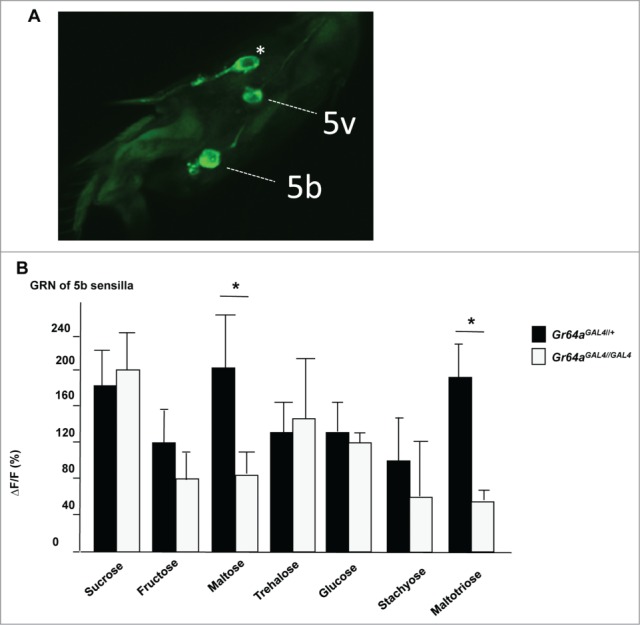
Sweet taste neurons of Gr64aGAL4 mutant flies respond normally to most sugars. (A) Antibody staining of tarsi of Gr64f-GAL4;UAS-mCD8GFP shows 3 labeled sweet neurons; the two neurons associated with 5b and 5v sensilla were used for Ca2+ imaging. The additional, 5s associated sweet neuron is indicated with an asterisk. Note that Gr64f-GAL4 and Gr64aGAL4 are co-expressed in the sweet GNR of these sensilla.16 (B) Ca2+ responses of sweet GRNs associated with the 5b sensillum of Gr64aGAL4/+ (control) and Gr64aGAL4/GAL4 flies. Responses to maltose and maltotriose, but to none of the other sugars, were significantly reduced in homozygous mutants. All sugars were at 100 mM. Student's t-test: * P < 0.05; 4<N<8.
Generating a sugar-blind Drosophila strain
Null alleles (i.e., lack of function alleles), such as Gr64aGAL4, are useful when determining the contribution of a single gene to a particular taste trait. However, for delineating sufficiency (i.e., which genes together may encode for functional sugar receptors) null alleles are of limited value. Sufficiency is best assessed with the help of heterologous expression systems, which, unfortunately, have had little success in the context of insect taste receptors. An alternative approach to heterologous expression systems is the generation of an “empty neuron” system, whereby deletions/mutations are introduced in every Gr gene expressed in a particular neuronal subtype. Such an “empty neuron” system has been powerfully employed in the Drosophila olfactory system, where it was used to unambiguously identify the ligands for numerous olfactory receptors.22 While single GRNs express many more Gr genes than olfactory neurons express Or genes, we sought to test whether neurons of flies lacking all 8 sugar Gr genes could be used as a “sugar Gr deficient neuron system.” We therefore generated a strain in which all 8 sugar Gr genes carried null alleles (octuple mutant). Variations of this octuple mutant strain, also referred to as “sugar-blind” strain (or ▵8Grsugar/▵8Grsugar), were also equipped with a GRN specific GAL4 driver and a transgene for either the calcium indicator GCaMP6.0 (octuple mutant DRIVER strain) or one or more sugar Gr transgenes (octuple mutant REPORTER strains; Table 1). When octuple mutant flies from these strains are crossed, the effects on the cellular and behavioral responses of single or pair wise combinations of sugar Gr genes in otherwise sugar-blind flies can be quantitatively assessed using Ca2+ imaging and the proboscis extension reflex (PER) assay, respectively. To verify suitability of the ▵8Grsugar/▵8Grsugar strain, we first examined the sugar-induced neuronal responses in 2 types of GRNs, one expressing the non-canonical fructose receptor Gr43a (associated with the 5v sensilla) and one lacking expression of that gene (5b sensilla), of octuple mutant flies (as well as heterozygous control flies).16 No neural activity was observed in 5b associated sweet GRNs in homozygous flies upon stimulation with any sugar solution tested, whereas control flies responded robustly to all sugars (Fig. 3A). In contrast, the 5v-associated GRN, which express Gr43a, was activated when stimulated with fructose and sucrose, to a level approximating that of heterozygous control flies. These observations are consistent with our previous analysis of the Gr43a, which showed that this receptor functions independently of the sugar Gr proteins as a fructose sensor.11 Moreover, they suggest that the “sugar Gr deficient neuron system” is adequate to determine the response profile upon re-introduction of sugar Gr genes.
