Abstract
Homing and engraftment, a determining factor in hematopoietic stem cell transplantation success is defined as a process through which hematopoietic stem/progenitor cells (HSPCs) lodge recipient bone marrow. SDF-1/CXCR4 axis acts as a principle regulator in homing and engraftment, however, CXCR4 signaling is dependent upon expression of CXCR4 and its ligand SDF-1, which is highly dynamic. Hence, present investigation was aimed to explore the potential of CXCR4 constitutive active mutants (CXCR4-CAMs) in overcoming the limitation of CXCR4 signaling and up-modulate its efficiency in homing and engraftment. Regulated transgene expression study of these mutants revealed their significantly enhanced cell adhesion efficiency to endothelium and extracellular matrix protein. This altogether indicates promising prospects of CXCR4-CAMs in research aimed to improve HSPCs engraftment efficiency.
Keywords: HSPCs, CXCR4, homing, engraftment
Abbreviations
- HSPCs
Hematopoietic Stem/ Progenitor Cells
- BM
Bone Marrow
- TM3
Transmembrane three domain
- Ser
Serine
- Ala
Alanine
- Asn
Asparagine
- CAMs
Constitutive Active Mutants
- MCS
Multi Cloning Site
- IMDM
Iscove's Modified Dulbecco Media
- FBS
Fetal Bovine Serum
- Conc.
Concentration
- BSA
Bovine Serum Albumin
- ECM
Extracellular matrix
- BMEC
Bone marrow endothelial cells
- HUVECs
Human Umbilical Vein Endothelial cells
- FN
Fibronectin
- LIF
Leukemia Inhibitory Factor
Introduction
SDF-1/CXCR4 axis plays a key role in bone marrow (BM) homing and engraftment of HSPCs, a biological process through which intravenously transfused HSPCs reach and lodge the BM niche of recipient. However, the low homing and engraftment efficiency of HSPCs act as a limiting factor in wide range implications and success of hematopoietic stem cell transplantation (HSCT).1 Gene knockout studies of SDF-1 and CXCR4 as well as transfusion of human HSPCs in murine recipients revealed the principle role of SDF-1/CXCR4 signaling in homing and engraftment.2-5 CXCR4 (a G protein coupled receptor) is expressed on surface of HSPCs which upon binding to its ligand SDF-1, switches to active conformation, highly permissible for interaction to G protein and other downstream effectors, thus resulting in CXCR4 downstream signaling.6 This signaling cascade regulates the integrins mediated cell adhesion on BM endothelium and subsequent transmigration and lodgment of cells into BM stroma that altogether elicit homing and engraftment.7,8 Functional activation of CXCR4 signaling is therefore dependent on CXCR4 interaction to SDF-1, thus indicating the necessity of optimal hematopoietic microenvironment wherein both receptor and ligand should express fully. However, expression of SDF-1/CXCR4 axis is highly variable and regulated by several factors in BM.1,9 Under present scenario, specific modification of CXCR4 to make it active without ligand binding to induce autonomous signaling may prove beneficial via overcoming the necessity of consistent optimal expression of ligand and receptor.
NYSS is a highly conserved motif in transmembrane three (TM3) domain of CXC chemokine receptors, and play a crucial role in CXCR4 signaling through regulation of dynamic conformational equilibrium of receptor between inactive to active state.10 Conversion of Asn-119 of this motif in CXCR4 to Ser or Ala induces active conformation of CXCR4 which results its autonomous signaling. Autonomous coupling of these CAMs to G protein subunits is further augmented by SDF-1 binding, thus, indicates the consistent optimal active conformation of receptor.11 Hence, the regulated implication of CXCR4-CAMs may turn the cell as a more potent player over its native type in the cellular events crucial to elicit homing and engraftment process. Therefore, in present study we used Tet-on inducible gene expression system for regulated transgene expression of CXCR4-CAMs (N119A and N119S) in hematopoietic stem progenitor cell line K-562, and assessed the response of CXCR4 mutant expressing K-562 cells in the key cellular events of homing that are cell adhesion/binding ability to extracellular matrix (ECM) protein fibronectin and to endothelial cells as compared with wild type.
