Abstract
The detection of temperature is one of the most fundamental sensory functions across all species, and is critical for animal survival. Animals have thus evolved a diversity of thermosensory mechanisms allowing them to sense and respond to temperature changes (thermoreception). A key process underlying thermoreception is the translation of thermal energy into electrical signals, a process mediated by thermal sensors (thermoreceptors) that are sensitive to a specific range of temperatures. In disease conditions, the temperature sensitivity of thermoreceptors is altered, leading to abnormal temperature sensation such as heat hyperalgesia. Therefore, the identification of thermal sensors and understanding their functions and regulation hold great potential for developing novel therapeutics against many medical conditions such as pain.
Keywords: pain, somatosensory transduction, TRP ion channels, thermoreception, temperature
Introduction
Temperature affects nearly every aspect of function in organisms ranging from cell metabolism to animal behaviors. Animals have thus developed various robust sensory mechanisms permitting them to select their preferred temperatures, while avoiding thermal extremes, an essential process for animals to main temperature homeostasis.
The perception of temperature is initiated by the activation of thermoreceptors on peripheral nerve endings in mammals. However, the molecular entity of thermoreceptors has been a mystery for a long time. A breakthrough was achieved when the first temperature sensitive ion channel TRPV1 was cloned.1 This breakthrough stimulated considerable interest in hunting for other temperature sensitive ion channels over the years, leading to the identification of thermally sensitive TRPV2, TRPV3, TRPV4, TRPM8 and TRPA1. These thermo-sensitive ion channels belong to a large transient receptor potential (TRP) ion channel superfamily, they are thus also dubbed as Thermo-TRP ion channels (Fig. 1). Interestingly, equivalent temperature-sensitive ion channels and thermosensory mechanisms were also discovered in other organisms such as Drosophila.2 These thermo-sensitive ion channels, therefore, offer a molecular gateway for our understanding of thermal sensation and signaling.
Figure 1.
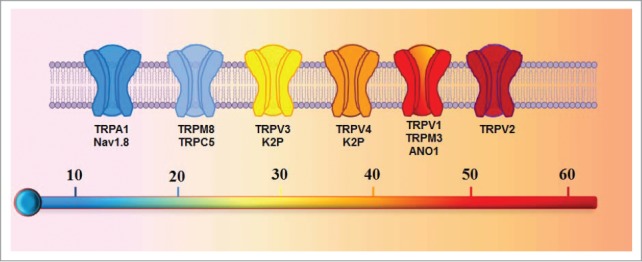
A schematic diagram depicting the temperature sensitive ion channels. Ion channels are ordered according to their relative activation threshold to temperatures.
Thermosensation and Thermoreceptors in Mammals
In mammals, temperature sensation is carried by specialized sensory neurons in the Dorsal Root Ganglia (DRG) and the trigeminal ganglia, which project their terminals to both peripheral tissues (e.g. skin) and the spinal cord in the central nervous system (CNS). These temperature-responding sensory neurons are thus the key to our understanding of a broad range of temperature sensation extending from heat, warm to cold.
Heat detection
An inward current triggered by noxious heat ( > 42°C) was first observed from a subpopulation of DRG neurons.3 The molecule responsible for this heat-activated current was soon identified as TRPV1 using the expression cloning strategy.1 Indeed, when expressed in a heterologous cell system, TRPV1 was activated by heat with a similar thermal threshold of 42°C, and also by capsaicin, a known ingredient from hot chilli peppers causing a burning heat sensation.1 Moreover, mice deficient for TRPV1 exhibited impaired responses to noxious heat and showed reduced heat hyperalgesia caused by inflammation.4,5 These findings argue for TRPV1 as a heat sensor responsible for detecting heat temperature in mice. However, nerve fibers isolated from TRPV1-deficient mice responded to heat normally.6 Moreover, the heat avoidance behavior of mice was not impaired by deleting TRPV1 over the temperature range between 40°C and 50°C evaluated in a 2 temperature preference assay,7 but completely eliminated by ablating TRPV1-expresing (TRPV1+) neurons or by silencing TRPV1+ fibers.7-10 These results support the idea that there are other yet unknown molecular sensors within TRPV1+ neurons that mediate noxious heat detection.
What molecule functions as an additional heat sensor? In search for homologous genes to TRPV1, TRPV2 was identified as another heat-activated ion channel expressed on sensory neurons, albeit with a much higher heat activation threshold ( >52°C).11 However, the majority of TRPV2+ cutaneous nerve fibers did not respond to heat,6,12 and TRPV2-deficient mice exhibited no deficits in response to noxious heat over a broad heat range.13 Therefore, it is not likely that TRPV2 functions as a heat sensor.
TRPM3 and calcium activated chloride channel anoctamin 1 (ANO1) are another 2 recently identified ion channels that respond to noxious heat (Fig. 1). TRPM3 and ANO1 exhibit steep temperature dependence and can be directly activated by heat over 40°C and 44°C, respectively, when they were heterologous expressed in HEK293 cells.14,15 Of interest, both TRPM3 and ANO1 are mainly expressed in small diameter nociceptive neurons and the majority of them also co-express TRPV1, suggesting a role of these ion channels in heat nociception. Indeed, responses to noxious heat in mice was significantly reduced by either deleting TRPM3 or ANO1,14,15 similar to that observed in TRPV1-deficient mice.4,5 However, there remains a large proportion of heat responding neurons after deleting TRPM3 combined with pharmacologically blocking TRPV1.14 It remains to be determined whether blocking TRPV1 and TRPM3 together with ANO1 can further eliminate remaining fractions of heat-responding neurons. Collectively, these data suggest that sensory neurons employ multiple and redundant heat sensors within TRPV1+ neurons to transduce noxious heat, presumably robust thermosensory mechanisms are required for reliably detecting and avoiding damaging stimuli, such as extreme heat, which otherwise can cause irreversible tissue injury.
