Abstract
Mucormycosis is an uncommon but severe fungal infection, typically observed in immunocompromized patients. We report a case of acute lymphoblastic leukemia complicated by rhino-oculo-cerebral mucormycosis in a pediatric patient. Combination lipid polyene-echinocandin therapy, along with surgical debridement appeared to be effective. Nevertheless, a severe relapse occurred during posaconazole prophylaxis; antifungal therapy, hemimaxillectomy and suspension of chemotherapy were performed. Although mucormycosis is a frequently lethal infection, prompt diagnosis and aggressive treatment can be successful even in cases of relapse.
Key words: Mucormycosis, pediatrics, leukemia
Introduction
Mucormycosis (also called Zygomycosis) is a rare, life-threatening invasive fungal infection caused by organisms belonging to the Mucorales order of the Zygomycetes class; after aspergillosis it is the second most common form of mycosis caused by filamentous fungi in immunocompromized patients. The infection is typically considered opportunistic and community-acquired, although the true incidence of mucormycosis in hematology units is probably underestimated because of difficulties in ante-mortem diagnosis and the low autopsy rate in cancer patients. Risk factors include uncontrolled diabetes, cancer, organ transplant, neutropenia and skin trauma. A prompt diagnosis, immediate antifungal treatment, together with an aggressive surgical approach, are essential to prevent dissemination, although survival remains very poor in immunocompromized patients. We report a case of T-cell acute lymphoblastic leukemia (ALL) complicated by recurrent rhino-oculo-cerebral mucormycosis, successfully managed by timely administration of medical and surgical intervention.
Case Report
A twelve year old girl was receiving the first part of reinduction chemotherapy according to AIEOP-BFM ALL 2009 protocol for high-risk T cell ALL (dexamethasone days 1-15, vincristine and doxorubicin days 1, 7).
On day eleven she developed fever and rapidly progressive multi-organ toxicity; according to the Common Terminology Criteria for Adverse Events Version 4.0, the toxicity was grade four hematological (severe pancytopenia), grade four metabolic (insulin-resistant diabetes, severe hypertriglyceridemia), grade three hepatobiliary (hyperbilirubinemia 4.5 mg/dL), grade three musculoskeletal (severe myositis with rhabdomyolysis) and grade two neurological painful peripheral neuropathy). Microbiological investigations detected Epstein Barr virus (EBV) polimerase chain reaction test and Candida antigen serum positivity; moreover, extended-spectrum beta-lactamases-producing Escherichia Coli was isolated in blood, urine and pharyngeal swab. Although broad-spectrum antibiotics and liposomal amphotericin B (3 mg/kg) were immediately administered (no treatment for EBV was established), a left periorbital swelling appeared, quickly progressing to severe rhino-orbital cellulitis and the girl suffered a self-limiting seizure forty-eight hours later. Contrast-enhanced brain magnetic resonance imaging showed multiple altered signal areas in the right frontal and bilateral cerebellar white matter (Figure 1); computed tomography scan of the paranasal sinuses showed peri-orbital soft tissues edema and left maxillary sinusitis with concomitant bone erosion. Despite aggressive supportive therapies, the patient’s general conditions and local infection rapidly worsened and the right upper mucosa of the mouth appeared bloated. Diagnostic rhino-endoscopy detected a huge necrotic pansinusitis and microscopic examination revealed massive fungal infarction in all specimens. At histological confirmation of the suspicion of mucormycosis, the liposomal amphotericin B dose was increased to 7.5 mg/kg/day, caspofungin was introduced and serial rhino-endoscopic surgical debridement along with dacryocystorhinostomy were performed. Standard tissue cultures of specimens were disappointing (Figure 2). On the following days, hematological parameters and clinicalradiological findings progressively improved. Hyperbaric oxygen therapy was proposed but rejected in view of the pain. On day 39 the patient complained of a sudden shooting pain in the left parotid region. Angio magnetic resonance imaging showed a thrombotic pseudoaneurysm of the left internal maxillary artery, that resolved during subsequent urgent selective angiography. On day sixty, cerebral and the palatal lesions and cellulitis had completely disappeared, as well as any histological evidence of fungi in rhino-endoscopic biopsies. Liposomal amphotericin B was replaced by oral posaconazole (400 mg/bid with target dose >0.7 mg/L) and tapered maintenance chemotherapy was resumed (daily 6-mercaptopurine and weekly methotrexate). Six months later, during continuous posaconazole treatment, the girl exhibited a rapid reactivation of the palatal fungal localization, revealed through spontaneous avulsion of three teeth and wide maxillary bone exposure; computed tomography scan showed necrosis of the whole left upper maxilla. High-dose liposomal amphotericin B treatment was resumed and the girl underwent left hemimaxillectomy with a palatal and orbital base prosthesis implant. Chemotherapy was definitely stopped and liposomal amphotericin B was continued for fifty-eight days and then replaced by posaconazole. At present, six months from the hemimaxillectomy, the girl shows no functional deficits and a mild cosmetic impairment, she is in complete hematological remission and continues to take posaconazole.