Results and Discussion
CXCR4 transgene expression in stable transfected K-562 cells
The K-562 cell population is highly undifferentiated hematopoietic stem progenitor cells,12 and is CXCR4 null at m-RNA transcript level,13 hence, provides opportunity for transgene expression study of CXCR4-transgene constructs without any interference of host endogenous CXCR4 gene. Further, to analyze the function of transgene of interest, it is critical to regulate the target gene expression in mammalian cell system or preclinical models in a conditional manner. The ability to turn the transgene gene expression on or off in the selected cell line at specific times, allows the flexibility in dissecting the target gene functions in various biological process. Pioneering studies in conditional transgene expression have brought about the development of variety of controlled gene expression systems. Among them, the tetracycline-controlled expression systems (e.g., Tet-off system and Tet-on system) have been used extensively in vitro and in vivo.14,15 Therefore, using Tet-on inducible gene expression system we derived three different subsets of CXCR4 stable K-562 cells which express the wild type (wtCXCR4-pTRE2hyg), mutant 1(N119ACXCR4-pTRE2hyg) and mutant 2 (N119SCXCR4-pTRE2hyg) gene respectively in each subset. Further, doxycycline dependent CXCR4 target gene activation in stable transfected cells was confirmed by immunofluorescence microscopic analysis which detected the doxycycline inducible regulated expression of CXCR4 surface protein at 48 h of incubation in all the subsets as evident by their significantly increased fluorescence intensity as compared with control (doxycycline induced untransfected K-562 cells) (Fig. 1).
Figure 1.
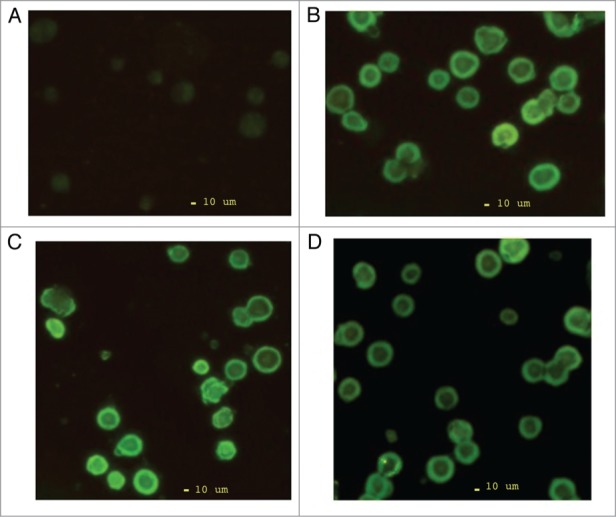
Immunofluorescence microscopy of doxycycline induced CXCR4 surface protein expression in stable transfected K-562 cells. (A) Control untransfected K-562 cells, (B) Wild type CXCR4 stable transfected K-562 cells, (C) N119ACXCR4-pTRE2hyg stable transfected K-562 cells, (D) N119SCXCR4-pTRE2hyg stable transfected K-562 cells.
CXCR4 mutants revealed significantly enhanced cell adhesion to endothelial cells as compared with native type
Following intravenous transfusion of HSPCs, their journey to home recipient BM mainly relies upon three consecutive cellular events principally mediated by CXCR4 signaling; 1- firm adhesion of HSPCs on BMEC, 2- their subsequent trans-endothelial migration and chemotaxis toward SDF-1 gradient in BM stroma, and 3-lodgment of transmigrated HSPCs into BM stroma through binding to BM ECM protein, mainly fibronectin.1,9 BMEC form inner lining of blood vessels and serve as the cellular interface between the circulating blood and vessel wall. The role of SDF-1/CXCR4 axis has shown to be crucial for translating the rolling of HSPCs into firm adhesion over BMEC by increasing the adhesiveness of integrins to bind endothelial ligands.8 This event in cell homing is not only essential for preventing the back movement of HSPCs to circulation, but also an essential prerequisite cellular step to proceed further the next step of cell transmigration and subsequent lodgment in BM. We therefore, aimed to assess the endothelium adhesion efficiency of K-562 cells expressing CXCR4-CAMs in comparison to wild type CXCR4 expressing cells by endothelial cell adhesion assay using human umbilical vein endothelial cells (HUVECs) monolayer. The cytokine activated HUVECs monolayer is a well established vascular endothelial cell system which act as a standard model for BM endothelium in ex vivo study.16,17 As evident (Fig. 2) K-562 cells expressing wild type CXCR4, Mutant 1 (N119ACXCR4) and mutant 2 (N119SCXCR4) showed 35%, 66%, and 52% cell adhesion respectively as compared with 11% of control untransfected K-562 cells. Further, in comparative analysis among CXCR4-CAMs, mutant 1 (N119A) and mutant 2 (N119S) expressing cells have revealed significantly increased cell adhesion as compared with wild type (P < 0.05), thus indicating the improved ability of mutants expressing cells to adhere on endothelium vasculature as compared with wild type.