Warm sensation
The identification of heat transducers prompted the search for sensors responsible for detecting warm temperatures. The attempt led to the cloning of TRPV3 by several labs around similar time.16-18 TRPV3 was activated by innocuous warm temperatures (>33°C).16,18,19 However, TRPV3 was mainly expressed in skin and keratinocytes without significant expression in DRG.18,19 The unique TRPV3 expression profile led to the proposal that TRPV3 acts as a warm receptor in the skin responsible for detecting physiological range of temperatures. Indeed, in one report, mice lacking TRPV3 exhibited deficits in response to both innocuous and noxious heat.20 However, these deficits were not observed in another TRPV3-null mice line with a different gene background.21 TRPV3 may thus have only an assisting role in mediating warm and/or heat perception. In support for this idea, mice with TRPV3 overexpressed in keratinocytes did not display significant altered thermosensory behaviors until functions of the heat receptor TRPV1 were masked by a pharmacological inhibitor.22 A more recent study employing TRPV3 and TRPV1 double knockout mice provided more direct evidence supporting the notion that skin derived-TRPV3 and sensory neuron-localized TRPV1 have a cooperative role in mediating warm and heat temperature sensation.23
TRPV4 was initially recognized as an osmolality sensor.24,25 It was soon found that TRPV4 can also be activated by warm temperatures over 27°C.26,27 Interestingly, similar to TRPV3, TRPV4 is highly expressed in skin epidermal keratinocytes, but not in DRG.28,29 As expected, both TRPV3 and TRPV4 contribute to different components of currents elicited by warm temperatures in primary skin keratinocytes,19,30 suggesting that keratinocytes may act in concert with sensory neurons to transduce thermal information. As predicted, TRPV4-deficient mice displayed deficits in detecting warmer temperatures.31,32 Puzzlingly, TRPV3/TRPV4 double knock-out mice did not exhibit significant deficits in either thermo-sensory behaviors or thermal nociception.33 These studies suggest that there are other as-yet-unknown significant warm sensing mechanisms that may compensate warm sensation.
In addition to acting on thermo-sensitive ion channels on the cell membrane, temperature rises can also cluster and activate STIM1, an ER Ca2+ sensor, leading to the activation of the store-operated ion channel Orai1 and Ca2+ influx,34 implying that STIM1 also acts as a intracellular heat sensor. However, it remains to be established whether this heat signaling mechanism contributes to warm and/or heat transduction in somatosensory neurons.
Cold sensation
Following the identification of the heat-activated TRPV1 channel, it was suggested that there exists a similar thermoreceptor for detecting cold temperatures, because a moderate cooling can directly elicit an inward ionic current from a subpopulation of sensory neurons.35 Indeed, molecule responsible for mediating the cold-induced current was later on identified as the TRPM8 ion channel.36,37 TRPM8 can be activated by a broad range of cold temperatures ranging from innocuous cooling (<26°C) to noxious cold ( <16°C), and also by cooling compounds such as menthol. Consistently, mice lacking TRPM8 lost the ability to sense cold (up to 15°C) and exhibited pronounced deficits in cold-avoiding behaviors.38-40 Furthermore, pain induced by noxious cold was also prevented by either genetically deleting TRPM8 or by pharmacological blocking TRPM8,41,42 in line with TRPM8 activation by noxious cold. These studies conclusively demonstrated that TRPM8 is a bona fide principal cold sensor in animals. However, the ability to detect noxious cold largely remains in TRPM8-deficient mice, suggesting that there are other significant unknown cold sensing mechanisms.
The attempt to seek another cold sensor for transducing noxious cold led to the identification of TRPA1 using a bioinformatic approach.43 TRPA1 is indeed can be activated by an average of 17.5°C, much lower than that of TRPM8.43 However, this proposal caused a continued debate surrounding the cold sensitivity of TRPA1, with some supporting, while others disapproving.44 A recent study demonstrated that TRPA1 is sensitive to cold even when reconstituted into lipid bilayers, lending strong support to the idea that TRPA1 is cold-sensitive intrinsically.45 However, there is again no consensus on whether TRPA1 contributes to acute noxious cold sensation in animals, with some endorsing,46,47 and others not.7,42,48,49 Despite these differences, it is agreed that TRPA1 does play a significant role in pathological cold signaling, such as cold hypersensitivity associated with nerve injury and chemotherapy.48,50-52 In contrast to the controversial role of TRPA1 in cold transduction, TRPA1 was well documented as a polymodal nociceptor for integrating various environmental and endogenous damaging stimuli such as mustard oil and oxidative stress that elicit pain.53 Interestingly, in contrast to the cold-sensitive mammalian TRPA1, invertebrate TRPA1, such as rattlesnake and Drosophila TRPA1, is heat sensitive.54,55 The robust heat sensitivity of rattlesnake TRPA1 was proposed to enable rattlesnake to use infrared radiation to detect warm-blooded prey.54
TRPC5 is another TRP ion channel reported to respond to innocuous cold temperature (<37°C) (Fig. 1).56 However, there are no changes in temperature-sensing behaviors in TRPC5-null mice, thus TRPC5 may only act as a thermal modulator in cold transduction.
In summary, thermo-TRP ion channels function as thermo-sensors for detecting different spectrum of temperatures. But there are also other unknown mechanisms cooperative for sensing different ranges of temperatures.
Modulation of Thermal Sensors
Thermosensors have their inherent thermal activation threshold. The threshold for temperature activation, however, can be modulated by a variety of factors (e.g., inflammatory mediators), leading to abnormal thermo-sensation, such as heat hyperalgesia induced by inflammation. Therefore, understanding thermal modulation of thermosensors is crucial for elucidating abnormal thermo-sensation associated with diseases such as pain. Here I discuss the modulation of TRPV1 and TRPM8, 2 well-accepted thermo-sensitive ion channels, under both physiological and pathological conditions. TRPA1 modulation will also be discussed due to its significant role in pathological cold signaling. However, as TRPV2 and TRPC5 do not function as thermo-sensors, and either TRPV3 or TRPV4 alone does not contribute significantly to thermo-sensation, they are thus not the focus of this review.
Modulation of TRPV1
Physiological modulation
TRPV1 is believed to be intrinsically heat sensitive. However, different populations of TRPV1+ neurons exhibit differential heat sensitivities, and capsaicin-responding neurons are not always sensitive to heat.57,58 The varied heat sensitivities of TRPV1 in sensory DRG neurons under the basal condition suggest that there exist additional thermal modulators.
We have recently discovered that PKCβII is such a crucial modulator that causes varied heat-induced responses across different populations of TRPV1+ neurons.59 Here, PKCβII is co-expressed in only a subset of TRPV1+ neurons, and markedly enhances their responses by phosphorylating TRPV1 at T705. Interestingly, co-expressed PKCβII is constitutively active as a result of direct binding to TRPV1 and forming a local TRPV1-PKCβII complex59 (Fig. 2). Therefore, different basal phosphorylation at T705 may underlie varied heat sensitivities of TRPV1, and TRPV1-PKCβII complex-containing neurons may represent a subset of hypersensitive nociceptive neurons.
Figure 2.