Figure 1.
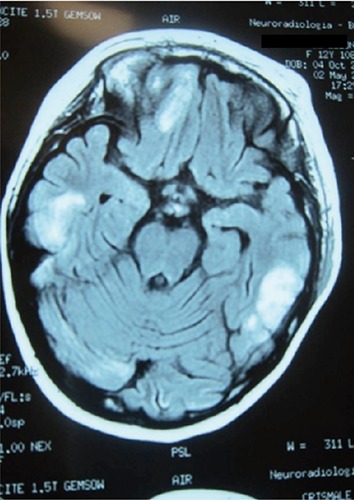
Brain magnetic resonance imaging: multiple contrast-enhanced cerebral lesions.
Figure 2.
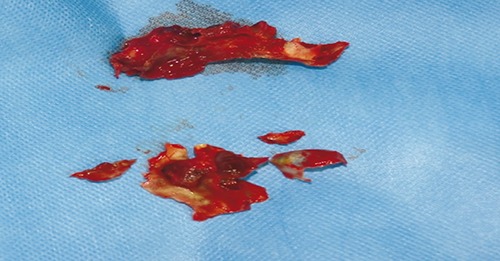
Tissue specimens for histological examination.
Discussion
Mucormycosis is five- to ten-fold less common than other fungal infections such as aspergillosis. Untreated or misdiagnosed infection in immunocompromized hosts can have a rapidly fatal outcome and the majority of patients still die within twelve weeks from diagnosis. A prompt diagnosis is hampered by nonspecific symptoms and signs (bloody nasal discharge, eye or facial pain, conjunctival suffusion, blurry vision, soft tissue swelling), mimicking more common infectious etiologies, unreliable serologic or polymerase-chain-reaction based tests, rarely positive blood and infected tissues cultures, that are often inconsistent or contaminated. Moreover, the frequent isolation of bacteria from infected tissues may dissuade clinicians from suspecting a concomitant invasive fungal infection, thus delaying targeted therapy. Therefore, the diagnosis of mucormycosis requires a high grade of suspicion and close communication between clinicians and microbiologists; histopathologic identification of the causative fungus is essential. Together with an early diagnosis, the reversal of underlying predisposing factors (if feasible) and a multidisciplinary approach are critical for a successful outcome; high-dose liposomal amphotericin B therapy and aggressive debridement of necrotic tissues are widely recommended.1 The central role of iron metabolism in the pathogenesis of mucormycosis suggests the use of iron chelators as adjunctive therapy and case reports suggest that hyperbaric oxygen may be beneficial, too. Nevertheless, data on mucormycosis in pediatric immunocompromized patients are limited. Dabritz et al.2 reported twelve pediatric cases of mucormycosis collected from Germany and Austria between January 2004 and December 2008. Three patients with isolated soft tissues infection survived, whereas seven out of nine with pulmonary and rhino-cerebral involvement died.2 Similarly, a retrospective study of eleven immunocompromized pediatric cancer patients treated in France from 1991 to 2011 affected by mucormycosis was carried out by Phulpin-Weibel and colleagues; eight children survived.3 Four immunocompromized patients with nasal sinuses mucormycosis undergoing multiple aggressive debridement were described by Rassi et al.; in the postoperative course one child died of the disease, one developed persistent unilateral blindness, one was lost to follow-up and the last one was cured with no sequelae.4 Other single-center pediatric cases have recently been described, including a seven-year-old girl with ALL and rhino-cerebral mucormycosis, two children with acute leukemia and intraoral mucormycosis and four children with ALL and cutaneous mucormycosis; all patients were successfully treated with amphotericin B and surgical debridement.5-8 Further case reports describe a child with acute leukemia and isolated muscular mucormycosis, one with ALL and fatal gastrointestinal mucormycosis, a nine-year-old boy with ALL and isolated hepatic mucormycosis requiring surgical excision and combined liposomal amphotericin B and posaconazole therapy. Wehl et al. reported a boy with ALL relapse experiencing rhino-cerebral-mucormycosis who could not receive surgical debridement because of extensive involvement; his long-term survival of fifteen months was attributed to the long-term administration of liposomal amphotericin B, early neutrophil recovery and slow leukemia progression.9 All papers highlight that patients with mucormycosis have a high mortality rate, especially in cases of multi-organ involvement and delayed diagnosis; standard treatment is a medical-surgical approach (Table 1). In our case, the histopathologic diagnosis was rapid but our patient exhibited many risk factors and comorbidities. Mainly, she was heavily pre-treated for high-risk ALL and received fluconazole antifungal prophylaxis (6 mg/kg) in Induction during neutropenia (absolute neutrophil count <500 mm-3).