Figure 2.
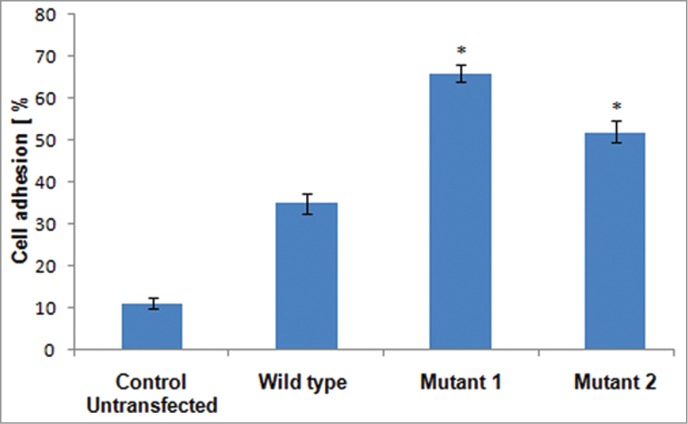
A bar graph depicts % cell adhesion of mutant 1 (N119ACXCR4) and mutant 2 (N119SCXCR4) expressing cells as compared with wildtype CXCR4 expressing cells on the human umbilical vein endothelial cells (HUVECs) monolayer. The results shown represent average of triplicates plus or minus SD (Error bars indicate standard deviation and * indicates P < 0.05 with respect to wild type).
CXCR4 mutants revealed significantly enhanced cell binding ability to fibronectin as compared with native type
After extravasations through BM vascular endothelium, HSPCs must adhere in BM stroma via interaction with ECM proteins in order to retain and home finally in BM niche. FN protein is a main component of ECM, and HSPCs interaction to FN through VLA-4 and VLA-5 receptors is shown to be regulated by SDF-1/CXCR4 axis resulting in cell adherence and anchorage to BM niche.8 We therefore studied the FN binding/adhesive ability of stable transfected cells by fibronectin cell adhesion assay. As shown (Fig. 3) CXCR4 stable K-562 cells expressing wild type CXCR4, mutant 1 (N119ACXCR4) and mutant 2 (N119SCXCR4) showed 22%, 49.5%, and 43.6% cell adhesion to FN respectively as compared with 6.6% of control untransfected cells. In similar manner to endothelial cell adhesion, we found significantly increased FN cell adhesion of mutant 1 (N119ACXCR4-pTRE2hyg) and mutant 2 (N119SCXCR4-pTRE2hyg) as compared with wild type CXCR4 (P < 0.05), thus indicating their up-modulated cell binding ability to FN matrix protein.
Figure 3.
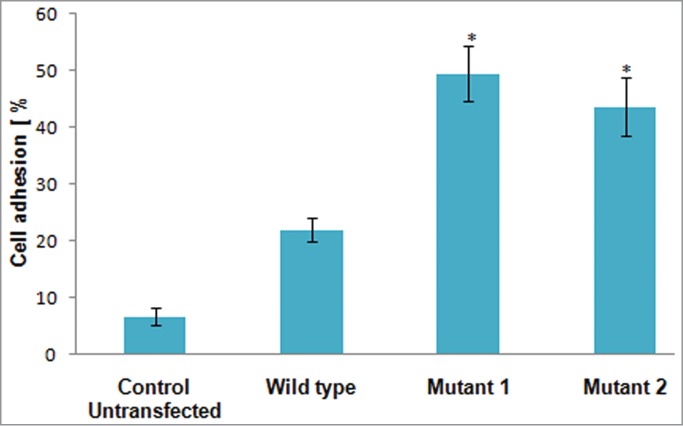
A bar graph showing % cell adhesion of mutant 1 (N119ACXCR4) and mutant 2 (N119SCXCR4) expressing cells as compared with wildtype CXCR4 expressing cells on fibronectin coated 24-well plate. The results shown represent average of triplicates plus or minus SD (Error bars indicate standard deviation and * indicates P < 0.05 with respect to wild type).