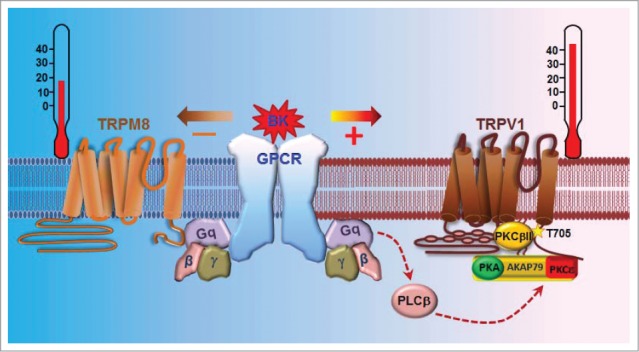
Summary of distinct modulation of TRPV1 and TRPM8 by inflammatory mediators activating Gq coupled receptors. The inflammatory mediator BK sensitizes hyperalgesia-mediated TRPV1, but inhibits analgesia-mediated TRPM8, resulting in inflammatory hyperalgesia. The sensitization of TRPV1 is caused by the phosphorylation of TRPV1 at S502/S801 (not depicted) by PKCε, which is anchored adjacent to TRPV1 by the scaffolding protein AKAP79/150 forming a macro-signaling complex. However, the inhibition of TRPM8 is mediated by a direct action of activated Gq on TRPM8 independently of the PLC signaling. Note that the basal thermal sensitivity of TRPV1 is determined by the basal phosphorylation of TRPV1 at T705 by PKCβII, which binds to TRPV1 forming another local protein complex with TRPV1.
The membrane lipid PIP2 is another critical factor involved in regulating the heat sensitivity of TRPV1. However, there is a continuing controversy regarding the exact role of PIP2 in TRPV1 activation, with some supporting an inhibiting role,60,61 and some advocating an stimulating effect,62-69 whereas others favoring both activating and inhibiting roles depending on certain conditions.70,71 Different approaches used for manipulating the cellular PIP2 level may underlie the difference, with some including PIP2 into the whole-cell recording pipette,65,67 and some applying PIP2 directly to an inside-out excised patch,63,68,69 whereas others reconstituting PIP2 and purified TRPV1 in an artificial liposome or into planar lipid bilayers.61,64 It should be noted that most of these studies are conducted in expression or reconstitution system, which may also contribute to variable conclusions. A missing study is to determine the role of PIP2 in native sensory neurons. In this respect, it will be interesting to know whether different levels of PIP2 are present in different populations of TRPV1+ neurons and thus influence their heat sensitivity.
The negatively charged head groups of PIP2 underlie most of its functional effect. PIP2 acts primarily by binding to positively charged residues on ion channels through the head groups. The identification of PIP2 effector regions or sites on TRPV1 is thus important for elucidating the acting mechanisms of PIP2. In this regard, both a distal C-terminal region (777∼820) and a TRP domain in the proximal C-terminal region (682∼725), rich in polybasic residues, were identified as the PIP2 binding region.63,72 A recent study further identified R575 and R579 in the S4-S5 linker, and K694 in the TRP domain, as specific PIP2 binding sites on TRPV1 using molecular docking simulation based on the resolved TRPV1 structure.69 It is interesting to note that PIP2 was predicted to bind at the interface between the transmembrane domain and the cytoplasmic domains of TRPV1, lined with the identified basic residues, similar to that observed in the structure of Kir2.2.69 However, how PIP2 exactly binds to TRPV1 can only be answered after resolving the structure of TRPV1 in complex with PIP2.
Pathological modulation
TRPV1 is activated by noxious heat. In disease conditions such as inflammation, the heat activation threshold of TRPV1 is markedly lowered down so that even pleasant warm temperatures can be felt to be very painful, a process known as heat hyperalgesia. It is caused by the sensitization of TRPV1 by a variety of inflammatory mediators released during tissue injury and inflammation, including bradykinin (BK),60,73 prostaglandin E2 (PGE2),74,75 nerve growth factor (NGF),60,76 ATP,77 substance P,78 cytokines (e.g. IL-6),79 chemokines (e.g., CCL3),80 endothelin-181 and proteases.82–84 Most of these agents bind to G protein-coupled receptors (GPCR) that couple to either Gs and/or Gq, leading to the activation of PKA and PKCε, which then phosphorylates TRPV1 at S116 and S502/S801, respectively, leading to the sensitization of TRPV185 (Fig. 2). Mutating these PKA and/or PKCε phosphorylation sites markedly impaired TRPV1 sensitization induced by these agents,85 suggesting that TRPV1 phosphorylation at these sites is critical for inflammatory heat hyperalgesia.
Interestingly, the same mutation of PKCε phosphorylation sites (S502/S801), however, did not affect the basal TRPV1 thermal sensitivity, which is determined by phosphorylation at T705 by PKCβII.59 On the other hand, mutating PKCβII phosphorylation site T705 had no effect on sensitizing TRPV1 induced by BK. Therefore, PKCβII and PKCε control basal thermal sensitivity and sensitization of TRPV1, respectively, by phosphorylating distinct PKC sites. Notably, TRPV1 phosphorylation by PKCε depends on the scaffolding protein AKAP79/150, which anchors both PKA and PKCε in close proximity to TRPV1 by binding to the C terminus of TRPV1, thus assembled into a macro-protein signaling complex.74,75,86 Correspondingly, the sensitization of TRPV1 induced by both PKA and PKCε was blunted either by knocking down AKAP79/150 or by disrupting mutual interactions between TRPV1 and AKAP79/150.75,87,88 Importantly, inflammatory heat hyperalgesia was inhibited by interfering with the interaction between TRPV1 and AKAP79.89,90 These studies suggest a possible novel analgesic approach by antagonizing the TRPV1-AKAP79/150 interaction.
Intriguingly, another complex formed between TRPV1 and GABAB1 receptor was recently identified.91 Here, activated GABAB1 inhibits TRPV1 sensitization and inflammatory pain caused by inflammatory mediators by preventing TRPV1 phosphorylation. It will be interesting to know whether GABAB1 acts by interfering in the interaction between TRPV1 and AKAP79/150.
The responsiveness of TRPV1 to heat is not only influenced by the thermal gating of TRPV1, but also affected by the number of ion channels trafficking to the cell membrane. The dynamic trafficking of TRPV1 is a tightly-regulated process. Many protein kinases, such as PKC, PKA, Src kinase and cyclin-dependent kinase 5, were shown to promote the forward trafficking of TRPV1 to the cell membrane, contributing to thermal hyperalgesia.75,76,92 On the other hand, inhibition of TRPV1 internalization induced prolonged thermal hyperalgesia.93
Taken together, both enhanced gating and trafficking of TRPV1 are responsible for enhanced TRPV1 responses to heat, leading to inflammatory hyperalgesia.