Table 1.
Strains generated and used in this study. List of strains used for the examination of phenotypes of (i) mutations in single sugar Gr genes (top six lines) and of transgene rescue in octuple mutant (Gr5aLEXA; ΔGr61a ΔGr64a-f) background (bottom four lines). R1 is an X linked genomic construct that contains two essential non-Gr genes missing in the ΔGr64a-f deletion.
| Genotype | Description | Remarks |
|---|---|---|
| Gr5aLEXA;+;+ | Gr5a null allele | Coding Region Replaced by LEXA |
| +;+; Gr64aGAL4 | Gr64a null allele | Coding Region Replaced by GAL4 |
| +;+; Gr64bLEXA | Gr64b null allele | Coding Region Replaced by LEXA |
| +;+; Gr64cLEXA | Gr64c null allele | Coding Region Replaced by LEXA |
| +;+; Gr64eLEXA | Gr64e null allele | Coding Region Replaced by LEXA |
| +;+; Gr64fLEXA | Gr64f null allele | Coding Region Replaced by LEXA |
| R1,Gr5aLexA;+;Δ61a, Δ64a-f | All sugar Grs deleted | sugar blind strain |
| R1,Gr5aLexA; Gr61a-GAL4:GCamP6m/Cyo; ΔGr61a, ΔGr64a-f | Driver line for the rescue experiment in octuple mutant background | Yields sugar blind flies suitable for Ca2+ imaging when crossed to sugar blind strain |
| R1,Gr5aLEXA; UAS-Gr64X/Cyo; ΔGR61a, ΔGR64a-f | UAS lines used for single rescue experiment in octuple mutant background | Yields flies with single sugar Gr suitable for Ca2+ imaging when crossed to sugar blind strain |
| Gr5aLEXA; UAS-Gr64X,UAS-Gr64Y/Cyo ; ΔGr61a, ΔGr64a-f/TM6b | Reporter strains used for double Rescue experiment in octupleMutant background | Yields flies with two sugar Gr suitable for Ca2+ imaging when crossed to sugar blind strain |
UAS-Gr64f construct is on the X chromosome (recombined onto R1, Gr5aLEXA chromosome) and UAS-Gr5a construct is on third chromosome (recombined onto ΔGr61a, ΔGr64a-f chromosome).
Figure 3.
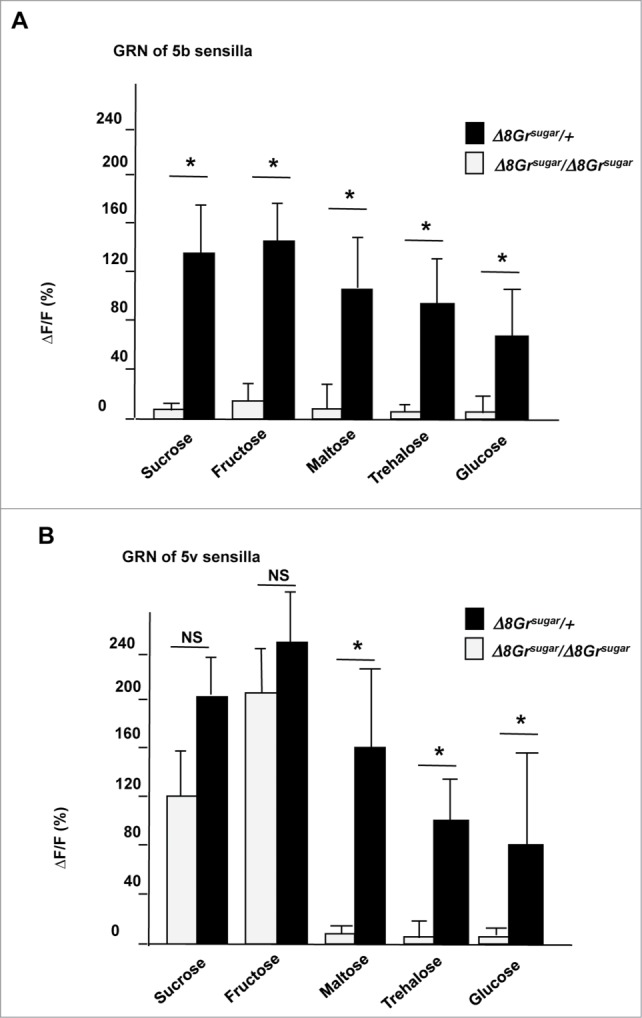