Taken together, these results revealed that CXCR4 null untransfected cells are though able to bind and adhere on endothelial cells and matrix protein fibronectin, the % cell adhesion is much below (3.2-fold in endothelial cell adhesion, and 3.3-fold in fibnonectin adhesion) than the cells expressing wild type CXCR4 gene. Supporting the dogma of pivotal role of CXCR4 signaling in homing and engraftment, this altogether indicate that firm adhesion of cells either to endothelium or matrix protein fibronectin is though not solely dependent on CXCR4 signaling, but, is indeed regulated by a co-operative mechanism of homing pathways wherein CXCR4 signaling cascade act as a dominant player to significantly regulate the mechanistic of homing and engraftment.
Further in focus of our study, it is noteworthy that mutants expressing cells revealed significantly increased endothelial cell adhesion as well as fibronectin adhesion efficiency as compared with wild type expressing cells. This may indicate the upregulation of CXCR4 downstream signaling pathways in these mutants, which altogether regulate the functional activation of cell adhesion molecules; integrins and focal adhesion proteins, ultimately resulting in up-modulated adhesion potential of mutants in comparison to wild type. This postulation is strengthened further by differential gene expression profile of CXCR4 stable K-562 transfected cells through microarray comparative study which revealed the upregulation of group of genes in these mutants with respect to native type that have crucial role in CXCR4 mediated homing and engraftment via acting as downstream effectors or positive regulators of CXCR4 signaling. Among these in particular the genes which encode the proteins acting as molecular players of MAPK, PI3K pathways, as well as the cytokine LIF have been shown to play the role in cell adhesion of hematopoietic and/or non-hematopoietic cells.18-22 We can thus postulate that CXCR4 mutants have improved cell adhesion potential as compared with wild type via likely activation of CXCR4 mediated downstream effectors and pathways which altogether regulate the cell adhesion machinery in these cells to bind their endothelial receptors and BM matrix proteins. Further, the % increase of cell adhesion to FN as well as endothelium was found comparable but not significantly different among these two mutants of CXCR4. This may indicate their similar mode of action in up-modulation of adhesion efficiency, and signals further studies in this direction that would be definitely helpful to provide additional insights to decode fully the molecular mechanism of these mutants, as well as to unravel their multi-dimensional hidden aspects. Moreover, to extend this investigative idea to the next phase of research is further important as it offers a better prospect over previously tried other method of virus mediated CXCR4 overexpression.23 In comparison to viral mediated CXCR4 overexpression, this approach may be helpful in not only overcoming the limitation of necessary requirement of constant maintenance of optimal level ligand availability in BM microenvironment which is difficult to maintain especially in various adverse conditions, but is also free from risk of malignant transformation of viral vector in later stages. Ultimately, the regulated up-modulation of CXCR4 signaling may have potential prospects in improving the HSPCs engraftment in BM transplantation as well as in gene therapy and stem cell regenerative medicine, as the backbone of these two areas are based on the specific infusion or targeted delivery of stem cells in body which fundamentally implies the similar cell molecular events of directed cell trafficking, adhesion and lodgment in specific niche.
Conclusion
In conclusion, this study was aimed to establish an approach for up-modulation of homing and engraftment mechanistic via targeting its key molecular regulator. Present investigation was thus performed to explore the regulated implication of CXCR4-CAMs and evaluate their functional efficiency in cellular processes that are crucial in journey of cells to home and engraft in BM. Although this is a preliminary study but indeed a pioneering that enlightens the potential of CXCR4-CAMs as a molecular players in homing and engraftment by revealing their significantly improved cell adhesion efficacy to endothelium and matrix protein over native type. Altogether, present study serves as a opening point in research strategy aimed to develop and unwind the promising role of CXCR4-CAMs in cell molecular process that elicit homing and engraftment, and indicates further investigations that may ultimately prove breakthrough to improve the HSPCs homing and engraftment efficiency in clinical transfusion, as well as in regenerative medicine for targeted delivery of corrected stem/progenitor cells.
Materials and Methods
Sub-cloning of CXCR4 gene into Tet-on gene expression vector
Wild type CXCR4-cDNA present in pcDNA3 vector was kindly gifted from Dr. S.C. Peiper (Henry Vogt Cancer Research Institute). This CXCR4 cDNA of 1.1 kb was sub-cloned into Multi Cloning Site (MCS) of pTRE2hyg between Nhe1 and Sal1 restriction sites.
In vitro site directed mutagenesis
The site specific substitution mutation of CXCR4 wild type gene to derive the CXCR4-CAMs; Asn119 to Ser (N119SCXCR4-pTRE2hyg) and Asn119 to Ala (N119ACXCR4-pTRE2hyg) was done by in vitro site directed mutagenesis system (Invitrogen, USA) following manufacturer protocol.