Modulation of TRPM8
Physiological Modulation
TRPM8 responds to both innocuous and noxious cold and exhibits different cold activation threshold across different populations of sensory neurons. Based on the different activation threshold, TRPM8+ neurons were classified into 2 main categories, with one subpopulation activated by a low-threshold (LT) cold (>26°C) and another responding to a high-threshold (HT) cold (<24C°).94,95 However, the mechanisms that govern different cold threshold among TRPM8+ neurons are not completely understood. In one study, different levels of TRPM8 expression was proposed to be one of the mechanisms, because LT TRPM8+ neurons are often associated with higher TRPM8 responses and vice versa.95 The same study also implicated different expression of shaker-like Kv1 channels in setting the threshold of TRPM8+ neurons, with LT neurons containing lower expression of outward K+ currents and HT neurons associated with higher level of K+ currents. A further analysis of TRPM8+ neurons identified TASK3, a 2-pore -domain K+ leak channel (K2P), to be highly enriched in TRPM8+ neurons and critical for specifying the threshold of HT TRPM8+ neurons.96 However, in other studies, A type K+ currents and voltage gated Na+ currents were thought to be critical in specifying cold activation threshold of TRPM8+ neurons.94,97,98 It is possible that a complex interplay and concerted action of different ion conductance shape the excitability of TRPM8+ neurons. What remains little known is why TRPM8 per se exhibits different cold sensitivities in different subpopulation of neurons and what determine the varied cold sensitivity of TRPM8.
PIP2 is a well-established factor critical for maintaining TRPM8 activity by binding to the TRP domain in the C terminus of TRPM8.99,100 Addition of synthesized PIP2 activates TRPM8, whereas depletion of PIP2 inhibits TRPM8 by inducing a 5-phosphatase.101,102 Interestingly, different basal temperatures can alter the interaction of PIP2 with TRPM8, which was thought to be responsible for changes in temperature thresholds for TRPM8 activation induced by different pre-exposed ambient temperatures.103 Furthermore, the metabolic products of membrane lipids due to phospholipase A2 activation can alter TRPM8 thermal sensitivity. For example, lysophospholipids (LPLs) shifts TRPM8 cold activation threshold toward warm temperature, whereas another product, arachidonic acid, inhibits TRPM8 activation by cold.104,105 There is also evidence showing that TRPM8 thermal responses are inhibited by lipid rafts, a cholesterol-rich membrane micro-domain where TRPM8 tends to reside.106 It is thus tempting to wonder whether these different lipids are crucial in specifying different cold sensitivities of TRPM8 in sensory neurons.
Pathological modulation
It is known that a moderate cooling (innocuous cold) inhibits pain mediating an analgesia effect, but noxious cold causes pain. Paradoxically, TRPM8 can mediate both processes.38–40 During inflammatory condition, TRPM8 sensitivity is susceptible to alteration by inflammatory mediators, leading to inflammatory hyperalgesia and cold hypersensitivity. Of note, a brief application of BK rapidly inhibited TRPM8 in DRG neurons, an event presumably leading to the inhibition of TRPM8-mediated analgesia and thus contributing to inflammatory hyperalgesia.107,108 The effect is mainly mediated by the BK receptor B2R, a Gq-coupled GPCR. However, the underlying mechanisms for BK-induced TRPM8 inhibition had been unclear. It had been suggested to be caused by either depletion of PIP2 due to activation of PLCβ or by activation of downstream PKC.107,108 However, we found that neither of these mechanisms is critical, instead activated Gq directly inhibits TRPM8 by binding to the channel forming a local protein complex independently of downstream GPCR signaling109 (Fig. 2). Notably, PIP2 cannot activate TRPM8 anymore in the presence of activated Gq,109 suggesting that Gq is a potent regulator of TRPM8 activity. Interestingly, activated Gq and G11 inhibit TRPM8 to a markedly different degree, despite they have similar capability of inducing PIP2 hydrolysis,110 further supporting the idea that direct inhibition of TRPM8 by Gq is separable from PIP2 hydrolysis-mediated TRPM8 inhibition. However, it is not known whether these 2 mechanisms act concomitantly to inhibit TRPM8 during activation of a Gq-coupled receptor. In contrast to BK-induced TRPM8 inhibition, artemin, a glial cell-derived neurotrophic factor, sensitizes TRPM8-mediated cold responses in mice, leading to cold hypersensitivity.111 However, the sensitizing effect of artemin was not demonstrated at the cellular level and the underlying potential signaling mechanisms remain to be established. The opposing effects of BK and artemin may be caused by the colocalization of their respective acting receptors (i.e. B2R and GFRα3) in analgesia- and pain-mediating TRPM8+ neurons, respectively, thereby contributing to inflammatory hyperalgesia and cold hypersensitivity, separately.
Modulation of TRPA1
As a key damage sensing ion channel, it is not surprising that TRPA1 is targeted by many inflammatory mediators (e.g. BK and PGE2), leading to pain hypersensitivity. Similar to TRPV1, TRPA1 can be potentiated by BK and PGE2,112,113 which activates Gq and Gs-coupled GPCR, respectively, resulting in the activation of phospholipase C (PLC) and PKA. Blocking PLC and PKA prevented the sensitization of TRPA1 induced by BK,112 and activation of PLC and PKA evoked TRPA1-mediated hyperalgesia.113 Mechanistically, activation of PLC/PKA pathways enhanced trafficking of TRPA1 to the cell membrane,114 suggesting that the PLC and PKA pathways potentiates TRPA1 by promoting forward trafficking of the channel. Interestingly, several downstream signaling messengers of the Gq-PLC pathway such as Ca2+, diacylglycerol (DAG) and arachidonic acid (AA) can directly activate TRPA1,115-117 and was suggested to be a mechanism underlying BK-elicited excitation of sensory neurons and pain.117 A similar direct action on TRPA1 was also observed with prostaglandins (PG). However, PG excites TRPA1 via 15d-PGJ2, a metabolite of PGD2, without the involvement of intracellular signaling.118-120 Puzzlingly, none of these studies investigated whether these modulation mechanisms can alter the cold sensitivity of TRPA1.
