Abstract
We found recently that mTORC1 regulates the biosynthesis of the ubiquitin E3 ligase Nedd4–1, but not the close homolog Nedd4–2. This regulatory process plays a key role in promoting neurite growth in neurons of the mammalian central nervous system. The molecular mechanism underlying this rather specific regulation likely involves a pyrimidine-rich sequence stretch near the putative transcriptional start site within the 5′ untranslated region of the Nedd4–1 mRNA, which may play a crucial role in directing the assembly of the protein translation machinery. We postulate that the Nedd4–1 mRNA is a major target of the local translation machinery within neurons that can be translated in a spatially and temporally controlled manner in response to various stimuli. Based on this model, neuronal Nedd4–1 may not only be involved in the regulation of neurite growth but also in axon guidance, spine formation, and synaptic plasticity.
Keywords: mTORC1, Nedd4–1, translation
Protein ubiquitination is one of the core regulatory principles in neuronal development.1 During the process of protein ubiquitination, E3 ubiquitin ligases play a central role as they determine the substrate specificity. Nedd4–1 is an E3 ubiquitin ligase that was originally identified as one of several Neuronal precursor cell Expressed and Developmentally Downregulated genes. During the development of neurons, Nedd4–1 is an important positive regulator of neurite outgrowth. In this regard, multiple potential protein substrates of Nedd4–1 have been identified, such as Rap2A and IGF1-R.2,3 However, little is known about the regulation of Nedd4–1.
We recently reported that in mammalian central nervous system neurons, Nedd4–1 expression is regulated at the translational level by mammalian target of rapamycin complex 1 (mTORC1). Aberrant upregulation of mTORC1 activity in neurons by deletion of PTEN, a lipid phosphatase that antagonizes phosphoinositide-3-kinase-dependent (PI3K-dependent) signaling by converting phosphatidylinositol-3,4,5-trisphosphate (PtdInsP3) into phosphatidylinositol-4,5-bisphosphate (PtdInsP2), leads to increased translation of Nedd4–1 mRNAs and concomitant upregulation of Nedd4–1 protein levels. In turn, inhibition of mTORC1 activity in PTEN KO neurons by the mTORC1 inhibitor rapamycin reverts the aberrant increase in Nedd4–1 expression.4
Regulation of Nedd4–1 expression by mTORC1-dependent signaling
Intriguingly, mTORC1 activity only regulates the expression of Nedd4–1 but not of Nedd4–2, the closest homolog of Nedd4–1 in the mammalian genome,4 but the molecular mechanism underlying this isoform preference is unknown. mTORC1 promotes global cap-dependent protein synthesis mainly by mediating the phosphorylation of eukaryotic initiation factor 4E binding protein 1 and 2 (4E-BP1 and 4E-BP2). 4E-BPs bind to eukaryotic initiation factor 4E (elF4E) to prevent the formation of protein initiation complex and protein translation. Phosphorylation of 4E-BPs leads to decreased affinity of 4E-BPs to eIF4E. This enables eIF4E to be anchored to the 5′-cap structure of mRNAs, promotes the formation of protein initiation complex, and initiates protein synthesis.5 Of note, the 5′ untranslated region (5'UTR) of the Nedd4–1 mRNA (GenBank accession number: NM_010890) contains a pyrimidine-rich sequence stretch that is related to the 5′ terminal oligopyrimidine (5′TOP) motif. This 5′TOP motif is important for anchoring elF4E to the 5′-cap structure of mRNAs, to direct the assembly of the translation machinery, and to initiate protein synthesis. The pyrimidine-rich sequence in the 5′UTR of Nedd4–1 mRNA may play a similar role as the 5′TOP motif in initiating the translation of Nedd4–1 mRNAs in an mTORC1 activity-dependent manner (Fig. 1). Further evidence supporting this model includes a study employing ribosome profiling to examine the translational efficiency of specific mRNAs in the presence of an mTORC1 inhibitor, which showed that the translational efficiency of Nedd4–1 mRNA is reduced in non-neuronal cells upon mTORC1 inhibition.6
Figure 1.
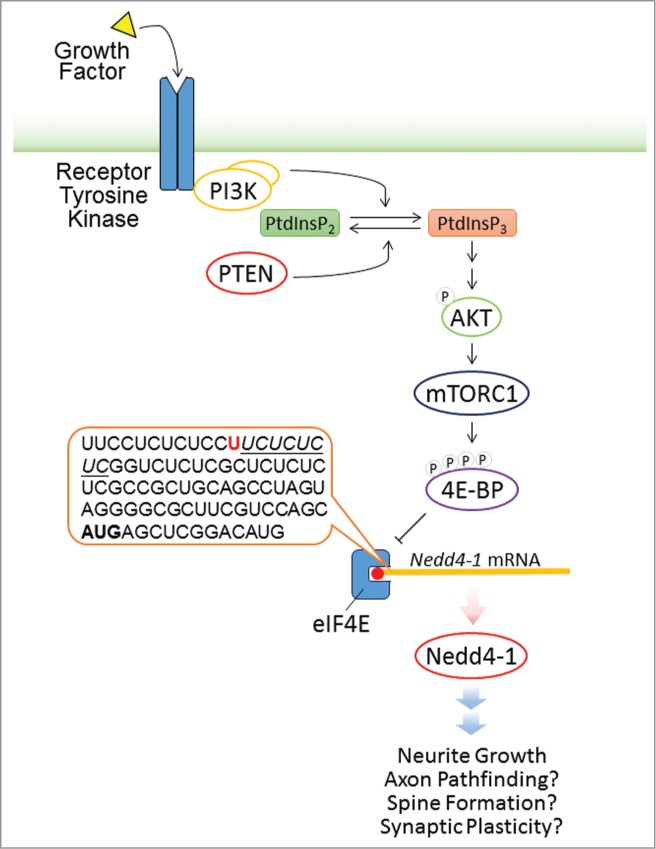
Model of the Regulation of Nedd4–1 Translation by mTORC1. In response to growth factor signaling, PI3K catalyzes the phosphorylation of PtdInsP2 to generate PtdInsP3. PTEN converts PtdInsP3 back to PtdInP2 and thus antagonizes the effect of PI3K. Elevated PtdInsP3 levels lead to the phosphorylation and activation of AKT, which then activates mTORC1. Phosphorylation of 4E-BPs by mTORC1 reduces the binding of 4E-BPs to eIF4E, enabling eIF4E to bind to the 5'UTR of target mRNAs and thus initiate protein synthesis. The 5'UTR of Nedd4–1 mRNA (see sequence in the dialog box) contains a pyrimidine-rich sequence stretch (underlined) after the putative transcriptional start site (red), which is related to the 5' terminal oligopyrimidine (5'TOP) motif. This pyrimidine-rich sequence may play a key role in starting the translation of Nedd4–1 mRNAs in an mTORC1 activity-dependent manner. Nedd4–1 is a positive regulator of neurite growth and may have additional roles in regulating axon pathfinding, spine formation, and synaptic plasticity.