Cell lines and stable transfection
Chronic myelogenous leukemia K-562 cell line (ATCC No. CCL-243TM) was obtained from National Centre of Cell Science, Pune, India. Cells were maintained in IMDM (Invitrogen, USA) supplemented with 10% fetal bovine serum (Gibco, USA), and antibiotics; penicillin and streptomycin (HiMedia, India), in a humidified CO2 incubator under 37 °C temp., 5% CO2 and 98% humidity (Thermo Scientific, USA). Double stable transfection of cells with pTet-on plasmid DNA and recombinant CXCR4-pTRE2hyg plasmid construct was done by using lipofectamine-2000 (Invitrogen, USA) following manufacturer protocol.
Immunofluorescence microscopy
Cells were seeded into 24-well plate at cells seeding density of 0.5 × 106 cells/ml and induced for CXCR4 transgene expression by 1μg/ml doxycycline (Sigma, USA). Antibody staining was done by incubation of cells with 10 μg/ml mouse anti-human CXCR4 monoclonal antibody (clone 12G5) (BD Biosciences, USA) for two hours at 4 °C. PBS washed twice and incubated again with 200 μl of 1:100 FITC-conjugated rat anti-mouse IgG (Sigma, USA) for two hours at 4 °C in dark, washed again as earlier. Finally, antibody stained cells were analyzed for fluorescence intensity under fluorescence microscope (Olympus IX51, Japan).
Endothelial cell adhesion assay
Assay was performed using endothelial cell adhesion assay kit (Milipore, USA). Briefly, human umbilical vein endothelial cells (HUVECs) obtained from Promo Cell (Germany), were maintained in endothelial cell growth medium (Promo Cell, Germany) supplemented with 15% FBS. To set up assay, 5.0x103 HUVECs cells/well were plated to 96 well-white plate (Nunc, Thermo Fisher Scientific, USA) and incubated for 48–72 h. 4–6 h prior to assay, pre-seeded HUVECs monolayer was activated by TNF-α (10 ng/ml), then, 5 × 104 calcein AM pre-labeled doxycycline induced test cells were added to each HUVECs pre-seeded well, incubated for two hours in dark followed by two times PBS washing. Finally, fluorescence intensity of cells was measured by 96 well fluorimeter at excitation and emission range of 485/530 nm (BioTek).
Fibronectin cell adhesion assay
Assay was performed as described by peled et al.8 Briefly, PBS (500 μl) containing 20 μg/ml human fibronectin (FN) (Chemicon, USA) was placed in 24-well plates (Falcon, Becton Dickinson, USA) and incubated overnight at 4 °C. Wells were PBS washed, blocked with 1000 μl 2.5% BSA in PBS, and incubated for one hour at room temperature. After washing with adhesion medium (IMDM supplemented with 0.2% BSA), 5 × 104 doxycycline induced test cells were added to FN pre-coated wells and allowed to adhere for 30 min at 37 °C in a CO2 incubator. After washing adherent cells were collected with medium containing 0.01% EDTA (Sigma, USA) by gentle shaking and counted microscopically.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). The statistical significance of differences between means was assessed using student t test considering the P value < 0.05 as significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are thankful to Director, Institute of Nuclear Medicine and Allied Sciences (INMAS-DRDO) for his constant support and encouragement, and Defense Research and Development Organisation (DRDO), Delhi (India), for the research grant. We also thank Dr S.C. Peiper (Henry Vogt Cancer Research Institute, USA) for providing the wild type CXCR4-pcDNA3 plasmid construct. Menka Sharma in particular thanks DRDO, Delhi (India), for the award of Ph.D. research fellowship.
