Abstract
Smooth muscle myosin light chain kinase (MLCK) plays a crucial role in artery contraction, which regulates blood pressure and blood flow distribution. In addition to this role, MLCK contributes to Ca2+ flux regulation in vascular smooth muscle (VSM) and in non-muscle cells, where cytoskeleton has been suggested to help Ca2+ channels trafficking. This conclusion is based on the use of pharmacological inhibitors of MLCK and molecular and cellular techniques developed to down-regulate the enzyme. Dissimilarities have been observed between cells and whole tissues, as well as between large conductance and small resistance arteries. A differential expression in MLCK and ion channels (either voltage-dependent Ca2+ channels or non-selective cationic channels) could account for these observations, and is in line with the functional properties of the arteries. A potential involvement of MLCK in the pathways modulating Ca2+ entry in VSM is described in the present review.
Keywords: Myosin light chain kinase, TRP, voltage-dependent calcium channels, vascular smooth muscle
Abbreviations
- VSMC
vascular smooth muscle cell
- VSM
vascular smooth muscle
- SMC
smooth muscle cell
- ER
endoplasmic reticulum
- SR
sarcoplasmic reticulum
- MLCK
myosin light chain kinase
- VOC
voltage-operated Ca2+ (channel)
- ROC
receptor-operated Ca2+ (channel)
- SOC
store-operated Ca2+ (channel)
- CaM
calmodulin
- TRP
transient receptor potential (channel)
- siRNA
small interfering RNA
- [Ca2+]cyt
cytosolic Ca2+ concentration
Introduction
Smooth muscle contractility is controlled by the phosphorylation status of myosin light chain (LC20), which is determined by the balance between the activity of myosin light chain kinase (MLCK) and myosin light chain phosphatase. More recently, the contribution of cytoskeleton remodelling to facilitate the transmission or maintenance of force has been recognized.1 The major role in the development of contraction is played by Ca2+ and results from the Ca2+ dependence of MLCK activity. An increase in Ca2+ and Ca2+/Calmodulin (CaM) complex activates smooth muscle MLCK (smMLCK, referred to as MLCK; a product of 130 kDa from mylk1 gene).2,3 Without Ca2+ or CaM, MLCK cannot be activated.4 In vascular smooth muscle cells (VSMC), cytosolic Ca2+ concentration ([Ca2+]cyt) increases as a result of Ca2+ release from intracellular Ca2+ stores located in the sarcoplasmic reticulum (SR) and of Ca2+ entry from the extracellular space through selective voltage-operated Ca2+ (VOC) channels and non-selective cation channels.5,6 Nevertheless, there are still several gaps in our understanding of the regulation of Ca2+ signaling in VSM. Particularly, the nature of the channels involved in Ca2+ entry and the mechanism of their activation remain unclear, disputed or not investigated. Interest in the involvement of MLCK and cytoskeleton in Ca2+ channels activation in smooth muscle and non-muscle cells has increased in recent years. This review provides an overview of the current state of knowledge on the contribution of MLCK to Ca2+ channels regulation mechanisms in VSM from large to small arteries.
Vascular smooth muscle: from large to small arteries
Mechanisms of [Ca2+]cyt increase vary according to vessel types and excitatory stimuli, probably because the expression of contractile proteins differs from proximal to more distal arteries.7 Time course of contractile response to a vasoconstrictor agonist differs in conduit versus resistance arteries. This correlates with either tonic smooth muscles that develop slower rates of force activation and relaxation, as observed in the aorta, or phasic smooth muscles that display faster rates of force activation and relaxation, as found in portal vein and in the microcirculation.7,8 In addition, resistance arteries, which compose the microcirculation, exhibit myogenic tone, this is the ability to contract in response to change in intraluminal pressure and is closely related to resistance microarteries intrinsic role in blood supply and blood pressure regulation.9 Significant differences in agonist-induced [Ca2+]cyt increase in VSMC from large conductance vs. small resistance arteries stem from the smaller contribution of intracellular Ca2+ release from the SR10 and the higher contribution of voltage-dependent Ca2+ entry11,12 observed in small resistance artery compared to large conduit artery contraction.
Calcium channels expression in vascular smooth muscle
In response to vasoconstrictor agonist, Ca2+ entry from the extracellular space occurs through VOC channels activated by membrane depolarization, and non-selective cation channels, most of them members of the transient receptor potential canonical (TRPC) channels family. TRPC channels are activated following receptor occupancy (and called receptor-operated cation channels or ROC) or by internal Ca2+ stores depletion inducing capacitative Ca2+ entry (store-operated cation channels or SOC). They simultaneously induce the entry of Na+ and Ca2+ triggering cell membrane depolarization and [Ca2+]cyt increase.5,6
All TRPC isoforms are found in VSM, with the exception of TRPC2 and TRPC7.13,14 The expression level of TRPC members is varying depending on the vessel type.15 Commonly, TRPC1 and TRPC6 are highly expressed.16-21 In general TRPC4 is detected at a lower expression level than TRPC1 and TRPC6 as described in rat aorta,17,20 resistance mesenteric artery,16 cerebral artery,19 renal artery20 and is not detected in caudal artery.18 TRPC3 level is higher in rat cerebral artery,19,22 caudal artery18,23 and renal artery20 than in the conductance artery aorta.17,20 TRPC3 is expressed in rat resistance mesenteric artery but its level of expression is disputed.16,21 While TRPC5 is not detected in rat resistance mesenteric artery,16,21 a slight signal is observed in aorta17,20 as well as in renal artery.17,20.
Voltage-dependent L-type (CaV1.2), P-/Q-type (CaV2.1) and T-type (CaV3.1 and CaV3.2) Ca2+ channels are expressed in VSMC. They are characterized by distinct pharmacological and electrophysiological properties.24 However, their relative distribution varies along the vascular tree.25 CaV1.2, CaV2.1, CaV3.1 and CaV3.2 are expressed in a quite similar manner in aorta, while in resistance mesenteric artery the expression of CaV2.1, CaV3.1 and CaV3.2 is higher than that of CaV1.2.25-28 Similarly, although L-type and T-type Ca2+ channels are present in SMC from cerebral artery, T-type Ca2+ channels are predominantly expressed.29 Gustafsson et al.30 failed to detect L-type channels mRNA in rat mesenteric arterioles <40 μm in diameter while T-type channels are expressed, suggesting that T-type channels might be associated with the regulation of myogenic tone in resistance arteries.
Culture is reported to strongly affect TRPC and VOC channels expression. In cultured rat mesenteric myocytes, all TRPC proteins are up-regulated compared to freshly dissociated cells.31 In addition, cultured cells lose their expression of L-type VOC channels, while the expression of T-type VOC channels is increased.32 Conversely, in the aortic cell line A7r5, L-type, P-/Q-type and T-type VOC channels are expressed and mRNA content of TRPC1, TRPC4 and TRPC6 is detected at a high level, while the expression of TRPC2, TRPC5 and TRPC7 is controversial.33-35
TRPC and VOC channels are not static molecules. They operate in association with other intracellular molecules required for their trafficking and insertion in the plasma membrane and modulating their activity within signal transduction complexes. Interactions of channel proteins with regulatory proteins have already been considered in detail in several reviews.13,15,36,37 In addition, modulation of Ca2+ channels activation by several protein kinases (PK) such as PKC, PKG, PKA, Src tyrosine kinase and Ca2+/CaM-dependent protein kinase II (CaMKII) has also been described based on the effects of protein kinases inhibitors on TRPC and VOC channels activity.38-40 How MLCK contributes to Ca2+ channel activation is the issue raised in the present review.
Calcium channels regulation by MLCK
Controversial contribution of MLCK to calcium channels regulation in cultured or freshly isolated cells
Growing evidence highlights a role of MLCK in [Ca2+]cyt increase in several smooth muscle and non-muscle cellular models. Indeed, concurring studies suggest that MLCK is involved in the activation of Ca2+ channels (Table 1). They were performed in different cell types, measuring either cytosolic Ca2+ or cation current using pharmacological inhibitors to block MLCK activity (Table 2 for IC50 or Ki values) or molecular techniques to depress the kinase expression.
Table 1.
Effect of MLCK inhibition on Ca2+ signaling in cultured or freshly isolated cells
| Cell type | MLCK Inhibition | Stimulating agent | Effect on [Ca2+]cyt | References |
|---|---|---|---|---|
| Freshly isolated rabbit portal vein myocytes | ML-9 (5 μM), ML-7 (5 μM), MLCK(11-19)amide (5 μM) | NA, GTPγS, OAG | ↘Icat | 43 |
| AV25 (10 μM) | No effect on Icat(ATP) | |||
| Freshly isolated rabbit mesenteric artery myocytes | WT (20 μM) | AngII | ↘Icat | 122 |
| OAG | No effect | |||
| Freshly isolated guinea-pig gastric myocytes | ML-7 (3 μM) | CCh | ↘ICCh | 50 |
| Voltage | ↘IBa (L-type VOC) | |||
| Freshly isolated human monocytes/ macrophages | ML-9 (10-100 μM), WT (1-100 μM), MLCK antisense | TG, CPA | ↘CCE | 44 |
| Freshly isolated murine ileal myocytes | ML-7 (5 μM), ML-9 (10 μM) | CCh | ↘ICCh | 42 |
| Cultured bovine adrenal glomerulosa cells | WT (10 μM), ML9 (100 μM), KT5926 (100 μM), MS-347a (30 μM) | AngII | ↘Ca2+ entry | 123 |
| Iono, TG, KCl | No effect | |||
| Cultured porcine aortic endothelial cells | ML-9 (1-100 μM) | BK, TG | ↘CCE | 124,125 |
| ML-7 (0.1-30 μM) | 126 | |||
| MLCK antisense | 41 | |||
| Cultured rat pulmonary artery endothelial cells | ML-9 (100 μM) | TG | ↘CCE | 127 |
| Cultured rat pulmonary artery smooth muscle cells | ML-9 (10-100 μM), ML-7 (1-100 μM) | Hypoxia | ↘CCE | 128 |
| Cultured human HEK293 cells | ML-9 (100 μM) | TG | ↘CCE; ↘ICRAC |
49 |
| MLCK siRNA, WT (20 μM) | No effect | |||
| Cultured human HEK293 cells overexpressing TRPC5 | ML-7 (3 μM), MLCK-siRNA | CCh | ↘ICCh | 46 |
| Cultured human HEK293 cells overexpressing TRPC5 | ML-9 (3-50 μM), WT (1-10 μM), | CCh, ATP | ↘Ca2+ entry (no effect on Ca2+ release) | 45 |
| MLCK dominant negative | ↘ ICRAC | |||
| Cultured human HEK293 cells overexpressing TRPC6 | ML-9 (10 μM), ML-7 (10 μM) | CCh | ↘ICCh | 48 |
| WT (3 μM), MLCK dominant negative inhibitory peptide | No effect | |||
| Cultured rat aortic SMC | ML-7 (10 μM) | VP | No effect | 47 |
| Cultured rat aortic cell line A7r5 | MLCK-siRNA | VP | ↘Ca2+ entry | 410 |
| Voltage | ↘IBa (L- and T-type VOC) |
Abbreviations: NA: noradrenaline; VP: vasopressin; KCl: physiological solution enriched in K+; TG: thapsigargin; Iono: ionomycin; AngII: angiotensinII; BK: bradykinin; CCh: carbachol; CPA: cyclopiazonic acid; WT: wortmannin; OAG: 1-oleoyl-2-acetyl-sn-glycerol ; ↘ decrease; VOC: voltage-dependent Ca2+ channels; IBa: barium current; ICRAC: calcium-release activated calcium current; Icat: cationic current; CCE: capacitative Ca2+ entry.