Many sweet taste neurons of octuple mutant flies lack sugar responses. (A) 5b associated sweet GRNs of ▵8Grsugar/▵8Grsugar flies (homozygous mutant for all 8 sugar Gr genes) lack responses to any sugar tested, while imaging of the same neuron of sugar ▵8Grsugar/ +flies respond robustly to all sugars tested. (B) 5v associated sweet GRNs, which express the atypical fructose receptor Gr43a, of ▵8Grsugar/▵8Grsugar flies respond to sucrose and fructose, but not to maltose, trehalose, and glucose. Heterozygous flies show somewhat stronger responses to sucrose and fructose, indicating that receptors for these sugars are formed by sugar Gr proteins. All sugar concentrations were at 100 mM. Student's t-test: NS, Not Significant; * P < 0.05; 3<N<7.
Sugar receptors are encoded by 2 or more sugar Gr genes
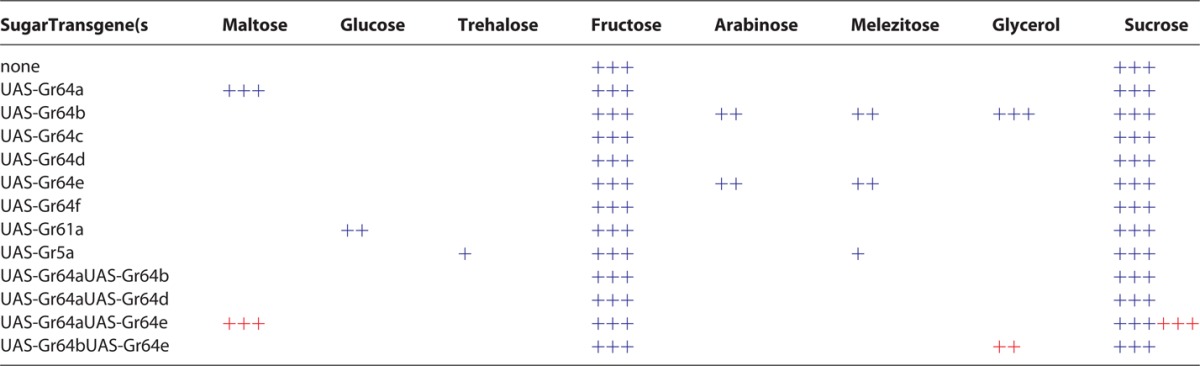
It was recently reported that olfactory neurons expressing any single sugar Gr gene are activated when bathed in a sugar solutions, and the authors suggested that single Grs function in the absence of other sugar Grs. This observation, however, contradicts evidence from numerous studies that strongly argue that functional sugar receptors are composed of 2 (or more) different sugar Gr proteins.6-8,15 To address whether or not single Gr proteins can mediate sugar responses, we expressed each of the 8 sugar Gr genes in sweet GRNs of octuple mutant flies and performed Ca2+ imaging experiments on the tarsal 5b sensilla. None of the Gr proteins, when expressed singly, led to a fluorescence increase after application of 8 different sugar solutions. Interestingly, when we measured cellular responses of the 5v associated Gr43a expressing neuron, expression of single sugar Gr genes was sufficient for activation following application of some sugars (Table 2). For example, expression of Gr64a alone elicited a maltose response in the Gr43a neuron, expression of Gr64b alone or Gr64e alone elicited responses to arabinose, melezitose, and glycerol, and expression of Gr61a alone elicited a glucose response. The interpretation of these results is that Gr43a can form complexes with sugar Gr proteins to form receptors for select sugars.