Thermo-Modulation by Other Ion Channels
Thermo-reception not only depends on the temperature sensitivity of thermos-sensors, but also relies on the membrane excitability and transducing capability of thermo-sensitive neurons, which is determined by several K+ channels and voltage-gated sodium channels, respectively. Therefore, activities of these channels can significantly influence thermo-reception. Of note, the 2 pore domains background K+ channels (K2P) TREK-1, TREK-2 and TRAAK are sensitive to temperature increases (Fig. 1).121 They are thus proposed to hyperpolarize both heat- and cold-sensitive neurons and antagonize the depolarizing effect evoked by thermo-sensors, leading to a shift of temperature threshold of thermo-sensitive neurons.122,123 Voltage-gated sodium channels (Nav), in particular Nav1. 8, also play a significant role in thermoreception (Fig. 1). Nav1.8 was found to be the only functional sodium channel that elicits firing of nerve fibers during cold condition, and was thus implicated in noxious cold transduction.98 Indeed, noxious cold sensation was lost in mice lacking Nav1.8.98,124 Taken together, combined actions of thermosensors, K+ and Na+ channels result in the generation of temperature-dependent nerve impulses which can then be propagated to the CNS, leading to thermoreception.
Concluding Remarks
Thermoreception is fundamental to animals. Many temperature-sensitive ion channels and receptors have been identified and some of them act as molecular thermometers involved in thermo-sensation. Under pathological conditions such as inflammation and tissue injury, the thermo-sensitivity of thermoreceptors was subjected to be regulated by a variety of factors, leading to thermal hyperalgesia. Thereby, thermo-transduction is governed by both thermal sensors and modulators. Despite rapid progress in our understanding of thermoreception, many questions remain. For example, what are molecular entities for detecting heat and noxious cold, independently of thermo-TRP ion channels? It is still not known whether LT TRPM8 neurons mediate cold analgesia and HT TRPM8 neurons cause cold pain. Understanding these fundamental questions will be critical for elucidating pathological thermo-sensations and open up novel targets for therapy of related diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389: 816–24; PMID:9349813; http://dx.doi.org/ 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 2.Barbagallo B, Garrity PA. Temperature sensation in Drosophila. Curr Opin Neurobiol 2015; 34C: 8–13; PMID:25616212; http://dx.doi.org/ 10.1016/j.conb.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A 1996; 93: 15435–9; PMID:8986829; http://dx.doi.org/ 10.1073/pnas.93.26.15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288: 306–13; PMID:10764638; http://dx.doi.org/ 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- 5.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, et al.. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000; 405: 183–7; PMID:10821274; http://dx.doi.org/ 10.1038/35012076 [DOI] [PubMed] [Google Scholar]
- 6.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci 2004; 24: 6410–5; PMID:15254097; http://dx.doi.org/ 10.1523/JNEUROSCI.1421-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pogorzala LA, Mishra SK, Hoon MA. The cellular code for Mammalian thermosensation. J Neurosci 2013; 33: 5533–41; PMID:23536068; http://dx.doi.org/ 10.1523/JNEUROSCI.5788-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenneis C, Kistner K, Puopolo M, Segal D, Roberson D, Sisignano M, Labocha S, Ferreiros N, Strominger A, Cobos EJ, et al.. Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. J Neurosci 2013; 33: 315–26; PMID:23283344; http://dx.doi.org/ 10.1523/JNEUROSCI.2804-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A 2009; 106: 9075–80; PMID:19451647; http://dx.doi.org/ 10.1073/pnas.0901507106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J 2011; 30: 582–93; PMID:21139565; http://dx.doi.org/ 10.1038/emboj.2010.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999; 398: 436–41; PMID:10201375; http://dx.doi.org/ 10.1038/18906 [DOI] [PubMed] [Google Scholar]
- 12.Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain 2008; 9: 298–308; PMID:18226966; http://dx.doi.org/ 10.1016/j.jpain.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci 2011; 31: 11425–36; PMID:21832173; http://dx.doi.org/ 10.1523/JNEUROSCI.1384-09.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, et al.. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011; 70: 482–94; PMID:21555074; http://dx.doi.org/ 10.1016/j.neuron.2011.02.051 [DOI] [PubMed] [Google Scholar]
- 15.Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F, et al.. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci 2012; 15: 1015–21; PMID:22634729; http://dx.doi.org/ 10.1038/nn.3111 [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al.. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002; 418: 181–6; PMID:12077604; http://dx.doi.org/ 10.1038/nature00882 [DOI] [PubMed] [Google Scholar]
- 17.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, et al.. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002; 418: 186–90; PMID:12077606; http://dx.doi.org/ 10.1038/nature00894 [DOI] [PubMed] [Google Scholar]
- 18.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, et al.. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002; 296: 2046–9; PMID:12016205; http://dx.doi.org/ 10.1126/science.1073140 [DOI] [PubMed] [Google Scholar]
- 19.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem 2004; 279: 21569–75; PMID:15004014; http://dx.doi.org/ 10.1074/jbc.M401872200 [DOI] [PubMed] [Google Scholar]
- 20.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005; 307: 1468–72; PMID:15746429; http://dx.doi.org/ 10.1126/science.1108609 [DOI] [PubMed] [Google Scholar]
- 21.Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain 2011; 7: 37; PMID:21586160; http://dx.doi.org/ 10.1186/1744-8069-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci 2008; 28: 13727–37; PMID:19091963; http://dx.doi.org/ 10.1523/JNEUROSCI.5741-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marics I, Malapert P, Reynders A, Gaillard S, Moqrich A. Acute heat-evoked temperature sensation is impaired but not abolished in mice lacking TRPV1 and TRPV3 channels. PLoS One 2014; 9: e99828; PMID:24925072; http://dx.doi.org/ 10.1371/journal.pone.0099828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000; 103: 525–35; PMID:11081638; http://dx.doi.org/ 10.1016/S0092-8674(00)00143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2000; 2: 695–702; PMID:11025659; http://dx.doi.org/ 10.1038/35036318 [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 2002; 277: 47044–51; PMID:12354759; http://dx.doi.org/ 10.1074/jbc.M208277200 [DOI] [PubMed] [Google Scholar]