Table 2.
IC50 or Ki values of pharmacological inhibitors on MLCK activity
| Pharmacological inhibitor | Target | IC50 or Ki (μM) |
|---|---|---|
| Wortmannin | acts at or near the catalytic domain of MLCK in a noncompetitive and irreversible manner; far more potent inhibitor of PI3K of Class 1 and Class 2132,150 | IC50: 1.9 |
| AV25 | targets the auto-inhibitory site of MLCK.51 | IC50: 0.2 |
| MLCK(11-19)amide | binds to the substrate site of MLCK.129 | Ki: 10 |
| KT5926 | targets the ATP-binding site of MLCK in a competitive mode and the substrate site of MLCK in a noncompetitive manner; far more potent inhibitor of CaMKII.151,152 | Ki: 0.0044 - 0.018 |
| K-252a | targets the ATP-binding site of MLCK; far more potent inhibitor of CaMKII.152,153 | Ki: 0.0018 - 0.020 |
| MS-347a | binds to the catalytic domain of MLCK in an irreversible manner.130 | IC50: 9.2 |
| ML-9 | targets the ATP-binding site of MLCK.101,152 | Ki: 3.9 IC50 (on NA-evoked Icat): 2 |
| ML-7 | targets the ATP-binding site of MLCK.101,152 | Ki: 0.3 IC50 (on NA-evoked Icat): 0.8 |
IC50 and Ki values with respect to MLCK activity, except for ML-7 and ML-9 where the IC50 values were obtained on noradrenaline (NA)-induced cationic current (Icat).
Most of the data obtained in cultured or freshly isolated cells suggest MLCK implication in a ROC/SOC-dependent Ca2+ entry. Using the synthetic naphtalenesulphonyl derivatives, ML-9 and ML-7, Watanabe et al.41 and Kim et al.42 showed that MLCK contributes to agonist-induced Ca2+ influx in endothelial cells and murine ileal myocytes, respectively. This observation has been confirmed by using MLCK antisense oligonucleotide.41 In rabbit portal vein myocyte, 5 μM of a substrate-specific peptide inhibitor of MLCK, MLCK(11-19)amide, inhibits the cation current activated by noradrenaline by 80%, and 10 μM of AV25, a peptide of 25 amino acids targeting the auto-inhibitory site of MLCK, depresses the amplitude of the cation current by 72%.43 MLCK is also reported to be involved in capacitative Ca2+ entry following thapsigargin or cyclopiazonic acid treatment in human monocytes/macrophages.44 In addition, by using a MLCK dominant negative mutant and RNA interference for MLCK, Shimizu et al.45 and Kim et al.,46 respectively, suggested the involvement of MLCK in the activation of TRPC channels responsible for a non-capacitative Ca2+ entry in HEK293 cells activated by carbachol.
Other researchers reported either no effect of MLCK inhibitor on [Ca2+]cyt increase, as observed in cultured aortic SMC (Fig. 1A)47 or a MLCK independent effect48,49 (Table 1). Smyth et al.49 showed that ML-9 inhibits capacitative Ca2+ entry by preventing the localization of stromal interaction molecule 1 (STIM1) into punctae structures between the plasma and endoplasmic reticulum (ER) membranes where it can interact and activate Ca2+ channels. This observation could not be confirmed with a small interfering RNA (siRNA) directed against MLCK, which suggests that the effect of ML-9 is not related to MLCK inhibition.49 Similarly, a direct effect of ML-9 on the channel protein, independently on change in MLCK activity, is suggested to explain the inhibition of TRPC6 current in transfected HEK293 cells.48
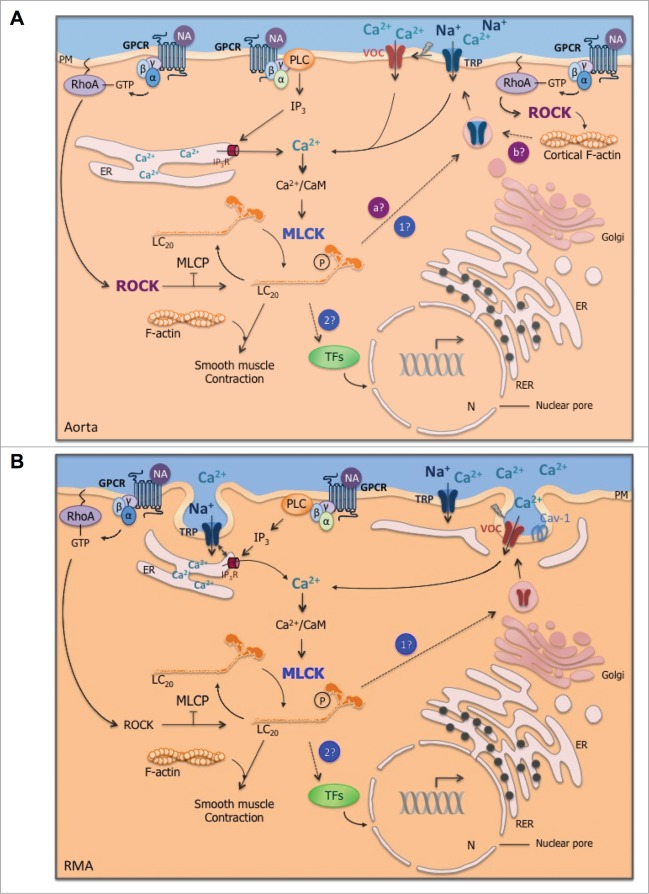
Figure 1.
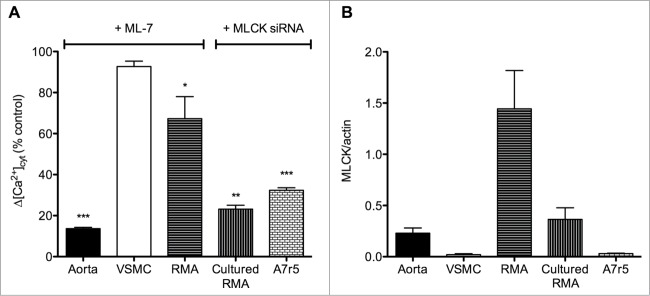
Effect of MLCK inhibition on agonist-induced cytosolic Ca2+ concentration ([Ca2+]cyt) increase in comparison with MLCK expression level in aorta, cultured aortic smooth muscle cells (VSMC), resistance mesenteric artery (RMA), cultured RMA (organ-cultured for 4 days) and the aortic cell line A7r5. (A) Δ[Ca2+]cyt response after agonist stimulation (noradrenaline 10 μM in RMA and cultured RMA and vasopressin 10 nM in aorta, VSMC and A7r5 cells) in the presence of the MLCK inhibitor (ML-7 at 3 μM in aorta and RMA and 10 μM in VSMC) or after MLCK down-regulation with anti-MLCK-siRNA (in cultured RMA and A7r5) compared to control stimulated conditions. The VOC channel antagonist verapamil (1 μM) was used to block the voltage-dependent component of the [Ca2+]cyt increase in response to vasopressin in aorta. (B) Mean values of Western blot data. MLCK expression was normalized to the actin content. Vertical bars represent the SEM (n = 3–7). *, **, *** Significant differences compared to control conditions. Results were extracted from published data.10,47 The expression level of MLCK is lower in VSMC compared to whole vascular smooth muscle (aorta or RMA) but this does not explain the weaker contribution of MLCK to Ca2+ channel regulation in VSMC. Indeed larger inhibition of Δ[Ca2+]cyt is observed in aorta compared to RMA, while the expression level of MLCK is lower.
Only 2 reports suggest a role of MLCK in voltage-dependent Ca2+ entry.10,50 We, recently, demonstrated that the decreased MLCK expression after anti-MLCK siRNA transfection in the aortic cell line A7r5 leads to the inhibition of L-type Ca2+ current and VOC-dependent Ca2+ entry (Fig. 1A).10 However, decreased Ca2+ flux is associated with decreased CaV1.2 expression in MLCK-depleted cells, preventing to confirm direct regulation of VOC channel activity by MLCK (Fig. 2).10
Figure 2.