Table 2.
Summary of single and double rescue Ca2+ imaging experiments. Sweet GRN responses using Ca2+ imaging observed in the 5b sensilla (lacking expression of Gr43a; red), and in the 5v sensilla (expressing Gr43a; blue) of sugar blind (Δ8Grsugar/Δ8Grsugar) flies expressing a (top) or pairs of select sugar Gr genes are shown. Empty fields indicate no statically significant increase compared sugar blind (Δ8Grsugar/Δ8Grsugar) flies; +, ++ and +++ indicate statistically significant increase compared to sugar blind flies with cellular response < 33%, 33 to 66% and > 66% compared to control flies (Δ8Grsugar/+), respectively. Note that the GRN of the 5v sensilla responds to sucrose and fructose, due to expression of the Gr43a gene in that neuron. Also note that expression of single UAS-Gr transgene in the 5b-associated GRN fails to restore responses to any sugar, while expression in the 5v-assocaited GRN leads to the recovery of some sugar responses. However, expression of pairs of UAS-Gr genes recovers select sugar response in the 5b-associated neuron (red). UAS transgenes were expressed under the control of Gr61a-GAL4.
 |
The experiments described thus far are consistent with the hypothesis that Gr proteins do not function as single receptors or homodimers, but are composed of at least 2 different Gr subunits. To test this idea further, we randomly chose 4 pairwise UAS-Gr combinations, expressed them in octuple mutant flies, and monitored activity in the sweet GRN of the 5b sensillum (which does not express Gr43a; Figure 4). Indeed, 2 combinations lead to strong neural responses to a select group of sugars. Specifically, the Gr64a/64e pair induced strong response to maltose and sucrose, but not to glucose, trehalose, fructose, arabinose, melezitose, and glycerol. In contrast, the Gr64b/64e pair was able to induce glycerol-specific responses, but did not mediate responses to the other 7 sugars we tested. We note that 2 other combinations of Gr proteins—Gr64a/Gr64b and Gr64a/Gr64d—failed to convey cellular responses in 5b associated sweet GRNs of octuple mutant flies when tested with any of the 8 sugars.
Figure 4.
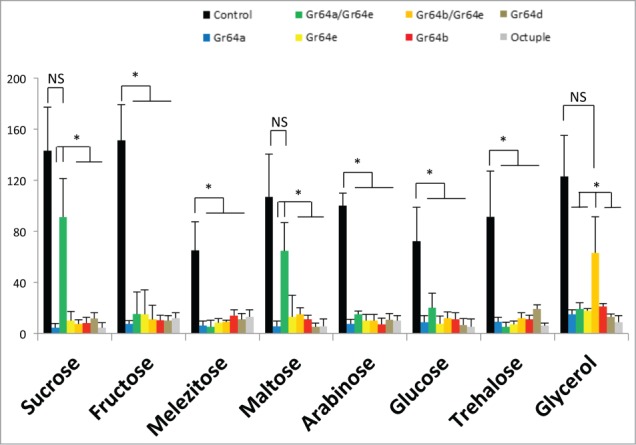
Two sugar Gr proteins are necessary to form functional sugar receptors 5b-associated sweet GRNs of octuple mutant flies (gray) expressing Gr64a (blue), Gr64b (red), Gr64d (tan), or Gr64e (yellow) do not respond to any of the 8 sugars we tested. However, when octuple mutant flies were provided with Gr64a and Gr64e (green), or Gr64b and Gr64e (orange), responses to maltose and sucrose, or glycerol, was recovered. All sugars were at 100mM. Student's t-test: P < 0.05; 3<N<7.
Conclusion
We have generated a number of precise sugar Gr mutations that can be used both as null alleles as well as expression alleles. We also generated a sugar-blind fly strain that lacks measurable sugar responses in sweet taste neurons (except in those expressing Gr43a), and we have explored the utility of the sugar-blind strain to answer some important, pressing questions. Indeed, one of the main findings from our study is that sugar receptors are multimeric complexes composed of 2 or more subunits, rejecting the suggestion derived from pseudo-heterologous expression studies that single sugar Gr genes can mediate sugar responses on their own.22 Using sugar-blind flies, we have determined the necessary components of 2 sugar receptors, one tuned to the disaccharides sucrose and maltose and one to the sugar alcohol glycerol.