Table 1.
Summary of all published papers reporting on mucormycosis.
| Author | Patients | Treatment | Risk factors | Deaths |
|---|---|---|---|---|
| Däbritz et al.2 | 12 | Surgical debridement + antifungal therapy (4); Amphotericin in B/posaconazole (5) | Glucocorticosteroids treatment/neutropenia (8), suline-dependent diabetes (2), soft tissue trauma (1), extracorporal membrane oxygenation (1) | 8 |
| Phulpin-Weibel et al.3 | 11 | Amphotericin B + Caspofungin (3) Posaconazole (2) Amphotericin B (5) | Neutropenia (6), immunosoppressive agents (3), steroids-induced diabetes mellitus (2) | 3 |
| Rassi et al.4 | 4 | A Amphotericin B and surgical debridement | Immunosoppressive agents | 1 |
| Popa et al.5 | 1 | A Amphotericin B and surgical debridement | Glucocorticosteroids treatment/neutropenia | 0 |
| Dogan et al.6 | 2 | Amp Amphotericin B and and surgical debridement | Glucocorticosteroids treatment/neutropenia | 0 |
| Cantatore-Francis et al.7 | 2 | Amp Amphotericin B and surgical debridement | Immunosoppressive agents | 0 |
| Gupta et al.8 | 2 | Amp Amphotericin B and surgical debridement | Glucocorticosteroids treatment/neutropenia | 0 |
| Wehl et al.9 | 1 | Amphotericin B | Blood transplantation | 0 |
Concomitantly, she experienced iatrogenic diabetes that was barely controlled despite insulin dosages of up to 1.8 U/kg per day and corticosteroid tapering. Granulocyte stimulating-factor was introduced to shorten the anticipated prolonged and profound neutropenia but Epstein Barr virus reactivation and beta-lactamases-producing Escherichia Coli colonization contributed to the deterioration of her general performance status. Liposomal amphotericin B was initially introduced as empiric antifungal therapy but then increased to 7.5 mg/kg per day when Mucor was identified; the liposomal amphotericin B formulation was chosen versus standard amphotericin B because of its predictable pharmacokinetics, superior central nervous system penetration and better toxicity profile. Even if Echinocandins demonstrate a modest in vitro activity against Mucorales, Reed et al. highlighted a survival advantage in diabetic patients with rhino-oculo-cerebral mucormycosis receiving this combination treatment (liposomal amphotericin B plus caspofungin).10 We decided to combine the two agents, predicting a severe course of the infection, and no relevant side effect was noted. The initial surgical approach was immediate but conservative endoscopic serial debridement aimed at removing as much necrotic matter as possible but sparing the deep orbital structures and visual function. Long-term posaconazole secondary therapy was chosen for its known activity against the fungus; therapeutic drug monitoring was instituted to prevent breakthrough relapse associated with inadequate blood concentrations. It is widely recognized that a fine balance between antifungal and antileukemic therapy is a critical issue. In our case, the planned high-dose chemotherapy schedule was abandoned in favor of a tailored antileukemic maintenance therapy prudentially instituted to minimize the risk of ALL relapse. An unexpected life-threatening fungal reactivation occurred despite continuous secondary prophylaxis and the mild immunosuppression allowed a more aggressive intervention (hemimaxillectomy); chemotherapy was definitely stopped to permit a full immunological recovery.