Local translation of Nedd4–1 in neurons and possible roles
Accumulating evidence indicates crucial roles of local protein synthesis in various aspects of normal neuronal development and function, including axon guidance, spine formation, and synaptic plasticity. All of these processes are highly dynamic and require spatially and temporally well-regulated and accurate changes in local protein levels in response to various stimuli.7-9 Locally regulated translation of mRNAs provides an efficient way to spatially and temporally control local protein composition, which is of particular importance in distal neurites, where proteins synthesized at the soma may not be an economic source. The finding that Nedd4–1 expression is regulated at the translational level is compatible with the notion that Nedd4–1 mRNAs are major targets of the local translational machinery in neurons. If so, it is very likely that Nedd4–1 does not only regulate neurite growth regulation, as we reported previously,2,4 but may have important additional roles in axon guidance, spine formation, and synaptic plasticity. Indeed, Drosophila melanogaster Nedd4 regulates Commisureless, which is an essential protein regulator of Slit/Robo signaling during axon pathfinding and midline crossing.10 Moreover, synaptic activation has been shown to cause a site-specific increase in Nedd4–1 expression, which may mediate the ubiquitination and endocytosis of the AMPA receptor subunit GluA1.11,12 In view of these findings, additional studies, ideally with higher spatiotemporal resolution, on how Nedd4–1 expression is regulated in response to extracellular signals is very likely to provide further important insights into the physiological roles of Nedd4–1 in neurons.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Nils Brose for his comments on the manuscript.
Funding
This work has been supported by grants from the German Research Foundation (SPP1365/KA3423/1–1; to H.K.) and the Fritz Thyssen Foundation (to H.K.).
References
- 1.Kawabe H, Brose N. The role of ubiquitylation in nerve cell development. Nat Rev Neurosci 2011; 12:251-68; PMID:21505515; http://dx.doi.org/ 10.1038/nrn3009 [DOI] [PubMed] [Google Scholar]
- 2.Kawabe H, Neeb A, Dimova K, Young SM Jr, Takeda M, Katsurabayashi S, Mitkovski M, Malakhova OA, Zhang D-E, Umikawa M, et al.. Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron 2010; 65:358-72; PMID:20159449; http://dx.doi.org/ 10.1016/j.neuron.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao XR, Lill NL, Boase N, Shi PP, Croucher DR, Shan H, Qu J, Sweezer EM, Place T, Kirby PA, et al.. Nedd4 controls animal growth by regulating IGF-1 signaling. Sci Signal 2008; 1:ra5; PMID:18812566; http://dx.doi.org/ 10.1126/scisignal.1160940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsia H-E, Kumar R, Luca R, Takeda M, Courchet J, Nakashima J, Wu S, Goebbels S, An W, Eickholt BJ, et al.. Ubiquitin E3 ligase Nedd4-1 acts as a downstream target of PI3K/PTEN-mTORC1 signaling to promote neurite growth. Proc Natl Acad Sci 2014; 111:13205-10; PMID:25157163; http://dx.doi.org/ 10.1073/pnas.1400737111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gingras A-C, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 1999; 68:913-63; PMID:10872469; http://dx.doi.org/ 10.1146/annurev.biochem.68.1.913 [DOI] [PubMed] [Google Scholar]
- 6.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012; 485:109-13; PMID:22552098; http://dx.doi.org/ 10.1038/nature11083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt CE, Schuman EM. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron 2013; 80:648-57; PMID:24183017; http://dx.doi.org/ 10.1016/j.neuron.2013.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci 2012; 13:308-24; PMID:22498899; http://dx.doi.org/ 10.1038/nrn3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell 2006; 127:49-58; PMID:17018276; http://dx.doi.org/ 10.1016/j.cell.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 10.Myat A, Henry P, McCabe V, Flintoft L, Rotin D, Tear G. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the Roundabout receptor. Neuron 2002; 35:447-59; PMID:12165468; http://dx.doi.org/ 10.1016/S0896-6273(02)00795-X [DOI] [PubMed] [Google Scholar]
- 11.Hou Q, Gilbert J, Man H-Y. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron 2011; 72:806-18; PMID:22153376; http://dx.doi.org/ 10.1016/j.neuron.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarz LA, Hall BJ, Patrick GN. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J Neurosci 2010; 30:16718-29; PMID:21148011; http://dx.doi.org/ 10.1523/JNEUROSCI.3686-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