Surprisingly, while the Gr43a fructose receptor functions on its own in the brain and probably in other chemosensory organs,11 as well as in heterologous expression systems,23 it is capable of forming additional sugar receptors when combined with other Gr proteins (Table 2). This observation suggests that some Grs, albeit none of the sugar Grs, function as homomultimers, but in combination with other Gr proteins, they can combine to form receptors with novel ligand properties.
Our imaging analysis of Gr64a mutant flies, together with behavioral studies of single Gr64 mutant flies,16 demands re-evaluation of the promoted model of sweet taste, which suggested that 2 multimeric receptors composed by only 3 Gr proteins (including Gr64a) function as the major, if not sole, receptors for sweet chemicals. Indeed, the conspicuous expression of Gr64a in nutrient sensing neurons in the brain, along with the absence in labial palp neurons,16 suggests that the main function for this gene is likely the sensing of an internal sugar, rather than a dietary one.
Lastly, whether the GRNs in the octuple mutant strains represent a true empty neuron system remains to be determined. It is impossible to rule out that other Gr genes are expressed in sweet GRNs; moreover, a number of Ionotropic chemoreceptor genes are expressed in the gustatory system24-26 and it is not known whether they are expressed in sweet GRNs. Finally, sweet GRNs were recently shown to mediate taste response to fatty acids, suggesting expression of receptors in these neurons that recognize such chemicals.4 Regardless, the complete lack of sugar mediated responses in GRNs that lack expression of Gr43a should make the octuple mutant strain a powerful tool to analyze not only Drosophila sugar Gr genes, but also putative sugar Gr genes from other insect species.
Experimental Procedures
Molecular cloning of knock-in constructs
Targeting constructs for ends-out homologous recombination were based on the CMC-loxP-Gal411 and CMC-loxP-LexA::VP16 vectors. CMC-loxP-LexA::VP16 was obtained by first adding loxP sites into the AvrII the BstEII sites of the CMC vector.27 From the resulting plasmid (“CMC-loxP”), we cloned the LexA::VP16 sequence into the SpeI and AvrII sites yielding the targeting vector CMC-loxP-LexA::VP16. To generate gene-specific targeting constructs, PCR fragments flanking the gene being targeted were cloned into the TOPO-XL vector (Life Technologies) and then subcloned into the upstream and downstream multiple cloning sites of CMC-loxP-Gal4 (Gr64a) or CMC-loxP-LexA::VP16 (Gr5a, Gr64b, Gr64c, Gr64e, and Gr64f).
In most cases, restriction sites were introduced into the primer sequence used to generate the PCR fragments, with the following exceptions: (1) Gr5a 3′ flank—Internal SpeI site in the PCR product and a SpeI site in the TOPO vector were used to ligate fragment to NheI site in 3′ MCS. (2) Gr64b 3′ flank—Internal NheI site in the PCR product was used to ligate fragment to NheI site in 3′ MCS. (3) Gr64c 3′ flank—Internal NheI site in the PCR product was used to ligate fragment to NheI site in 3′ MCS (4) Gr64f 3′ flank—Internal NheI site in the PCR product was used to ligate fragment to NheI site in 3′ MCS.