Conclusions
Despite being a very rare infection, mucormycosis should be included in differential diagnosis when dealing with pediatric immunocompromized patients. Our case demonstrates that an early diagnosis, integrated care across several medical-surgical disciplines and a tailored and complex decision-making sequence can be life-saving. Nevertheless, these efforts do not totally protect against the risk of relapse, that can occur despite high grade surveillance, continuous secondary prophylaxis and de-escalated chemotherapy. Nevertheless, a reactivation of the infection does not rule out the chance for healing, although a complete suspension of chemotherapy is revealed as essential for a long term successful outcome in such cases. More extensive studies are needed to clarify the contribution of Echinocandins combination therapy.
Acknowledgments
The authors would thank Mrs. Babette Pragnell for language revision.
References
- 1.Lewis RE, Kontoyiannis DP. Epidemiology and treatment of mucormycosis. Future Microbiol 2013;8:1163-75. [DOI] [PubMed] [Google Scholar]
- 2.Däbritz J, Attarbaschi A, Tintelnot K, et al. Mucormycosis in paediatric patients: demographics, risk factors and outcome of 12 contemporary cases. Mycoses 2011;54:e785-8. [DOI] [PubMed] [Google Scholar]
- 3.Phulpin-Weibel A, Rivier A, Leblanc T, et al. Focus on invasive mucormycosis in paediatric haematology oncology patients: a series of 11 cases. Mycoses 2013;56:236-40. [DOI] [PubMed] [Google Scholar]
- 4.Rassi SJ, Melkane AE, Rizk HG, Dahoui HA. Sinonasal mucormycosis in immuno-compromised pediatric patients. J Pediatr Hematol Oncol 2009;31:907-10. [DOI] [PubMed] [Google Scholar]
- 5.Popa G, Blag C, Sasca F. Rhinocerebral mucormycosis in a child with acute lymphoblastic leukemia: a case report. 2008;30:163-5. [DOI] [PubMed] [Google Scholar]
- 6.Dogan MC, Leblebisatan G, Haytac MC, et al. Oral mucormycosis in children with leukemia: report of 2 cases. Quintessence Int 2007;38:515-20. [PubMed] [Google Scholar]
- 7.Cantatore-Francis JL, Shin HT, Heilman E, Glick SA. Primary cutaneous zygomycosis in two immunocompromised children. Pediatr Dermatol 2007;24:257-62. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A, Jain S, Agrawal C, Gauri K. Successful outcome of mucormycosis in two children on induction therapy for acute lymphoblastic leukemia. Indian J Med Paediatr Oncol 2013;34:313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wehl G, Hoegler W, Kropshofer G, et al. Rhinocerebral mucormycosis in a boy with recurrent acute lymphoblastic leukemia: long-term survival with systemic antifungal treatment. J Pediatr Hematol Oncol 2002;24:492-4. [DOI] [PubMed] [Google Scholar]
- 10.Reed C, Bryant R, Ashraf S, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis 2008;47:364-71. [DOI] [PMC free article] [PubMed] [Google Scholar]