- 27.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 2002; 22: 6408–14; PMID:12151520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander R, Kerby A, Aubdool AA, Power AR, Grover S, Gentry C, Grant AD. 4alpha-phorbol 12,13-didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4. Br J Pharmacol 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 2003; 39: 497–511; PMID:12895423; http://dx.doi.org/ 10.1016/S0896-6273(03)00462-8 [DOI] [PubMed] [Google Scholar]
- 30.Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem 2003; 278: 32037–46; PMID:12783886; http://dx.doi.org/ 10.1074/jbc.M303251200 [DOI] [PubMed] [Google Scholar]
- 31.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci 2005; 25: 1304–10; PMID:15689568; http://dx.doi.org/ 10.1523/JNEUROSCI.4745.04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem 2004; 279: 35133–8; PMID:15187078; http://dx.doi.org/ 10.1074/jbc.M406260200 [DOI] [PubMed] [Google Scholar]
- 33.Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain 2011; 7: 37; PMID:21586160; http://dx.doi.org/ 10.1186/1744-8069-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao B, Coste B, Mathur J, Patapoutian A. Temperature-dependent STIM1 activation induces Ca(2)+ influx and modulates gene expression. Nat Chem Biol 2011; 7: 351–8; PMID:21499266; http://dx.doi.org/ 10.1038/nchembio.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid G, Flonta ML. Physiology. Cold current in thermoreceptive neurons. Nature 2001; 413: 480; PMID:11586349; http://dx.doi.org/ 10.1038/35097164 [DOI] [PubMed] [Google Scholar]
- 36.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416: 52–8; PMID:11882888; http://dx.doi.org/ 10.1038/nature719 [DOI] [PubMed] [Google Scholar]
- 37.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al.. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108: 705–15; PMID:11893340; http://dx.doi.org/ 10.1016/S0092-8674(02)00652-9 [DOI] [PubMed] [Google Scholar]
- 38.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 2007; 54: 371–8; PMID:17481391; http://dx.doi.org/ 10.1016/j.neuron.2007.02.024 [DOI] [PubMed] [Google Scholar]
- 39.Colburn RW, Lubin ML, Stone DJ Jr., Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007; 54: 379–86; PMID:17481392; http://dx.doi.org/ 10.1016/j.neuron.2007.04.017 [DOI] [PubMed] [Google Scholar]
- 40.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007; 448: 204–8; PMID:17538622; http://dx.doi.org/ 10.1038/nature05910 [DOI] [PubMed] [Google Scholar]
- 41.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One 2011; 6: e25894; PMID:21984952; http://dx.doi.org/ 10.1371/journal.pone.0025894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010; 150: 340–50; PMID:20542379; http://dx.doi.org/ 10.1016/j.pain.2010.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al.. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003; 112: 819–29; PMID:12654248; http://dx.doi.org/ 10.1016/S0092-8674(03)00158-2 [DOI] [PubMed] [Google Scholar]
- 44.Caspani O, Heppenstall PA. TRPA1 and cold transduction: an unresolved issue? J Gen Physiol 2009; 133: 245–9; PMID:19237589; http://dx.doi.org/ 10.1085/jgp.200810136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moparthi L, Survery S, Kreir M, Simonsen C, Kjellbom P, Hogestatt ED, Johanson U, Zygmunt PM. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc Natl Acad Sci U S A 2014; 111: 16901–6; PMID:25389312; http://dx.doi.org/ 10.1073/pnas.1412689111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A 2009; 106: 1273–8; PMID:19144922; http://dx.doi.org/ 10.1073/pnas.0808487106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aubdool AA, Graepel R, Kodji X, Alawi KM, Bodkin JV, Srivastava S, Gentry C, Heads R, Grant AD, Fernandes ES, et al.. TRPA1 is essential for the vascular response to environmental cold exposure. Nat Commun 2014; 5: 5732; PMID:25501034; http://dx.doi.org/ 10.1038/ncomms6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, Segreti JA, Han P, Zhang XF, Niforatos W, et al.. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011; 152: 1165–72; PMID:21402443; http://dx.doi.org/ 10.1016/j.pain.2011.01.049 [DOI] [PubMed] [Google Scholar]
- 49.de OC, Garami A, Lehto SG, Pakai E, Tekus V, Pohoczky K, Youngblood BD, Wang W, Kort ME, Kym PR, et al.. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J Neurosci 2014; 34: 4445–52; PMID:24671991; http://dx.doi.org/ 10.1523/JNEUROSCI.5387-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol 2006; 200: 112–23; PMID:16546170; http://dx.doi.org/ 10.1016/j.expneurol.2006.01.031 [DOI] [PubMed] [Google Scholar]
- 51.Vetter I, Touska F, Hess A, Hinsbey R, Sattler S, Lampert A, Sergejeva M, Sharov A, Collins LS, Eberhardt M, et al.. Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J 2012; 31: 3795–808; PMID:22850668; http://dx.doi.org/ 10.1038/emboj.2012.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.del CD, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D'Amours M, Deering N, et al.. TRPA1 contributes to cold hypersensitivity. J Neurosci 2010; 30: 15165–74; PMID:21068322; http://dx.doi.org/ 10.1523/JNEUROSCI.2580-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol 2013; 75: 181–200; PMID:23020579; http://dx.doi.org/ 10.1146/annurev-physiol-030212-183811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature 2010; 464: 1006–11; PMID:20228791; http://dx.doi.org/ 10.1038/nature08943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature 2003; 423: 822–3; PMID:12815418; http://dx.doi.org/ 10.1038/423822a [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, Uysal S, Pfeifer JD, Riccio A, Clapham DE. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci U S A 2011; 108: 18114–9; PMID:22025699; http://dx.doi.org/ 10.1073/pnas.1115387108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirschstein T, Busselberg D, Treede RD. Coexpression of heat-evoked and capsaicin-evoked inward currents in acutely dissociated rat dorsal root ganglion neurons. Neurosci Lett 1997; 231: 33–6; PMID:9280161; http://dx.doi.org/ 10.1016/S0304-3940(97)00533-8 [DOI] [PubMed] [Google Scholar]
- 58.Vyklicky L, Vlachova V, Vitaskova Z, Dittert I, Kabat M, Orkand RK. Temperature coefficient of membrane currents induced by noxious heat in sensory neurones in the rat. J Physiol 1999; 517(Pt 1):181–92; PMID:10226158; http://dx.doi.org/ 10.1111/j.1469-7793.1999.0181z.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L, Hasan R, Zhang X. The basal thermal sensitivity of the TRPV1 ion channel is determined by PKCbetaII. J Neurosci 2014; 34 8246–58; PMID:24920628; http://dx.doi.org/ 10.1523/JNEUROSCI.0278-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001; 411 957–62; PMID:11418861; http://dx.doi.org/ 10.1038/35082088 [DOI] [PubMed] [Google Scholar]