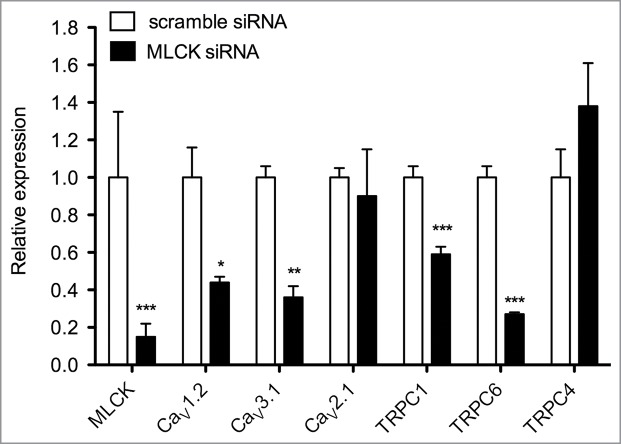
Effect of MLCK depletion on genes expression in A7r5 cells. Relative mRNA expression of L-type (CaV1.2), P/Q type (CaV2.1) and T-type (CaV3.1) voltage-dependent Ca2+ channels and TRPC1, TRPC6 and TRPC4 non-selective cation channels in anti-MLCK-siRNA-transfected A7r5 cells (black bars) and scramble-siRNA-transfected A7r5 cells (open bars) (n = 5-8). mRNA level of each gene was normalized to the level of RPL32 mRNA and compared to the value in scramble-siRNA-transfected cells. Results are expressed as mean ± SEM (vertical bars). * P < 0.05, ** P < 0.01, *** P < 0.001 vs. scramble-siRNA-transfected cells. Data on MLCK and voltage-dependent Ca2+ channels were extracted from published data.10 MLCK downregulation in A7r5 cells is associated with a decrease in the mRNA expression of several Ca2+ channels.
MLCK regulation of cytosolic calcium increase in agonist stimulated smooth muscle tissues
Few studies have investigated the involvement of MLCK in Ca2+ signaling in isolated artery or smooth muscle tissue (Table 3). The use of inhibitory peptides in whole artery is quite difficult due to their high molecular weight. In rat caudal artery, 75 μM of the inhibitory peptide AV25 is required to markedly inhibit the contractile tension without affecting the LC20 phosphorylation status.51 Wortmannin, a more potent inhibitor of phosphoinositide 3-kinase (PI3K) than of MLCK, does not affect the cytosolic Ca2+ response to high KCl in rat aorta,52 rabbit aorta,53 guinea-pig ureter54 or human myometrium,55 where response to oxytocin is also not affected. This could be explained by the relatively low concentration in wortmannin used, whereas a higher concentration depresses the Ca2+ response to high KCl and vasoconstrictor agonist in porcine carotid artery,56 this was confirmed when inhibiting MLCK with ML-9. Involvement of MLCK in Ca2+ channels activation in smooth muscle is also disputed by the observation that in fura-2-loaded guinea-pig tracheal smooth muscle, ML-9 inhibits the increase in [Ca2+]cyt and the contraction induced by 60 mM KCl, methacholine or thapsigargin, while wortmannin inhibits the contraction elicited by these stimuli without affecting [Ca2+]cyt. Similarity between the effects of ML-9 and the cation channel blocker SKF-96365 suggests that ML-9 acts as a potent inhibitor of Ca2+-permeable channels independently on MLCK inhibition in tracheal smooth muscle.57
Table 3.
Effect of MLCK inhibition on Ca2+ signaling in smooth muscle tissues
| Tissue type | Inhibitor | Stimulating agent | Effect on [Ca2+]cyt | References |
|---|---|---|---|---|
| Rat aorta | WT (1 μM) | KCl, Phe | No effect | 52 |
| Rabbit aorta | WT (1 μM) | KCl, DPB | No effect | 53 |
| Rat aorta | ML-7 (3 μM) | NA | ↘Ca2+ influx | 47 |
| Rat resistance mesenteric artery | ML-7 (3 μM), MLCK-siRNA | NA, KCl | ↘Ca2+ influx; ↘IBa (L-type VOC) |
10 |
| Porcine carotid artery | ML-9 (10-100 μM), WT (10 μM) | KCl | ↘Ca2+ influx | 56 |
| Porcine aortic endothelial cells (in valvular strips) | ML-9 (30-100 μM) | TG | ↘CCE | 131 |
| WT (10 μM) | No effect | |||
| Guinea-pig tracheal smooth muscle | ML-9 (10-100μM) | KCl, MC, TG | ↘Ca2+ influx | 57 |
| WT (3 μM) | No effect | |||
| Guinea-pig ureter | WT (4 μM) | KCl | No effect | 54 |
| Human myometrium | WT (4 μM) | Spontaneous contraction, oxytocin | No effect | 55 |
Abbreviations: NA: noradrenaline; VP: vasopressin; KCl: physiological solution enriched in K+; DPB: 12-deoxyphorbol 13-isobutyrate; MC: methacholine; TG: thapsigargin; Phe: phenylephrine; WT: wortmannin; ↘ decrease; IBa: barium current; CCE: capacitative Ca2+ entry.
In opposition, in isolated rat aorta, the more potent MLCK inhibitor, ML-7, decreases the non-voltage-dependent and non-capacitative Ca2+ entry induced by vasopressin (Fig. 1A), and inhibits LC20 phosphorylation.47 In rat resistance mesenteric artery, where contraction is mainly dependent on VOC activation, ML-7 depresses the voltage-dependent Ca2+ entry in response to noradrenaline (Fig. 1A) and the depolarization-activated L-type current.10 These results were confirmed with a siRNA directed against MLCK, which was transfected by reversible permeabilization in resistance mesenteric artery.10 After 3 days of artery culture, MLCK protein content was depressed by approximately 50%, which was associated with similar reduction of VOC-dependent Ca2+ signals in response to noradrenaline (Fig. 1A) or high KCl. As also observed in A7r5 cells, the decrease in Ca2+ responses in MLCK-down-regulated arteries is associated with a decrease in the mRNA expression of CaV1.2 channel, though this decrease was not statistically significant.10
Factors limiting the identification of calcium channels regulation by MLCK
Data summarized above indicate that the degree of MLCK contribution to the regulation of Ca2+ channels activity is variable among arteries and cell models and that no precise Ca2+ channel type can be identified as the target of MLCK activity.
The first difficulty lies in the lack of specific pharmacological inhibitors against MLCK. Selectivity of those inhibitors is controversial and should be viewed cautiously. Most of them are far more potent inhibitors of other kinases: wortmannin against PI3K,58 KT5926 and K-252a against CaMKII.59 The more efficient compound is the synthetic naphtalenesulphonyl derivative ML-7, which is approximatively 2.5 fold more potent than ML-9 to reduce noradrenaline-induced cation current.43 However, effect of pharmacological inhibitor was not always confirmed in knockdown models and direct interaction with Ca2+ channel has been suggested in several studies.48,57
Down-regulation of MLCK protein is an alternative method to the use of chemical inhibitors. However, interpretation of changes in Ca2+ movements can be biased by the fact that depletion in MLCK protein is associated with a decrease in several Ca2+ channels expression, as described below.
The complexity of excitation-response coupling mechanisms and the different contribution of voltage-dependent and non-voltage-dependent Ca2+ channels to contraction according to the origin and the type of the vessel contribute to the difficulty of drawing a global model accounting for MLCK effect.11,12 Identification of the channel targeted by MLCK is also hindered by the low selectivity of Ca2+ channels pharmacological inhibitors. The activity of TRPC is usually measured using compounds as SKF-96365, flufenamic acid or gadolinium, none of these being specific. The function of TRPC has then been highlighted by using downregulation techniques with oligonucleotide antisense or siRNA transfection methods or knockout (KO) animal models. However, KO models are reported to lead to compensatory overexpression as exemplified by the up-regulation of TRPC3 in aorta and cerebral artery from TRPC6 KO mice60 or to conflicting results as observed for TRPC1, which does not appear to be an obligatory component of SOC or stretch-activated cation channels in cerebral artery from TRPC1−/- mice, but is required for ROC and SOC in siRNA transfected aortic cells17 and in endothelial cells from TRPC4−/- mice or STIM1 mutant.61 Selectivity of VOC channels blockers is also questionable.27,62 Unfortunately the use of CaV1.2 KO mice is compromised as mice die before birth, probably due to heart defect.63
The level of MLCK expression might also influence the degree of MLCK contribution to Ca2+ flux regulation. A comparison of MLCK protein expression in aorta versus small resistance mesenteric artery shows that the relative MLCK content is higher in resistance artery than in aorta (Fig. 1B).64 The activity of MLCK is also different according to the vessel type, being 1.9-fold lower in tonic smooth muscle compared to phasic smooth muscle.65 The higher activity of MLCK is likely to occur in phasic smooth muscle where faster contractile responses are observed.65 As shown on Figure 1 and as previously demonstrated in cultured tracheal SMCs,66 the level of MLCK expression is lower in cultured aortic SMC compared to aorta and resistance mesenteric artery, and Ca2+ entry is also less affected by MLCK inhibition. This observation could suggest that a lower level of MLCK expression is associated with a weaker contribution of MLCK to Ca2+ channels regulation. However, this model does not fit with the larger inhibition of Ca2+ entry by ML-7 in aorta compared to resistance mesenteric artery (Fig. 1A).
Eventually, the intracellular organization of SMC from large and small artery could affect Ca2+ entry mechanisms by favoring interactions and cross-regulation between intracellular compartments. Intracellular distribution of different pools of MLCK as well as of Ca2+ ions might influence the contribution of MLCK to the regulation of the contractile machinery and the Ca2+ entry mechanisms. Further experiments would allow confirming this hypothesis.
The apparent absence of Ca2+ channel selectivity in the effect of MLCK could reflect an action of the kinase on a process in the activation pathway that might be shared by several types of channels. By regulating the translocation of the channel protein and its functional insertion in the membrane through a modulation of cytoskeleton filaments, MLCK could contribute to the activation of several types of Ca2+ channels, but also of other signaling molecules for which trafficking is an important step in the pathway leading to their activation.