Primer Gr5a 5′ Flank Sense—CGTACGCCGCAACTGG-AAATGGAAATCTGA
Primer Gr5a 5′ Flank Antisense—ACTAGTTGTGTACAAGCTCTAAATCCTGACTAAACG Primer Gr5a 3′ Flank Sense—GGTGACCCACCCTTCAATCTTGATTAGACGCAC Primer Gr5a 3′ Flank Antisense —GCTAGCGTTTTTACGCCTGCTGTCTGCTG Primer Gr64a 5′ Flank Sense—GGCGCGCCCTGTCGTTGGTTCTCCAGCAGC Primer Gr64a 5′ Flank Antisense—CGTACGGACGCTGGTCCCT-TTTGCACTGAC Primer Gr64a 3′ Flank Sense—GCGG-CCGCTGGACAACAATAGCCACCAACACC Primer Gr64a 3′ Flank Antisense—GCTAGCCAAGCCGCACTTCCCACATAGG Primer Gr64b 5′ Flank Sense—GGCGCGCCGCAA-ATGGGGGAAGATCATTACTGGG
Primer Gr64b 5′ Flank Antisense—CGTACGGGCCAAA-CTAGCACTAACCAAACGAC
Primer Gr64b 3′ Flank Sense—GCGGCCGCATCC-TAGAATTTACTACTCGTATCTCCAATTCAAGAACG
Primer Gr64b 3′ Flank Antisense—GCTAGCCTCACTTT-TCGAACTGGCATCAAAGC
Primer Gr64c 5′ Flank Sense—GGCGCGCCGTAGCTATAT-TACTACTGCCCTACGTTCACTG
Primer Gr64c 5′ Flank Antisense—ACTAGTGGCTTGACT-GTTGGGTAGCAAATG
Primer Gr64c 3′ Flank Sense—GCGGCCGCTTCTAG-TTTGAAATTTGCATTCTGTCGCACCTTC
Primer Gr64c 3′ Flank Antisense—GCTAGCCTTTTCTT-CAGCCGCCTCAACTTG
Primer Gr64e 5′ Flank Sense—GGCGCGCCGTGAGTT-GAGAAATGACTTTACACAGCTTAG
Primer Gr64e 5′ Flank Antisense—ACTAGTGTTCCGTA-CTCGACTGACAACCAATC
Primer Gr64e 3′ Flank Sense—GCGGCCGCATTTTGTG-GAAGTGGCAGGGGGTTAAG
Primer Gr64e 3′ Flank Antisense—GCTAGCGATGCGGA-TGTGTCCCAGTACTTG
Primer Gr64f 5′ Flank Sense—GGCGCGCCGTGGAGTGCAAGCTGGATGCGAAC Primer Gr64f 5′ Flank Antisense—ACTAGTCCTAGGACCTGCTGGGGTAAACTG Primer Gr64f 3′ Flank Sense—GCGGCCGCCCGCTAGAGAGATTCTACGTGTGTCCG Primer Gr64f 3′ Flank Antisense—GCTAGCCTTATGGCGGACACTGCAATCCTGG.
The transgenes were excised and linearized as described by Miyamoto et al.11 and potential relocation onto the third chromosome for the Gr64GAL4/LEXA constructs and the X for the Gr5aLEXA construct was evaluated based on segregation from respective chromosome balancers. Between 2 and 10 lines with integration on the respective chromosome were generated and genomic DNA of homozygous flies with putatively recombined alleles was isolated. To determine whether the coding sequence of the respective Gr genes was precisely replaced with either that of LEXA or GAL4, we performed PCR using a primer within the targeting construct and a primer complementary to a sequence just upstream of downstream of the targeting construct for each Gr gene.
Ca2+ imaging of tarsal taste sensilla
Preparation of forelegs and Ca2+ imaging of taste sensilla was performed as described by Miyamoto et al.12. Concentration of all sugar was 100 mM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Tetsuya Miyamoto for advice on Ca2+ imaging experiments. AY, JS and HA designed the experiments. JS generated the targeting constructs. JS, CJ, and AY produced the knock-in alleles, and JS and CJ performed the molecular analysis on the targeted alleles. AY carried out all Ca2+ imaging experiments. HA wrote the paper.