- 61.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 2013; 77: 667–79; PMID:23439120; http://dx.doi.org/ 10.1016/j.neuron.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klein RM, Ufret-Vincenty CA, Hua L, Gordon SE. Determinants of molecular specificity in phosphoinositide regulation. Phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2) is the endogenous lipid regulating TRPV1. J Biol Chem 2008; 283: 26208–16; PMID:18574245; http://dx.doi.org/ 10.1074/jbc.M801912200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. Localization of the PIP2 sensor of TRPV1 ion channels. J Biol Chem 2011; 286: 9688–98; PMID:21224382; http://dx.doi.org/ 10.1074/jbc.M110.192526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun X, Zakharian E. Regulation of the temperature-dependent activation of Transient Receptor Potential Vanilloid 1 by phospholipids in planar lipid bilayers. J Biol Chem 2015; 290: 4741–7; PMID:25561742; http://dx.doi.org/ 10.1074/jbc.M114.611459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 2007; 54: 905–18; PMID:17582331; http://dx.doi.org/ 10.1016/j.neuron.2007.05.027 [DOI] [PubMed] [Google Scholar]
- 66.Sowa NA, Street SE, Vihko P, Zylka MJ. Prostatic acid phosphatase reduces thermal sensitivity and chronic pain sensitization by depleting phosphatidylinositol 4,5-bisphosphate. J Neurosci 2010; 30: 10282–93; PMID:20685973; http://dx.doi.org/ 10.1523/JNEUROSCI.2162-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 2008; 133: 475–85; PMID:18455988; http://dx.doi.org/ 10.1016/j.cell.2008.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 2006; 128: 509–22; PMID:17074976; http://dx.doi.org/ 10.1085/jgp.200609576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poblete H, Oyarzun I, Olivero P, Comer J, Zuniga M, Sepulveda RV, Baez-Nieto D, Gonzalez LC, Gonzalez-Nilo F, Latorre R. Molecular determinants of phosphatidylinositol 4,5-Bisphosphate (PI(4,5)P2) binding to transient receptor potential V1 (TRPV1) channels. J Biol Chem 2015; 290: 2086–98; PMID:25425643; http://dx.doi.org/ 10.1074/jbc.M114.613620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senning EN, Collins MD, Stratiievska A, Ufret-Vincenty CA, Gordon SE. Regulation of TRPV1 ion channel by phosphoinositide (4,5)-bisphosphate: the role of membrane asymmetry. J Biol Chem 2014; 289: 10999–1006; PMID:24599956; http://dx.doi.org/ 10.1074/jbc.M114.553180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci 2007; 27: 7070–80; PMID:17596456; http://dx.doi.org/ 10.1523/JNEUROSCI.1866-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 2003; 300: 1284–8; PMID:12764195; http://dx.doi.org/ 10.1126/science.1083646 [DOI] [PubMed] [Google Scholar]
- 73.Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 1999; 23: 617–24; PMID:10433272; http://dx.doi.org/ 10.1016/S0896-6273(00)80813-2 [DOI] [PubMed] [Google Scholar]
- 74.Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 2008; 28: 4904–17; PMID:18463244; http://dx.doi.org/ 10.1523/JNEUROSCI.0233-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008; 59: 450–61; PMID:18701070; http://dx.doi.org/ 10.1016/j.neuron.2008.05.015 [DOI] [PubMed] [Google Scholar]
- 76.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 2005; 24: 4211–23; PMID:16319926; http://dx.doi.org/ 10.1038/sj.emboj.7600893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, et al.. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci 2003; 23: 6058–62; PMID:12853424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, Zhao ZQ. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. J Neurosci 2007; 27: 12067–77; PMID:17978048; http://dx.doi.org/ 10.1523/JNEUROSCI.0496-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, Vogl C, Sailer CA, Uceyler N, Brockhaus J, et al.. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci 2009; 29: 13473–83; PMID:19864560; http://dx.doi.org/ 10.1523/JNEUROSCI.1822-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A 2005; 102: 4536–41; PMID:15764707; http://dx.doi.org/ 10.1073/pnas.0406030102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plant TD, Zollner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, Furkert J, Stein C, Oksche A. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain 2007; 3: 35; PMID:18001466; http://dx.doi.org/ 10.1186/1744-8069-3-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci 2004; 24: 4293–9; PMID:15128843; http://dx.doi.org/ 10.1523/JNEUROSCI.0454-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vellani V, Kinsey AM, Prandini M, Hechtfischer SC, Reeh P, Magherini PC, Giacomoni C, McNaughton PA. Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurones. Mol Pain 2010; 6: 61; PMID:20875131; http://dx.doi.org/ 10.1186/1744-8069-6-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, et al.. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci 2004; 24: 4300–12; PMID:15128844; http://dx.doi.org/ 10.1523/JNEUROSCI.5679-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol 2012; 165: 787–801; PMID:21797839; http://dx.doi.org/ 10.1111/j.1476-5381.2011.01601.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 2008; 138: 604–16; PMID:18381233; http://dx.doi.org/ 10.1016/j.pain.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Efendiev R, Bavencoffe A, Hu H, Zhu MX, Dessauer CW. Scaffolding by a-kinase anchoring protein enhances functional coupling between adenylyl cyclase and TRPV1 channel. J Biol Chem 2012; PMID:23264624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain 2009; 146: 301–7; PMID:19767149; http://dx.doi.org/ 10.1016/j.pain.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fischer MJ, Btesh J, McNaughton PA. Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J Neurosci 2013; 33: 7407–14; PMID:23616546; http://dx.doi.org/ 10.1523/JNEUROSCI.3721-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Btesh J, Fischer MJ, Stott K, McNaughton PA. Mapping the binding site of TRPV1 on AKAP79: implications for inflammatory hyperalgesia. J Neurosci 2013; 33: 9184–93; PMID:23699529; http://dx.doi.org/ 10.1523/JNEUROSCI.4991-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hanack C, Moroni M, Lima WC, Wende H, Kirchner M, Adelfinger L, Schrenk-Siemens K, Tappe-Theodor A, Wetzel C, Kuich PH, et al.. GABA blocks pathological but not acute TRPV1 pain signals. Cell 2015; 160: 759–70; PMID:25679765; http://dx.doi.org/ 10.1016/j.cell.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 92.Xing BM, Yang YR, Du JX, Chen HJ, Qi C, Huang ZH, Zhang Y, Wang Y. Cyclin-dependent kinase 5 controls TRPV1 membrane trafficking and the heat sensitivity of nociceptors through KIF13B. J Neurosci 2012; 32: 14709–21; PMID:23077056; http://dx.doi.org/ 10.1523/JNEUROSCI.1634-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holland S, Coste O, Zhang DD, Pierre SC, Geisslinger G, Scholich K. The ubiquitin ligase MYCBP2 regulates transient receptor potential vanilloid receptor 1 (TRPV1) internalization through inhibition of p38 MAPK signaling. J Biol Chem 2011; 286: 3671–80; PMID:21098484; http://dx.doi.org/ 10.1074/jbc.M110.154765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thut PD, Wrigley D, Gold MS. Cold transduction in rat trigeminal ganglia neurons in vitro. Neuroscience 2003; 119: 1071–83; PMID:12831865; http://dx.doi.org/ 10.1016/S0306-4522(03)00225-2 [DOI] [PubMed] [Google Scholar]