Involvement of MLCK in calcium channels trafficking
Calcium channels translocation
Ca2+ channels activation requires their functional insertion in the plasma membrane. We currently know from studies essentially performed in non-VSMC models, that ion channels inserted in intracellular vesicles are translocated to plasma membrane following agonist or mechanical stimulation.67-69
Translocation of TRP channels to plasma membrane has been reported to occur within smooth muscle caveolae,70 which are omega-shaped membrane invaginations containing caveolin-1, the main smooth muscle caveolin, involved in the clustering of signaling molecules and in the regulation of receptors/mediators trafficking.71 Caveolae and caveolin-1 have been shown to spatially localize IP3R1 and TRPC3 in cerebral myocytes.72 Similarly, caveolin-1 is involved in potassium channels (BKCa) and L-type Ca2+ channels co-localization within caveolae in mesenteric artery SMC.73 On the opposite, in conductance artery L-type Ca2+ channels translocation is independent of caveolae and KCl-induced contraction is not affected in caveolin-1 KO models.70,74,75 This observation can be related to the different intracellular organization of resistance and conductance arteries, the SR being preferentially localized in cell periphery in the former, while in conductance artery it is more centrally located.76 It is worth mentioning that, in addition to their differential role in the regulation of Ca2+ flux, caveolae are reported to differentially contribute to RhoA activation and artery contraction in resistance artery77 and in aorta.74,75
Scaffolding proteins such as the vesicle-associated membrane protein 2 (VAMP2) and the soluble N-ethylmaleimide-sensitive factor associated protein (αSNAP),78 the protein Homer79 and the PDZ motif 80,81 found in TRPC that have been identified in cultured HEK293 cells and neuronal cells could be involved Ca2+ channels trafficking in vascular smooth muscle. The only partner identified so far in A7r5 cells, primary cerebral artery myocytes and intact cerebral artery82 is PKCδ, which mediates the trafficking of TRPM4-containing vesicles to plasma membrane in response to pressure increase.
Translocation of the voltage-dependent Cav1.2 channels to plasma membrane involves the auxiliary subunits of the L-type VOC channel. The β subunit in HEK293 cells83-85 and the α2δ-1 subunit in cerebral artery SMC86 are essential interacting partners for the α1C subunit translocation. Binding of intracellular galectin-1, which is a carbohydrate-binding protein, to the α1-interacting domain of the α1C subunit can reduce channel expression at the plasma membrane in mesenteric artery as well as in A7r5 cells.87 But the relevance in small resistance artery where T-type channels instead of L-type channels are predominant and responsible for the myogenic tone remains elusive.
Recently, STIM1 activated by store depletion has been shown in HEK293 cells and A7r5 cells to bind to the C-terminal of CaV1.2 through its Ca2+-release activated Ca2+ domain and to inhibit CaV1.2 expression at the plasma membrane.88,89 There is an important discrepancy between this model and previous reports showing that store depletion-induced Ca2+ signal is depressed in the presence of the VOC Ca2+ channel blocker diltiazem in cultured aortic cells suggesting that Cav1.2 participates to capacitative Ca2+ entry.90 Similarly, 2 reports demonstrate that VOC channels contribute to the refilling of Ca2+ stores in A7r5 cells10 and guinea-pig intestinal smooth muscle.91
A potential role for MLCK in calcium channels trafficking
Although few data are available in whole vascular smooth muscle tissue, cytoskeleton elements appear to be ideal candidates to support Ca2+ channels trafficking in collaboration with scaffolding partners and regulatory proteins. In view of the role played by MLCK in cytoskeleton modulation, MLCK regulation of Ca2+ channel activity could implicate a contribution to channel protein trafficking.
In this line, MLCK through LC20 phosphorylation, and myosin motors are known to contribute to transport vesicles from the Golgi apparatus to the ER along actin filaments.92,93 The issue is still poorly investigated in VSM but Dey et al.94 demonstrated in CaV3.1 and CaV3.2 transfected HEK293 cells that brefeldin A, which disrupts the assembly of the Golgi apparatus, prevents the translocation of T-type Ca2+ channels to plasma membrane.
MLCK regulation of the actin cytoskeleton has been suggested in A7r5 cells, in which ML-7 partially blocks the disassembly of α-actin cables in response to [Ca2+]cyt increase by A23187 or thapsigargin.95,96 The role of the actin cytoskeleton in the regulation of smooth muscle contraction through the cross-bridge cycling process and in organizing and remodeling the submembranous cytoskeletal network97 is not disputed. Although the point is still controversial, the actin cytoskeleton has also been suggested to contribute to cytosolic Ca2+ regulation through trafficking and conformational coupling of plasma membrane and ER Ca2+ channels. Several studies demonstrate that actin depolymerization using cytochalasin-D (which sequesters G-actin monomers98) completely blocks the contractile tension in rat aorta12,99 and resistance mesenteric artery12,100 in response to agonist or pressure, without affecting the Ca2+ response.12 Conversely, in rat cerebral artery, pressure-induced [Ca2+]cyt increase is depressed in the presence of cytochalasin D.101 In cultured A7r5 cells94,102 and HEK293 cells,117 actin-depolymerizing agents also reduce L-type and T-type current, respectively. On the opposite, it has been shown that, in HEK293 cells,103 depolymerized actin contributes to TRPC3 channel activation, and in isolated gallbladder myocytes104 actin depolymerization with cytochalasin-D or latrunculin A (which blocks contractile tension by capping actin filaments98), enhances capacitative Ca2+ influx, while jasplakinolide, an activator of actin polymerization, decreases the influx of Ca2+, but none of these inhibitors affects the L-type Ca2+ influx.
The contribution of the cytoskeleton to Ca2+ channel activation is further suggested by the observation that Rho kinase (ROCK), a known modulator of cytoskeleton regulating proteins,105 is required for the non-voltage and non-capacitative Ca2+ entry in response to vasoconstrictor agonist in rat aorta and superior mesenteric artery,106 although the Ca2+ signal and its sensitivity to ROCK inhibition are not affected by cytochalasin D. The same observation is reported in rat penile small artery,107 but was not repeated in rat resistance mesenteric artery.12
In addition, in the mouse aorta cell line MOVAS, the non-selective cation channel TRPP2 has been shown to interact with filamin A, an actin-binding protein, to reduce stretch-activated cation (SAC) channels activity supporting the role of cortical F-actin cytoskeleton in the regulation of SAC channels in resistance arteries.108
From these elements, we can draw a hypothetical model describing the contribution of MLCK and myosin motors along with actin filaments and the Golgi-produced vesicles containing channels proteins, to Ca2+ channel activation (Fig. 3). In agreement with this model, myosin II activated by MLCK has been proposed to regulate the trafficking of aquaporin AQP2 vesicles to the apical plasma membrane in the rat renal collecting duct following vasopressin stimulation.109 One year later, a multiprotein complex was identified as a motor for the water channel AQP2 trafficking, in which the channel is organized with actin proteins, smooth muscle myosin light chains and non-muscle myosin heavy chains.110 In TRPC5 transfected HEK293 cells, MLCK inhibition by wortmannin impairs the translocation of TRPC5 to plasma membrane.45 But this effect can be attributed to the inhibition of PI3K by wortmannin.58 Indeed, in primary hippocampal neurons, PI3K is involved in TRPC5 homomeric channel translocation in collaboration with the Rho GTPase Rac1 and another phosphoinositide.111 Nevertheless, the implication of MLCK and cytoskeleton proteins in Ca2+ channels translocation is further supported by Shimizu et al.45 They showed, using ML-9 and a dominant-negative mutant of MLCK to inhibit MLCK, that MLCK inhibition in TRPC5 transfected HEK293 cells impairs the plasmalemmal localization of TRPC5.45
Figure 3.
(See previous page). Regulatory mechanisms of cytosolic Ca2+ concentration increase in response to agonist stimulation in aorta (A) and resistance mesenteric artery (RMA; B). In both representations, the stimulation of G protein-coupled receptor (GPCR) by vasoconstrictor agonist, for instance, noradrenaline (NA), activates phospholipase C (PLC), which produces inositol triphosphate (IP3). IP3 stimulates Ca2+ release from the endoplasmic reticulum (ER) and triggers cytosolic Ca2+ increase, which in association with calmodulin (CaM) activates myosin light chain kinase (MLCK). Phosphorylation of myosin light chains (LC20) by MLCK triggers cross-bridge cycling between actin and myosin filaments. (A) In aorta, in addition to smooth muscle contraction, agonist-induced MLCK activation triggers Ca2+ influx. We hypothesize that MLCK could be involved in Ca2+ channels trafficking (point 1 in blue) and/or could be responsible for the trafficking of transcription factors (TFs) within the nucleus (N; point 2 in blue), which in turn regulate the transcription of Ca2+ channels mRNA. Furthermore, in aorta, Rho kinase (ROCK) activated by RhoA-GTP following Gα12/13 activation contributes to non-voltage-dependent Ca2+ influx. ROCK could induce the trafficking of Ca2+ channels through myosin phosphorylation (point a in purple) as MLCK or through cortical F-actin polymerization (point b in purple). Caveolae do not appear to be directly involved in agonist-induced Ca2+ entry, while they contribute to contraction and ROCK activation. Further experiments are needed to validate or invalidate these potential Ca2+ regulation mechanisms (dotted lines) in conductance artery. (B) Conversely, in RMA, ROCK is not involved in Ca2+ entry, although ROCK contributes to smooth muscle contraction. MLCK is involved in voltage-dependent Ca2+ influx in response to a vasoconstrictor agonist. While caveolae are not required in agonist-induced Ca2+ entry in aorta, in RMA, the translocation of Ca2+ channels seems to involve the omega-shaped membrane invagination. We hypothezise that MLCK could contribute to voltage-dependent Ca2+ channels trafficking (point 1 in blue) and/or to the trafficking of transcription factors (TFs; point 2 in blue). The close proximity of the ER to the plasma membrane (PM) could make Ca2+ channel trafficking easier compared to aorta. Arrows are activating pathways; crossed out arrows are inhibiting processes; dotted lines are hypothetic pathways. RER: rough ER; Cav-1: caveolin-1; TRP: transient receptor potential channels; MLCP: myosin light chain phosphatase.