Funding
This work was supported by grants from the NIH (RO1-DC009014 and RO1-DC005606) to HA.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron 2014;81: 984-1000; PMID:24607224; http://dx.doi.org/ 10.1016/j.neuron.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res 1994;275: 3-26; PMID:8118845; http://dx.doi.org/ 10.1007/BF00305372 [DOI] [PubMed] [Google Scholar]
- 3.Toshima N, Hara C, Scholz CJ, Tanimura T. Genetic variation in food choice behaviour of amino acid-deprived Drosophila. J Insect Physiol 2014;69: 89-94; PMID:25010547; http://dx.doi.org/ 10.1016/j.jinsphys.2014.06.019 [DOI] [PubMed] [Google Scholar]
- 4.Masek P, Keene AC. Drosophila fatty acid taste signals through the PLC pathway in sugar-sensing neurons. PLoS Genet 20139: e1003710; PMID:24068941; http://dx.doi.org/ 10.1371/journal.pgen.1003710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toshima N, Tanimura T. Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. J Exp Biol 2012;215: 2827-2832; PMID:22837455; http://dx.doi.org/ 10.1242/jeb.069146 [DOI] [PubMed] [Google Scholar]
- 6.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol 2007;17: 1809-1816; PMID:17919910; http://dx.doi.org/ 10.1016/j.cub.2007.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci U S A 2007;104: 14110-14115; PMID:17715294; http://dx.doi.org/ 10.1073/pnas.0702421104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron 2007;56: 503-516; PMID:17988633; http://dx.doi.org/ 10.1016/j.neuron.2007.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell 2004;117: 981-991; PMID:15210117; http://dx.doi.org/ 10.1016/j.cell.2004.06.011 [DOI] [PubMed] [Google Scholar]
- 10.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol 2004;14: 1065-1079; PMID:15202999; http://dx.doi.org/ 10.1016/j.cub.2004.05.019 [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell 2012;151: 1113-1125; PMID:23178127; http://dx.doi.org/ 10.1016/j.cell.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto T, Chen Y, Slone J, Amrein H. Identification of a Drosophila glucose receptor using ca(2+) imaging of single chemosensory neurons. PLoS One 2013;8: e56304; PMID:23418550; http://dx.doi.org/ 10.1371/journal.pone.0056304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueno K, Ohta M, Morita H, Mikuni Y, Nakajima S, Yamamoto K, Isono K. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol 2001;11: 1451-1455; PMID:11566105; http://dx.doi.org/ 10.1016/S0960-9822(01)00450-X [DOI] [PubMed] [Google Scholar]
- 14.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci 2001;4: 1182-1186; PMID:11704765; http://dx.doi.org/ 10.1038/nn765 [DOI] [PubMed] [Google Scholar]
- 15.Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol 2008;18: 1797-1801; PMID:19026541; http://dx.doi.org/ 10.1016/j.cub.2008.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii S, Yavuz A, Slone J, Jagge C, Song X, Amrein H. Drosophila Sugar Receptors in Sweet Taste, Olfaction and Internal Nutrient Sensing. Current Biology 2015;15: 621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman EG, Wisotsky Z, Dahanukar A. Detection of sweet tastants by a conserved group of insect gustatory receptors. Proc Natl Acad Sci U S A 2014;111: 1598-1603; PMID:24474785; http://dx.doi.org/ 10.1073/pnas.1311724111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott K, Brady R Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 2001;104: 661-673; PMID:11257221; http://dx.doi.org/ 10.1016/S0092-8674(01)00263-X [DOI] [PubMed] [Google Scholar]
- 19.Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Current Biology : Cb 2001;11: 822-835; PMID:11516643; http://dx.doi.org/ 10.1016/S0960-9822(01)00258-5 [DOI] [PubMed] [Google Scholar]
- 20.Rong YS, Golic KG. A targeted gene knockout in Drosophila. Genetics 2001;157: 1307-1312; PMID:11238415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science 2000;288: 2013-2018; PMID:10856208; http://dx.doi.org/ 10.1126/science.288.5473.2013 [DOI] [PubMed] [Google Scholar]
- 22.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell 2004;117: 965-979; PMID:15210116; http://dx.doi.org/ 10.1016/j.cell.2004.05.012 [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci U S A. 2011;. 108(28): 11680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh TW, He Z, Gorur-Shandilya S, Menuz K, Larter NK, Stewart S, Carlson JR. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron 2014;83: 850-865; PMID:25123314; http://dx.doi.org/ 10.1016/j.neuron.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang YV, Ni J, Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science 2013;340: 1334-1338; PMID:23766326; http://dx.doi.org/ 10.1126/science.1234133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, Gibson TJ, Benton R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet 2010;6: e1001064; PMID:20808886; http://dx.doi.org/ 10.1371/journal.pgen.1001064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 2004;43: 703-714; PMID:15339651; http://dx.doi.org/ 10.1016/j.neuron.2004.08.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.