References
- 1. Sharma M, Afrin F, Satija N, Tripathi RP, Gangenahalli GU. Stromal-derived factor-1/CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev 2011; 20:933-46; PMID:21186999; http://dx.doi.org/ 10.1089/scd.2010.0263 [DOI] [PubMed] [Google Scholar]
- 2. Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 2002; 16:1992-2003; PMID:12357350; http://dx.doi.org/ 10.1038/sj.leu.2402684 [DOI] [PubMed] [Google Scholar]
- 3. Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A 1998; 95:9448-53; PMID:9689100; http://dx.doi.org/ 10.1073/pnas.95.16.9448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996; 382:635-8; PMID:8757135; http://dx.doi.org/ 10.1038/382635a0 [DOI] [PubMed] [Google Scholar]
- 5. Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999; 283:845-8; PMID:9933168; http://dx.doi.org/ 10.1126/science.283.5403.845 [DOI] [PubMed] [Google Scholar]
- 6. Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta 2007; 1768:952-63; PMID:17169327; http://dx.doi.org/ 10.1016/j.bbamem.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest 1999; 104:1199-211; PMID:10545519; http://dx.doi.org/ 10.1172/JCI7615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 2000; 95:3289-96; PMID:10828007 [PubMed] [Google Scholar]
- 9. Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood 2005; 106:1901-10; PMID:15890683; http://dx.doi.org/ 10.1182/blood-2005-04-1417 [DOI] [PubMed] [Google Scholar]
- 10. Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK. Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenergic receptor. Proc Natl Acad Sci U S A 2001; 98:5997-6002; PMID:11353823; http://dx.doi.org/ 10.1073/pnas.101126198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang WB, Navenot JM, Haribabu B, Tamamura H, Hiramatu K, Omagari A, Pei G, Manfredi JP, Fujii N, Broach JR, et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. J Biol Chem 2002; 277:24515-21; PMID:11923301; http://dx.doi.org/ 10.1074/jbc.M200889200 [DOI] [PubMed] [Google Scholar]
- 12. Lozzio BB, Lozzio CB. Properties and usefulness of the original K-562 human myelogenous leukemia cell line. Leuk Res 1979; 3:363-70; PMID:95026; http://dx.doi.org/ 10.1016/0145-2126(79)90033-X [DOI] [PubMed] [Google Scholar]
- 13. Gupta SK, Pillarisetti K. Cutting edge: CXCR4-Lo: molecular cloning and functional expression of a novel human CXCR4 splice variant. J Immunol 1999; 163:2368-72; PMID:10452968 [PubMed] [Google Scholar]
- 14. Gossen M, Bonin AL, Freundlieb S, Bujard H. Inducible gene expression systems for higher eukaryotic cells. Curr Opin Biotechnol 1994; 5:516-20; PMID:7765466; http://dx.doi.org/ 10.1016/0958-1669(94)90067-1 [DOI] [PubMed] [Google Scholar]
- 15. Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science 1995; 268:1766-9; PMID:7792603; http://dx.doi.org/ 10.1126/science.7792603 [DOI] [PubMed] [Google Scholar]
- 16. Bevilacqua MP, Gimbrone MA Jr.. Inducible endothelial functions in inflammation and coagulation. Semin Thromb Hemost 1987; 13:425-33; PMID:3122325; http://dx.doi.org/ 10.1055/s-2007-1003519 [DOI] [PubMed] [Google Scholar]
- 17. Rice GE, Munro JM, Bevilacqua MP. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med 1990; 171:1369-74; PMID:1691264; http://dx.doi.org/ 10.1084/jem.171.4.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. M S F A, Rp T G G. Regulated expression of CXCR4 constitutive active mutants revealed the up-modulated chemotaxis and up-regulation of genes crucial for CXCR4 mediated homing and engraftment of hematopoietic stem/progenitor cells. J Stem Cells Regen Med 2013; 9:19-27; PMID:24693205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 1998; 273:23169-75; PMID:9722546; http://dx.doi.org/ 10.1074/jbc.273.36.23169 [DOI] [PubMed] [Google Scholar]
- 20. Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res 2005; 65:9891-8; PMID:16267013; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1293 [DOI] [PubMed] [Google Scholar]
- 21. Inukai K, Funaki M, Anai M, Ogihara T, Katagiri H, Fukushima Y, Sakoda H, Onishi Y, Ono H, Fujishiro M, et al. Five isoforms of the phosphatidylinositol 3-kinase regulatory subunit exhibit different associations with receptor tyrosine kinases and their tyrosine phosphorylations. FEBS Lett 2001; 490:32-8; PMID:11172806; http://dx.doi.org/ 10.1016/S0014-5793(01)02132-9 [DOI] [PubMed] [Google Scholar]
- 22. Wysoczynski M, Jankowski K, Miekus K, Kucia M, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Leukemia inhibitory factor: a newly identified chemoattractant and regulator of metastasis of rhabdomyosarcomas and neuroblastomas to bone marrow. Blood (ASH Annual Meeting Abstracts) 2004; 104: Abstract 1278. [Google Scholar]
- 23. Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D, et al. Overexpression of CXCR4 on human CD34 +progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood 2004; 103:2942-9; PMID:15070669; http://dx.doi.org/ 10.1182/blood-2003-07-2607 [DOI] [PubMed] [Google Scholar]