Involvement of MLCK in calcium channels gene expression
Experimental evidence indicates that the decrease in MLCK expression is associated with a decrease in the expression of several types of Ca2+ channels as demonstrated for CaV1.2 and CaV3.1 in A7r5 cells and rat resistance mesenteric artery (Fig. 2).10 We also have observed in preliminary experiments, that TRPC1 and TRPC6 mRNA were decreased in MLCK-depleted A7r5 cells (Fig. 2), suggesting that different types of Ca2+ channels could be affected by MLCK down-regulation. A first explanation could be that alteration of Ca2+ influx in MLCK-depleted cells affects the pattern of gene expression, as gene transcription is highly dependent on a precise control of Ca2+ signaling.112,113 Indeed, activation of Ca2+ influx via L-type Ca2+ channels is reported to increase mRNA and protein expression of several TRPC channels in the guinea-pig gallbladder smooth muscle and mechanisms that decrease cellular Ca2+ level, also induce a downregulation of TRPC channels expression.114
An alternative explanation for the control of gene expression by MLCK is that MLCK could be involved in the trafficking of transcription factors into the nucleus. Several transcription factors have been implicated in the regulation of CaV1.2 gene expression, such as the cAMP-response element binding protein (CREB) and the nuclear factor κB (NF-κB), CaMKII being a major regulator of their activation.115 In endothelial cells, MLCK is reported to be involved in the nuclear translocation of NF-κB in response to tumor necrosis factor TNFα.116 In mouse lung, TRPC6-mediated Ca2+ entry activates non-muscle MLCK, which in turn increases lung vascular permeability and serves as scaffolding partner to allow NF-κB activation.117 The reciprocal modulation of MLCK and L-type Ca2+ channels expression is suggested by observations made in 2 separate studies showing that mRNA expression and density of L-type VOC channels and MLCK expression are upregulated in resistance mesenteric artery from hypertensive rats compared to normal Wistar Kyoto rats.118,119 Whether there is a link between these observations should be further investigated but could be in the line with the involvement of the myosin cytoskeleton in the transcriptional mechanisms controlling genes expression, as suggested in Figure 3.
Conclusion
It is well recognized that dysregulation of Ca2+ homeostasis in VSMC has major pathological consequences. Although main partners in the control of cytosolic Ca2+ are identified, several gaps remain in the understanding of their regulation. Several processes modulating the activity of plasmalemmal Ca2+ channels have been identified, leading to the emergence of a dynamic model, in which the cytoskeleton could play an important role. Recent evidence suggests that, by mediating the phosphorylation of myosin, MLCK might contribute to the regulation of Ca2+ homeostasis in response to agonist stimulation. Figure 3 summarizes the current knowledge about the role of MLCK in Ca2+ influx in stimulated conductance and resistance artery. An important problem in determining the role of MLCK in the regulation of Ca2+ channels is the discrepancy between studies performed in different cellular models or different arteries or tissues and between data obtained from patch-clamp recording of cation current and cytosolic Ca2+ measurement in cells or tissues loaded with fluorescent Ca2+ probes. Differences in cytoskeleton organization as reported in cultured cells, or in excitation-contraction coupling processes, as seen between conductance and resistance arteries, could be, at least partly, responsible for this variability.120,121
The potential interaction of MLCK with gene transcription suggested by the decreased expression of Ca2+ channels in MLCK-depleted cells should be further investigated. This action of MLCK should be considered when using MLCK knockdown models to study the involvement of MLCK in Ca2+ movements. As the specificity of pharmacological tools is always questionable, innovative approaches will be required to determine the role of MLCK and the cytoskeleton in the regulation of Ca2+ channels.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the Ministère de l’Education et de la Recherche Scientifique of the Belgian French Community (Action Concertée no 06/11-339) and the Fonds pour la Recherche Scientifique Médicale. A.M. was supported by a fellowship of the Fonds Spéciaux de Recherche (UCL). C.D. is senior research associate of the Fonds National de la Recherche Scientifique (FNRS).
References
- 1. Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med 2008; 12:2165-80; PMID:19120701; http://dx.doi.org/ 10.1111/j.1582-4934.2008.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herring BP, El-Mounayri O, Gallagher PJ, Yin F, Zhou J. Regulation of myosin light chain kinase and telokin expression in smooth muscle tissues. Am J Physiol Cell Physiol 2006; 291:C817-27; PMID:16774989; http://dx.doi.org/ 10.1152/ajpcell.00198.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takashima S. Phosphorylation of myosin regulatory light chain by myosin light chain kinase, and muscle contraction. Circ J 2009; 73:208-13; PMID:19110504; http://dx.doi.org/ 10.1253/circj.CJ-08-1041 [DOI] [PubMed] [Google Scholar]
- 4. Walsh MP. Vascular smooth muscle myosin light chain diphosphorylation: mechanism, function, and pathological implications. IUBMB Life 2011; 63:987-1000; PMID:21990256; http://dx.doi.org/ 10.1002/iub.527 [DOI] [PubMed] [Google Scholar]
- 5. Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2010; 2:a003962; PMID:20861159; http://dx.doi.org/ 10.1101/cshperspect.a003962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albert AP. Gating mechanisms of canonical transient receptor potential channel proteins: role of phosphoinositols and diacylglycerol. Adv Exp Med Biol 2011; 704:391-411; PMID:21290308; http://dx.doi.org/ 10.1007/978-94-007-0265-3_22 [DOI] [PubMed] [Google Scholar]
- 7. Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiol Genomics 2010; 42A:169-87; PMID:20736412; http://dx.doi.org/ 10.1152/physiolgenomics.00111.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogut O, Brozovich FV. Regulation of force in vascular smooth muscle. J Mol Cell Cardiol 2003; 35:347-55; PMID:12689814; http://dx.doi.org/ 10.1016/S0022-2828(03)00045-2 [DOI] [PubMed] [Google Scholar]
- 9. Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res 2008; 77:8-18; PMID:17764667 [DOI] [PubMed] [Google Scholar]
- 10. Martinsen A, Schakman O, Yerna X, Dessy C, Morel N. Myosin light chain kinase controls voltage-dependent calcium channels in vascular smooth muscle. Pflugers Arch 2014; 466:1377-89; PMID:24162233; http://dx.doi.org/ 10.1007/s00424-013-1380-3 [DOI] [PubMed] [Google Scholar]
- 11. Cauvin C, Lukeman S, Cameron J, Hwang O, van Breemen C. Differences in norepinephrine activation and diltiazem inhibition of calcium channels in isolated rabbit aorta and mesenteric resistance vessels. Circ Res 1985; 56:822-8; PMID:2408777; http://dx.doi.org/ 10.1161/01.RES.56.6.822 [DOI] [PubMed] [Google Scholar]
- 12. Martinsen A, Yerna X, Rath G, Gomez EL, Dessy C, Morel N. Different effect of Rho kinase inhibition on calcium signaling in rat isolated large and small arteries. J Vasc Res 2012; 49:522-33; PMID:22948674; http://dx.doi.org/ 10.1159/000341230 [DOI] [PubMed] [Google Scholar]
- 13. Large WA, Saleh SN, Albert AP. Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle. Cell Calcium 2009; 45:574-82; PMID:19324408; http://dx.doi.org/ 10.1016/j.ceca.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 14. Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res 2006; 99:119-31; PMID:16857972; http://dx.doi.org/ 10.1161/01.RES.0000233356.10630.8a [DOI] [PubMed] [Google Scholar]
- 15. Beech DJ. Characteristics of transient receptor potential canonical calcium-permeable channels and their relevance to vascular physiology and disease. Circ J 2013; 77:570-9; PMID:23412755; http://dx.doi.org/ 10.1253/circj.CJ-13-0154 [DOI] [PubMed] [Google Scholar]
- 16. Tai K, Vandenberg G, Hamaide MC, Wibo M, Morel N. Effect of organ culture on noradrenaline- evoked contraction, calcium signalling and TRPC expression in rat mesenteric artery. J Vasc Res 2009; 46:353-64; PMID:19142015; http://dx.doi.org/ 10.1159/000189796 [DOI] [PubMed] [Google Scholar]
- 17. Tai K, Hamaide MC, Debaix H, Gailly P, Wibo M, Morel N. Agonist-evoked calcium entry in vascular smooth muscle cells requires IP3 receptor-mediated activation of TRPC1. Eur J Pharmacol 2008; 583:135-47; PMID:18289524; http://dx.doi.org/ 10.1016/j.ejphar.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 18. Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res 2003; 93:839-47; PMID:14551243; http://dx.doi.org/ 10.1161/01.RES.0000100367.45446.A3 [DOI] [PubMed] [Google Scholar]
- 19. Bergdahl A, Gomez MF, Wihlborg AK, Erlinge D, Eyjolfson A, Xu SZ, Beech DJ, Dreja K, Hellstrand P. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol 2005; 288:C872-80; PMID:15561760; http://dx.doi.org/ 10.1152/ajpcell.00334.2004 [DOI] [PubMed] [Google Scholar]
- 20. Facemire CS, Mohler PJ, Arendshorst WJ. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol 2004; 286:F546-51; PMID:14678949; http://dx.doi.org/ 10.1152/ajprenal.00338.2003 [DOI] [PubMed] [Google Scholar]