- 95.Madrid R, de la Pena E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci 2009; 29: 3120–31; PMID:19279249; http://dx.doi.org/ 10.1523/JNEUROSCI.4778-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morenilla-Palao C, Luis E, Fernandez-Pena C, Quintero E, Weaver JL, Bayliss DA, Viana F. Ion channel profile of TRPM8 cold receptors reveals a role of TASK-3 potassium channels in thermosensation. Cell Rep 2014; 8: 1571–82; PMID:25199828; http://dx.doi.org/ 10.1016/j.celrep.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sarria I, Ling J, Xu GY, Gu JG. Sensory discrimination between innocuous and noxious cold by TRPM8-expressing DRG neurons of rats. Mol Pain 2012; 8: 79; PMID:23092296; http://dx.doi.org/ 10.1186/1744-8069-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 2007; 447: 855–8; PMID:17568746; http://dx.doi.org/ 10.1038/nature05880 [DOI] [PubMed] [Google Scholar]
- 99.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 2005; 8: 626–34; PMID:15852009; http://dx.doi.org/ 10.1038/nn1451 [DOI] [PubMed] [Google Scholar]
- 100.Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci 2005; 25: 1674–81; PMID:15716403; http://dx.doi.org/ 10.1523/JNEUROSCI.3632-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol 2006; 175: 377–82; PMID:17088424; http://dx.doi.org/ 10.1083/jcb.200607116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem 2009; 284: 1570–82; PMID:19019830; http://dx.doi.org/ 10.1074/jbc.M807270200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fujita F, Uchida K, Takaishi M, Sokabe T, Tominaga M. Ambient temperature affects the temperature threshold for TRPM8 activation through interaction of phosphatidylinositol 4,5-bisphosphate. J Neurosci 2013; 33: 6154–59; PMID:23554496; http://dx.doi.org/ 10.1523/JNEUROSCI.5672-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vanden Abeele F, Zholos A, Bidaux G, Shuba Y, Thebault S, Beck B, Flourakis M, Panchin Y, Skryma R, Prevarskaya N. Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J Biol Chem 2006; 281: 40174–82; PMID:17082190; http://dx.doi.org/ 10.1074/jbc.M605779200 [DOI] [PubMed] [Google Scholar]
- 105.Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci 2007; 27: 3347–55; PMID:17376995; http://dx.doi.org/ 10.1523/JNEUROSCI.4846-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morenilla-Palao C, Pertusa M, Meseguer V, Cabedo H, Viana F. Lipid raft segregation modulates TRPM8 channel activity. J Biol Chem 2009; 284: 9215–24; PMID:19176480; http://dx.doi.org/ 10.1074/jbc.M807228200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci 2005; 25: 11322–9; PMID:16339027; http://dx.doi.org/ 10.1523/JNEUROSCI.3006-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Linte RM, Ciobanu C, Reid G, Babes A. Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp Brain Res 2007; 178: 89–98; PMID:17006682; http://dx.doi.org/ 10.1007/s00221-006-0712-3 [DOI] [PubMed] [Google Scholar]
- 109.Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, McNaughton PA. Direct inhibition of the cold-activated TRPM8 ion channel by Galpha(q). Nat Cell Biol 2012; 14: 851–8; PMID:22750945; http://dx.doi.org/ 10.1038/ncb2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li L, Zhang X. Differential inhibition of the TRPM8 ion channel by Galphaq and Galpha 11. Channels (Austin) 2013; 7: 115–8; PMID:23334401; http://dx.doi.org/ 10.4161/chan.23466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J Neurosci 2013; 33: 12543–52; PMID:23884957; http://dx.doi.org/ 10.1523/JNEUROSCI.5765-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 2008; 131: 1241–51; PMID:18356188; http://dx.doi.org/ 10.1093/brain/awn060 [DOI] [PubMed] [Google Scholar]
- 113.Dall'Acqua MC, Bonet IJ, Zampronio AR, Tambeli CH, Parada CA, Fischer L. The contribution of transient receptor potential ankyrin 1 (TRPA1) to the in vivo nociceptive effects of prostaglandin E(2). Life Sci 2014; 105: 7–13; PMID:24607781; http://dx.doi.org/ 10.1016/j.lfs.2014.02.031 [DOI] [PubMed] [Google Scholar]
- 114.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 2009; 64: 498–509; PMID:19945392; http://dx.doi.org/ 10.1016/j.neuron.2009.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004; 41: 849–57; PMID:15046718; http://dx.doi.org/ 10.1016/S0896-6273(04)00150-3 [DOI] [PubMed] [Google Scholar]
- 116.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 2007; 10: 277–9; PMID:17259981; http://dx.doi.org/ 10.1038/nn1843 [DOI] [PubMed] [Google Scholar]
- 117.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006; 124: 1269–82; PMID:16564016; http://dx.doi.org/ 10.1016/j.cell.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 118.Cruz-Orengo L, Dhaka A, Heuermann RJ, Young TJ, Montana MC, Cavanaugh EJ, Kim D, Story GM. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol Pain 2008; 4: 30; PMID:18671867; http://dx.doi.org/ 10.1186/1744-8069-4-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Taylor-Clark TE, Undem BJ, Macglashan DW Jr., Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol 2008; 73: 274–81; PMID:18000030; http://dx.doi.org/ 10.1124/mol.107.040832 [DOI] [PubMed] [Google Scholar]
- 120.Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A 2008; 105: 12045–50; PMID:18687886; http://dx.doi.org/ 10.1073/pnas.0802354105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol 2005; 564: 103–16; PMID:15677687; http://dx.doi.org/ 10.1113/jphysiol.2004.081059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, et al.. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J 2009; 28: 1308–18; PMID:19279663; http://dx.doi.org/ 10.1038/emboj.2009.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pereira V, Busserolles J, Christin M, Devilliers M, Poupon L, Legha W, Alloui A, Aissouni Y, Bourinet E, Lesage F, et al.. Role of the TREK2 potassium channel in cold and warm thermosensation and in pain perception. Pain 2014; 155: 2534–44; PMID:25239074; http://dx.doi.org/ 10.1016/j.pain.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 124.Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 2008; 321: 702–5; PMID:18669863; http://dx.doi.org/ 10.1126/science.1156916 [DOI] [PubMed] [Google Scholar]