- 21. Hill AJ, Hinton JM, Cheng H, Gao Z, Bates DO, Hancox JC, Langton PD, James AF. A TRPC-like non-selective cation current activated by α1-adrenoceptors in rat mesenteric artery smooth muscle cells. Cell Calcium 2006; 40:29-40; PMID:16697039; http://dx.doi.org/ 10.1016/j.ceca.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 22. Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 2002; 90:248-50; PMID:11861411; http://dx.doi.org/ 10.1161/hh0302.105662 [DOI] [PubMed] [Google Scholar]
- 23. Mita M, Ito K, Taira K, Nakagawa J, Walsh MP, Shoji M. Attenuation of store-operated Ca2+ entry and enhanced expression of TRPC channels in caudal artery smooth muscle from Type 2 diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol 2010; 37:670-8; PMID:20337661; http://dx.doi.org/ 10.1111/j.1440-1681.2010.05373.x [DOI] [PubMed] [Google Scholar]
- 24. Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 2000; 16:521-55; PMID:11031246; http://dx.doi.org/ 10.1146/annurev.cellbio.16.1.521 [DOI] [PubMed] [Google Scholar]
- 25. Ball CJ, Wilson DP, Turner SP, Saint DA, Beltrame JF. Heterogeneity of L- and T-channels in the vasculature: rationale for the efficacy of combined L- and T-blockade. Hypertension 2009; 53:654-60; PMID:19237682; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.108.125831 [DOI] [PubMed] [Google Scholar]
- 26. Jensen LJ, Holstein-Rathlou NH. Is there a role for T-type Ca2+ channels in regulation of vasomotor tone in mesenteric arterioles? Can J Physiol Pharmacol 2009; 87:8-20; PMID:19142211; http://dx.doi.org/ 10.1139/Y08-101 [DOI] [PubMed] [Google Scholar]
- 27. Andreasen D, Friis UG, Uhrenholt TR, Jensen BL, Skott O, Hansen PB. Coexpression of voltage-dependent calcium channels CaV1.2, 2.1a, and 2.1b in vascular myocytes. Hypertension 2006; 47:735-41; PMID:16505211; http://dx.doi.org/ 10.1161/01.HYP.0000203160.80972.47 [DOI] [PubMed] [Google Scholar]
- 28. Hansen PB, Jensen BL, Andreasen D, Friis UG, Skott O. Vascular smooth muscle cells express the α1A subunit of a P-/Q-type voltage-dependent Ca2+ Channel, and It is functionally important in renal afferent arterioles. Circ Res 2000; 87:896-902; PMID:11073885; http://dx.doi.org/ 10.1161/01.RES.87.10.896 [DOI] [PubMed] [Google Scholar]
- 29. Abd El-Rahman RR, Brett SE, Harraz OF, Mufti RE, Goldman D, Welsh DG. Identification of L- and T- Type Ca2+ Channels in Rat Cerebral Arteries: Role in Myogenic Tone Development. Am J Physiol Heart Circ Physiol 2012; PMID:23103495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gustafsson F, Andreasen D, Salomonsson M, Jensen BL, Holstein-Rathlou N. Conducted vasoconstriction in rat mesenteric arterioles: role for dihydropyridine-insensitive Ca2+ channels. Am J Physiol Heart Circ Physiol 2001; 280:H582-90; PMID:11158955 [DOI] [PubMed] [Google Scholar]
- 31. Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol 2008; 295:C779-90; PMID:18596214; http://dx.doi.org/ 10.1152/ajpcell.00173.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch 2008; 456:769-85; PMID:18365243; http://dx.doi.org/ 10.1007/s00424-008-0491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem 2005; 280:39786-94; PMID:16204251; http://dx.doi.org/ 10.1074/jbc.M506064200 [DOI] [PubMed] [Google Scholar]
- 34. Maruyama Y, Nakanishi Y, Walsh EJ, Wilson DP, Welsh DG, Cole WC. Heteromultimeric TRPC6-TRPC7 channels contribute to arginine vasopressin-induced cation current of A7r5 vascular smooth muscle cells. Circ Res 2006; 98:1520-7; PMID:16690880; http://dx.doi.org/ 10.1161/01.RES.0000226495.34949.28 [DOI] [PubMed] [Google Scholar]
- 35. Moneer Z, Pino I, Taylor EJ, Broad LM, Liu Y, Tovey SC, Staali L, Taylor CW. Different phospholipase-C-coupled receptors differentially regulate capacitative and non-capacitative Ca2+ entry in A7r5 cells. Biochem J 2005; 389:821-9; PMID:15918794; http://dx.doi.org/ 10.1042/BJ20050145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beech DJ. Integration of transient receptor potential canonical channels with lipids. Acta Physiol (Oxf) 2012; 204:227-37; PMID:21624095; http://dx.doi.org/ 10.1111/j.1748-1716.2011.02311.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim H, Kim J, Jeon JP, Myeong J, Wie J, Hong C, Kim HJ, Jeon JH, So I. The roles of G proteins in the activation of TRPC4 and TRPC5 transient receptor potential channels. Channels (Austin) 2012; 6:333-43; PMID:22878724; http://dx.doi.org/ 10.4161/chan.21198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca2+ channels (CaV1.2a,b) by protein kinases. Am J Physiol Cell Physiol 2001; 281:C1743-56; PMID:11698232 [DOI] [PubMed] [Google Scholar]
- 39. Lee TS, Karl R, Moosmang S, Lenhardt P, Klugbauer N, Hofmann F, Kleppisch T, Welling A. Calmodulin kinase II is involved in voltage-dependent facilitation of the L-type CaV1.2 calcium channel: Identification of the phosphorylation sites. J Biol Chem 2006; 281:25560-7; PMID:16820363; http://dx.doi.org/ 10.1074/jbc.M508661200 [DOI] [PubMed] [Google Scholar]
- 40. Owsianik G, D'Hoedt D, Voets T, Nilius B. Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol 2006; 156:61-90; PMID:16634147 [PubMed] [Google Scholar]
- 41. Watanabe H, Tran QK, Takeuchi K, Fukao M, Liu MY, Kanno M, Hayashi T, Iguchi A, Seto M, Ohashi K. Myosin light-chain kinase regulates endothelial calcium entry and endothelium-dependent vasodilation. FASEB J 2001; 15:282-4; PMID:11156937 [DOI] [PubMed] [Google Scholar]
- 42. Kim BJ, Jeon JH, Kim SJ, So I. Role of calmodulin and myosin light chain kinase in the activation of carbachol-activated cationic current in murine ileal myocytes. Can J Physiol Pharmacol 2007; 85:1254-62; PMID:18066127; http://dx.doi.org/ 10.1139/Y07-118 [DOI] [PubMed] [Google Scholar]
- 43. Aromolaran AS, Albert AP, Large WA. Evidence for myosin light chain kinase mediating noradrenaline-evoked cation current in rabbit portal vein myocytes. J Physiol 2000; 524(Pt 3):853-63; PMID:10790163; http://dx.doi.org/ 10.1111/j.1469-7793.2000.00853.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tran QK, Watanabe H, Le HY, Pan L, Seto M, Takeuchi K, Ohashi K. Myosin light chain kinase regulates capacitative Ca2+ entry in human monocytes/macrophages. Arterioscler Thromb Vasc Biol 2001; 21:509-15; PMID:11304465; http://dx.doi.org/ 10.1161/01.ATV.21.4.509 [DOI] [PubMed] [Google Scholar]
- 45. Shimizu S, Yoshida T, Wakamori M, Ishii M, Okada T, Takahashi M, Seto M, Sakurada K, Kiuchi Y, Mori Y. Ca2+-calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J Physiol 2006; 570:219-35; PMID:16284075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim MT, Kim BJ, Lee JH, Kwon SC, Yeon DS, Yang DK, So I, Kim KW. Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells. Am J Physiol Cell Physiol 2006; 290:C1031-40; PMID:16306123; http://dx.doi.org/ 10.1152/ajpcell.00602.2004 [DOI] [PubMed] [Google Scholar]
- 47. Martinsen A, Baeyens N, Yerna X, Morel N. Rho kinase regulation of vasopressin-induced calcium entry in vascular smooth muscle cell: comparison between rat isolated aorta and cultured aortic cells. Cell Calcium 2012; 52:413-21; PMID:22883550; http://dx.doi.org/ 10.1016/j.ceca.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 48. Shi J, Takahashi S, Jin XH, Li YQ, Ito Y, Mori Y, Inoue R. Myosin light chain kinase-independent inhibition by ML-9 of murine TRPC6 channels expressed in HEK293 cells. Br J Pharmacol 2007; 152:122-31; PMID:17603544; http://dx.doi.org/ 10.1038/sj.bjp.0707368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smyth JT, Dehaven WI, Bird GS, Putney JW Jr. Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci 2008; 121:762-72; PMID:18285445; http://dx.doi.org/ 10.1242/jcs.023903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim YC, Kim SJ, Kang TM, Suh SH, So I, Kim KW. Effects of myosin light chain kinase inhibitors on carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Pflugers Arch 1997; 434:346-53; PMID:9211799; http://dx.doi.org/ 10.1007/s004240050407 [DOI] [PubMed] [Google Scholar]
- 51. Weber LP, Van Lierop JE, Walsh MP. Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. J Physiol 1999; 516(Pt 3):805-24; PMID:10200427; http://dx.doi.org/ 10.1111/j.1469-7793.1999.0805u.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takayama M, Ozaki H, Karaki H. Effects of a myosin light chain kinase inhibitor, wortmannin, on cytoplasmic Ca2+ levels, myosin light chain phosphorylation and force in vascular smooth muscle. Naunyn Schmiedeberg Arch Pharmacol 1996; 354:120-7; PMID:8857588; http://dx.doi.org/ 10.1007/BF00178711 [DOI] [PubMed] [Google Scholar]
- 53. Asano M, Matsunaga K, Miura M, Ito KM, Seto M, Sakurada K, Nagumo H, Sasaki Y, Ito K. Selectivity of action of staurosporine on Ca2+ movements and contractions in vascular smooth muscles. Eur J Pharmacol 1995; 294:693-701; PMID:8750735; http://dx.doi.org/ 10.1016/0014-2999(95)00616-8 [DOI] [PubMed] [Google Scholar]
- 54. Burdyga TV, Wray S. The effect of inhibition of myosin light chain kinase by Wortmannin on intracellular [Ca2+], electrical activity and force in phasic smooth muscle. Pflugers Arch 1998; 436:801-3; PMID:9716716; http://dx.doi.org/ 10.1007/s004240050705 [DOI] [PubMed] [Google Scholar]
- 55. Longbottom ER, Luckas MJ, Kupittayanant S, Badrick E, Shmigol T, Wray S. The effects of inhibiting myosin light chain kinase on contraction and calcium signalling in human and rat myometrium. Pflugers Arch 2000; 440:315-21; PMID:10898533; http://dx.doi.org/ 10.1007/s004240000305 [DOI] [PubMed] [Google Scholar]
- 56. Wingard CJ, Murphy RA. Inhibition of Ca2+-dependent contraction in swine carotid artery by myosin kinase inhibitors. Gen Pharmacol 1999; 32:483-94; PMID:10323490; http://dx.doi.org/ 10.1016/S0306-3623(98)00289-4 [DOI] [PubMed] [Google Scholar]
- 57. Ito S, Kume H, Honjo H, Kodama I, Katoh H, Hayashi H, Shimokata K. ML-9, a myosin light chain kinase inhibitor, reduces intracellular Ca2+ concentration in guinea pig trachealis. Eur J Pharmacol 2004; 486:325-33; PMID:14985055; http://dx.doi.org/ 10.1016/j.ejphar.2004.01.013 [DOI] [PubMed] [Google Scholar]
- 58. Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 2007; 408:297-315; PMID:17850214; http://dx.doi.org/ 10.1042/BJ20070797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hashimoto Y, Nakayama T, Teramoto T, Kato H, Watanabe T, Kinoshita M, Tsukamoto K, Tokunaga K, Kurokawa K, Nakanishi S, et al. Potent and preferential inhibition of Ca2+/calmodulin-dependent protein kinase II by K252a and its derivative, KT5926. Biochem Biophys Res Commun 1991; 181:423-9; PMID:1659814; http://dx.doi.org/ 10.1016/S0006-291X(05)81436-6 [DOI] [PubMed] [Google Scholar]
- 60. Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, et al. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol Cell Biol 2005; 25:6980-9; PMID:16055711; http://dx.doi.org/ 10.1128/MCB.25.16.6980-6989.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sundivakkam PC, Freichel M, Singh V, Yuan JP, Vogel SM, Flockerzi V, Malik AB, Tiruppathi C. The Ca2+ sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca2+ entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells (abstract). Mol Pharmacol 2012; 81:510-26; PMID:22210847; http://dx.doi.org/ 10.1124/mol.111.074658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Diochot S, Richard S, Baldy-Moulinier M, Nargeot J, Valmier J. Dihydropyridines, phenylalkylamines and benzothiazepines block N-, P/Q- and R-type calcium currents. Pflugers Arch 1995; 431:10-9; PMID:8584405; http://dx.doi.org/ 10.1007/BF00374372 [DOI] [PubMed] [Google Scholar]
- 63. Moosmang S, Lenhardt P, Haider N, Hofmann F, Wegener JW. Mouse models to study L-type calcium channel function. Pharmacol Ther 2005; 106:347-55; PMID:15922017; http://dx.doi.org/ 10.1016/j.pharmthera.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 64. Zhang H, Fisher SA. Conditioning effect of blood flow on resistance artery smooth muscle myosin phosphatase. Circ Res 2007; 100:730-7; PMID:17293476; http://dx.doi.org/ 10.1161/01.RES.0000260189.38975.35 [DOI] [PubMed] [Google Scholar]
- 65. Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem 1992; 267:14662-8; PMID:1321813 [PubMed] [Google Scholar]
- 66. Ma X, Wang Y, Stephens NL. Serum deprivation induces a unique hypercontractile phenotype of cultured smooth muscle cells. Am J Physiol 1998; 274:C1206-14; PMID:9612207 [DOI] [PubMed] [Google Scholar]
- 67. Kiselyov K, Shin DM, Kim JY, Yuan JP, Muallem S. TRPC channels: interacting proteins. Handb Exp Pharmacol 2007: 559-74; PMID:17217079; http://dx.doi.org/ 10.1007/978-3-540-34891-7_33 [DOI] [PubMed] [Google Scholar]
- 68. Ambudkar IS. Trafficking of TRP channels: determinants of channel function. Handb Exp Pharmacol 2007: 541-57; PMID:17217078; http://dx.doi.org/ 10.1007/978-3-540-34891-7_32 [DOI] [PubMed] [Google Scholar]
- 69. Simms BA, Zamponi GW. Trafficking and stability of voltage-gated calcium channels. Cell Mol Life Sci 2012; 69:843-56; PMID:21964928; http://dx.doi.org/ 10.1007/s00018-011-0843-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bergdahl A, Sward K. Caveolae-associated signalling in smooth muscle. Can J Physiol Pharmacol 2004; 82:289-99; PMID:15213728; http://dx.doi.org/ 10.1139/y04-033 [DOI] [PubMed] [Google Scholar]
- 71. Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 2009; 45:625-33; PMID:19324409; http://dx.doi.org/ 10.1016/j.ceca.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adebiyi A, Narayanan D, Jaggar JH. Caveolin-1 assembles type 1 inositol 1,4,5-trisphosphate receptors and canonical transient receptor potential 3 channels into a functional signaling complex in arterial smooth muscle cells. J Biol Chem 2011; 286:4341-8; PMID:21098487; http://dx.doi.org/ 10.1074/jbc.M110.179747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Suzuki Y, Yamamura H, Ohya S, Imaizumi Y. Caveolin-1 facilitates the direct coupling between large conductance Ca2+-activated K+ (BKCa) and CaV1.2 Ca2+ channels and their clustering to regulate membrane excitability in vascular myocytes. J Biol Chem 2013; 288:36750-61; PMID:24202214; http://dx.doi.org/ 10.1074/jbc.M113.511485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nuno DW, England SK, Lamping KG. RhoA localization with caveolin-1 regulates vascular contractions to serotonin. Am J Physiol Regul Integr Comp Physiol 2012; 303:R959-67; PMID:22955057; http://dx.doi.org/ 10.1152/ajpregu.00667.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001; 293:2449-52; PMID:11498544; http://dx.doi.org/ 10.1126/science.1062688 [DOI] [PubMed] [Google Scholar]
- 76. Nixon GF, Mignery GA, Somlyo AV. Immunogold localization of inositol 1,4,5-trisphosphate receptors and characterization of ultrastructural features of the sarcoplasmic reticulum in phasic and tonic smooth muscle. J Muscle Res Cell Motil 1994; 15:682-700; PMID:7706424; http://dx.doi.org/ 10.1007/BF00121075 [DOI] [PubMed] [Google Scholar]
- 77. Dubroca C, Loyer X, Retailleau K, Loirand G, Pacaud P, Feron O, Balligand JL, Levy BI, Heymes C, Henrion D. RhoA activation and interaction with Caveolin-1 are critical for pressure-induced myogenic tone in rat mesenteric resistance arteries. Cardiovasc Res 2007; 73:190-7; PMID:17150200; http://dx.doi.org/ 10.1016/j.cardiores.2006.10.020 [DOI] [PubMed] [Google Scholar]
- 78. Singh BB, Lockwich TP, Bandyopadhyay BC, Liu X, Bollimuntha S, Brazer SC, Combs C, Das S, Leenders AG, Sheng ZH, et al. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell 2004; 15:635-46; PMID:15327778; http://dx.doi.org/ 10.1016/j.molcel.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 79. Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, Yuan JP, Kiselyov K, Muallem S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium 2007; 42:363-71; PMID:17618683; http://dx.doi.org/ 10.1016/j.ceca.2007.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mery L, Strauss B, Dufour JF, Krause KH, Hoth M. The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci 2002; 115:3497-508; PMID:12154080 [DOI] [PubMed] [Google Scholar]
- 81. Harteneck C. Proteins modulating TRP channel function. Cell Calcium 2003; 33:303-10; PMID:12765677; http://dx.doi.org/ 10.1016/S0143-4160(03)00043-5 [DOI] [PubMed] [Google Scholar]
- 82. Crnich R, Amberg GC, Leo MD, Gonzales AL, Tamkun MM, Jaggar JH, Earley S. Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells. Am J Physiol Cell Physiol 2010; 299:C682-94; PMID:20610768; http://dx.doi.org/ 10.1152/ajpcell.00101.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature 1994; 368:67-70; PMID:7509046; http://dx.doi.org/ 10.1038/368067a0 [DOI] [PubMed] [Google Scholar]
- 84. Beguin P, Nagashima K, Gonoi T, Shibasaki T, Takahashi K, Kashima Y, Ozaki N, Geering K, Iwanaga T, Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature 2001; 411:701-6; PMID:11395774; http://dx.doi.org/ 10.1038/35079621 [DOI] [PubMed] [Google Scholar]
- 85. Buraei Z, Yang J. The β subunit of voltage-gated Ca2+ channels. Physiol Rev 2010; 90:1461-506; PMID:20959621; http://dx.doi.org/ 10.1152/physrev.00057.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell α2δ-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res 2009; 105:948-55; PMID:19797702; http://dx.doi.org/ 10.1161/CIRCRESAHA.109.203620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang J, Thio SS, Yang SS, Yu D, Yu CY, Wong YP, Liao P, Li S, Soong TW. Splice variant specific modulation of CaV1.2 calcium channel by galectin-1 regulates arterial constriction. Circ Res 2011; 109:1250-8; PMID:21998324; http://dx.doi.org/ 10.1161/CIRCRESAHA.111.248849 [DOI] [PubMed] [Google Scholar]
- 88. Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science 2010; 330:101-5; PMID:20929812; http://dx.doi.org/ 10.1126/science.1191027 [DOI] [PubMed] [Google Scholar]
- 89. Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 2010; 330:105-9; PMID:20929813; http://dx.doi.org/ 10.1126/science.1191086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ihara E, Hirano K, Hirano M, Nishimura J, Nawata H, Kanaide H. Mechanism of down-regulation of L-type Ca2+ channel in the proliferating smooth muscle cells of rat aorta. J Cell Biochem 2002; 87:242-51; PMID:12244576; http://dx.doi.org/ 10.1002/jcb.10295 [DOI] [PubMed] [Google Scholar]
- 91. Dessy C, Godfraind T. The effect of L-type calcium channel modulators on the mobilization of intracellular calcium stores in guinea-pig intestinal smooth muscle. Br J Pharmacol 1996; 119:142-8; PMID:8872367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Duran JM, Valderrama F, Castel S, Magdalena J, Tomas M, Hosoya H, Renau-Piqueras J, Malhotra V, Egea G. Myosin motors and not actin comets are mediators of the actin-based Golgi-to-endoplasmic reticulum protein transport. Mol Biol Cell 2003; 14:445-59; PMID:12589046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Valderrama F, Luna A, Babia T, Martinez-Menarguez JA, Ballesta J, Barth H, Chaponnier C, Renau-Piqueras J, Egea G. The golgi-associated COPI-coated buds and vesicles contain β/γ-actin. Proc Natl Acad Sci U S A 2000; 97:1560-5; PMID:10677499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dey D, Shepherd A, Pachuau J, Martin-Caraballo M. Leukemia inhibitory factor regulates trafficking of T-type Ca2+ channels. Am J Physiol Cell Physiol 2011; 300:C576-87; PMID:21178106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li C, Fultz ME, Parkash J, Rhoten WB, Wright GL. Ca2+-dependent actin remodeling in the contracting A7r5 cell. J Muscle Res Cell Motil 2001; 22:521-34; PMID:12038586 [DOI] [PubMed] [Google Scholar]
- 96. Fultz ME, Wright GL. Myosin remodelling in the contracting A7r5 smooth muscle cell. Acta Physiol (Oxf) 2003; 177:197-205; PMID:12558556 [DOI] [PubMed] [Google Scholar]
- 97. Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 2008; 295:C576-87; PMID:18596210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gerthoffer WT. Actin cytoskeletal dynamics in smooth muscle contraction. Can J Physiol Pharmacol 2005; 83:851-6; PMID:16333356 [DOI] [PubMed] [Google Scholar]
- 99. Saito SY, Hori M, Ozaki H, Karaki H. Cytochalasin D inhibits smooth muscle contraction by directly inhibiting contractile apparatus. J Smooth Muscle Res 1996; 32:51-60; PMID:8845566 [DOI] [PubMed] [Google Scholar]
- 100. Shaw L, Ahmed S, Austin C, Taggart MJ. Inhibitors of actin filament polymerisation attenuate force but not global intracellular calcium in isolated pressurised resistance arteries. J Vasc Res 2003; 40:1-10; discussion 10; PMID:12644721 [DOI] [PubMed] [Google Scholar]
- 101. Gokina NI, Osol G. Actin cytoskeletal modulation of pressure-induced depolarization and Ca2 +influx in cerebral arteries. Am J Physiol Heart Circ Physiol 2002; 282:H1410-20; PMID:11893578 [DOI] [PubMed] [Google Scholar]
- 102. Nakamura M, Sunagawa M, Kosugi T, Sperelakis N. Actin filament disruption inhibits L-type Ca2+ channel current in cultured vascular smooth muscle cells. Am J Physiol Cell Physiol 2000; 279:C480-7; PMID:10913014 [DOI] [PubMed] [Google Scholar]
- 103. Lockwich T, Singh BB, Liu X, Ambudkar IS. Stabilization of cortical actin induces internalization of transient receptor potential 3 (Trp3)-associated caveolar Ca2+ signaling complex and loss of Ca2+ influx without disruption of Trp3-inositol trisphosphate receptor association. J Biol Chem 2001; 276:42401-8; PMID:11524429 [DOI] [PubMed] [Google Scholar]
- 104. Morales S, Camello PJ, Rosado JA, Mawe GM, Pozo MJ. Disruption of the filamentous actin cytoskeleton is necessary for the activation of capacitative calcium entry in naive smooth muscle cells. Cell Signal 2005; 17:635-45; PMID:15683738 [DOI] [PubMed] [Google Scholar]
- 105. Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 2006; 98:322-34; PMID:16484628 [DOI] [PubMed] [Google Scholar]
- 106. Ghisdal P, Vandenberg G, Morel N. Rho-dependent kinase is involved in agonist-activated calcium entry in rat arteries. J Physiol 2003; 551:855-67; PMID:12853654; http://dx.doi.org/ 10.1113/jphysiol.2003.047050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Villalba N, Stankevicius E, Simonsen U, Prieto D. Rho kinase is involved in Ca2+ entry of rat penile small arteries. Am J Physiol Heart Circ Physiol 2008; 294:H1923-32; PMID:18223191; http://dx.doi.org/ 10.1152/ajpheart.01221.2007 [DOI] [PubMed] [Google Scholar]
- 108. Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell 2009; 139:587-96; PMID:19879844; http://dx.doi.org/ 10.1016/j.cell.2009.08.045 [DOI] [PubMed] [Google Scholar]
- 109. Chou CL, Christensen BM, Frische S, Vorum H, Desai RA, Hoffert JD, de Lanerolle P, Nielsen S, Knepper MA. Non-muscle myosin II and myosin light chain kinase are downstream targets for vasopressin signaling in the renal collecting duct. J Biol Chem 2004; 279:49026-35; PMID:15347643; http://dx.doi.org/ 10.1074/jbc.M408565200 [DOI] [PubMed] [Google Scholar]
- 110. Noda Y, Horikawa S, Katayama Y, Sasaki S. Identification of a multiprotein "motor" complex binding to water channel aquaporin-2. Biochem Biophys Res Commun 2005; 330:1041-7; PMID:15823548; http://dx.doi.org/ 10.1016/j.bbrc.2005.03.079 [DOI] [PubMed] [Google Scholar]
- 111. Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 2004; 6:709-20; PMID:15258588; http://dx.doi.org/ 10.1038/ncb1150 [DOI] [PubMed] [Google Scholar]
- 112. Cartin L, Lounsbury KM, Nelson MT. Coupling of Ca2+ to CREB activation and gene expression in intact cerebral arteries from mouse : roles of ryanodine receptors and voltage-dependent Ca2+ channels. Circ Res 2000; 86:760-7; PMID:10764409; http://dx.doi.org/ 10.1161/01.RES.86.7.760 [DOI] [PubMed] [Google Scholar]
- 113. Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res 2006; 98:868-78; PMID:16614312; http://dx.doi.org/ 10.1161/01.RES.0000216596.73005.3c [DOI] [PubMed] [Google Scholar]
- 114. Morales S, Diez A, Puyet A, Camello PJ, Camello-Almaraz C, Bautista JM, Pozo MJ. Calcium controls smooth muscle TRPC gene transcription via the CaMK/calcineurin-dependent pathways. Am J Physiol Cell Physiol 2007; 292:C553-63; PMID:16956967; http://dx.doi.org/ 10.1152/ajpcell.00096.2006 [DOI] [PubMed] [Google Scholar]
- 115. Ronkainen JJ, Hanninen SL, Korhonen T, Koivumaki JT, Skoumal R, Rautio S, Ronkainen VP, Tavi P. Ca2+-calmodulin-dependent protein kinase II represses cardiac transcription of the L-type calcium channel α1C-subunit gene (Cacna1c) by DREAM translocation. J Physiol 2011; 589:2669-86; PMID:21486818; http://dx.doi.org/ 10.1113/jphysiol.2010.201400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wadgaonkar R, Linz-McGillem L, Zaiman AL, Garcia JG. Endothelial cell myosin light chain kinase (MLCK) regulates TNFalpha-induced NFkappaB activity. J Cell Biochem 2005; 94:351-64; PMID:15526279; http://dx.doi.org/ 10.1002/jcb.20250 [DOI] [PubMed] [Google Scholar]
- 117. Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, et al. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J Exp Med 2012; 209:1953-68; PMID:23045603; http://dx.doi.org/ 10.1084/jem.20111355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension 2002; 40:214-9; PMID:12154116; http://dx.doi.org/ 10.1161/01.HYP.0000025877.23309.36 [DOI] [PubMed] [Google Scholar]
- 119. Han YJ, Hu WY, Chernaya O, Antic N, Gu L, Gupta M, Piano M, de Lanerolle P. Increased myosin light chain kinase expression in hypertension: Regulation by serum response factor via an insertion mutation in the promoter. Mol Biol Cell 2006; 17:4039-50; PMID:16822834; http://dx.doi.org/ 10.1091/mbc.E06-04-0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Eddinger TJ, Meer DP. Myosin II isoforms in smooth muscle: heterogeneity and function. Am J Physiol Cell Physiol 2007; 293:C493-508; PMID:17475667; http://dx.doi.org/ 10.1152/ajpcell.00131.2007 [DOI] [PubMed] [Google Scholar]
- 121. Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci 2006; 119:1769-80; PMID:16608882; http://dx.doi.org/ 10.1242/jcs.02805 [DOI] [PubMed] [Google Scholar]
- 122. Albert AP, Saleh SN, Large WA. Inhibition of native TRPC6 channel activity by phosphatidylinositol 4,5-bisphosphate in mesenteric artery myocytes. J Physiol 2008; 586:3087-95; PMID:18467363; http://dx.doi.org/ 10.1113/jphysiol.2008.153676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nakanishi S, Catt KJ, Balla T. Inhibition of agonist-stimulated inositol 1,4,5-trisphosphate production and calcium signaling by the myosin light chain kinase inhibitor, wortmannin. J Biol Chem 1994; 269:6528-35; PMID:8120005 [PubMed] [Google Scholar]
- 124. Watanabe H, Takahashi R, Zhang XX, Kakizawa H, Hayashi H, Ohno R. Inhibition of agonist-induced Ca2+ entry in endothelial cells by myosin light-chain kinase inhibitor. Biochem Biophys Res Commun 1996; 225:777-84; PMID:8780689; http://dx.doi.org/ 10.1006/bbrc.1996.1250 [DOI] [PubMed] [Google Scholar]
- 125. Takahashi R, Watanabe H, Zhang XX, Kakizawa H, Hayashi H, Ohno R. Roles of inhibitors of myosin light chain kinase and tyrosine kinase on cation influx in agonist-stimulated endothelial cells. Biochem Biophys Res Commun 1997; 235:657-62; PMID:9207215; http://dx.doi.org/ 10.1006/bbrc.1997.6856 [DOI] [PubMed] [Google Scholar]
- 126. Watanabe H, Takahashi R, Zhang XX, Goto Y, Hayashi H, Ando J, Isshiki M, Seto M, Hidaka H, Niki I, et al. An essential role of myosin light-chain kinase in the regulation of agonist- and fluid flow-stimulated Ca2+ influx in endothelial cells. FASEB J 1998; 12:341-8; PMID:9506478 [DOI] [PubMed] [Google Scholar]
- 127. Norwood N, Moore TM, Dean DA, Bhattacharjee R, Li M, Stevens T. Store-operated calcium entry and increased endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 2000; 279:L815-24; PMID:11053015 [DOI] [PubMed] [Google Scholar]
- 128. Wang J, Weigand L, Foxson J, Shimoda LA, Sylvester JT. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol 2007; 293:L674-85; PMID:17575009; http://dx.doi.org/ 10.1152/ajplung.00141.2007 [DOI] [PubMed] [Google Scholar]
- 129. Pearson RB, Misconi LY, Kemp BE. Smooth muscle myosin kinase requires residues on the COOH-terminal side of the phosphorylation site. Peptide inhibitors. J Biol Chem 1986; 261:25-7; PMID:3941075 [PubMed] [Google Scholar]
- 130. Nakanishi S, Ando K, Kawamoto I, Matsuda Y. MS-347a, a new inhibitor of myosin light chain kinase from Aspergillus sp. KY52178. J Antibiot 1993; 46:1775-81; PMID:8294233; http://dx.doi.org/ 10.7164/antibiotics.46.1775 [DOI] [PubMed] [Google Scholar]
- 131. Kuroiwa-Matsumoto M, Hirano K, Ahmed A, Kawasaki J, Nishimura J, Kanaide H. Mechanisms of the thapsigargin-induced Ca2+ entry in in situ endothelial cells of the porcine aortic valve and the endothelium-dependent relaxation in the porcine coronary artery. Br J Pharmacol 2000; 131:115-23; PMID:10960077; http://dx.doi.org/ 10.1038/sj.bjp.0703548 [DOI] [PMC free article] [PubMed] [Google Scholar]