Abstract
The flagellar pocket is a bulb-like invagination of the plasma membrane that encloses the base of the single flagellum in trypanosomes. It is the site of all endo- and exocytic activity in the parasite and has thus been proposed to be a therapeutic target. At the neck of the flagellar pocket is an electron-dense cytoskeletal structure named the flagellar pocket collar. The protein BILBO1 was the first characterized and remains the only known component of the flagellar pocket collar, with essential functions in the biogenesis of both the flagellar pocket and flagellar pocket collar. We recently reported that the filamentous assembly of Trypanosoma brucei BILBO1 (TbBILBO1) is mediated by its central coiled coil domain and C-terminal leucine zipper. Here, we discuss how TbBILBO1 might assemble at the flagellar pocket collar in T. brucei.
Keywords: BILBO1, cytoskeleton, flagellar pocket, flagellar pocket collar, parasite, protein assembly, Trypanosoma brucei
Abbreviations
- FP
flagellar pocket; FPC, flagellar pocket collar
Trypanosoma brucei is a protist parasite and the causative agent of Human African Trypanosomiasis (sleeping sickness).1 The single flagellum on the cell surface is essential for parasite motility, cell division, and immune evasion.2 The base of the flagellum is enclosed in a bulb-like invagination of the plasma membrane called the flagellar pocket (FP). The FP is the site of all endo-/exocytosis and thus essential for the survival of the parasite.3 At the neck of the FP is an electron-dense cytoskeletal structure termed the flagellar pocket collar (FPC, Fig. 1). A recent electron tomography study of the region around the FP suggested that the FPC might form a horseshoe shape, thus leaving a gap for placement of a specialized microtubule quartet that extends from alongside the basal body down the longitudinal axis of the cell to the cell anterior.4
Figure 1.
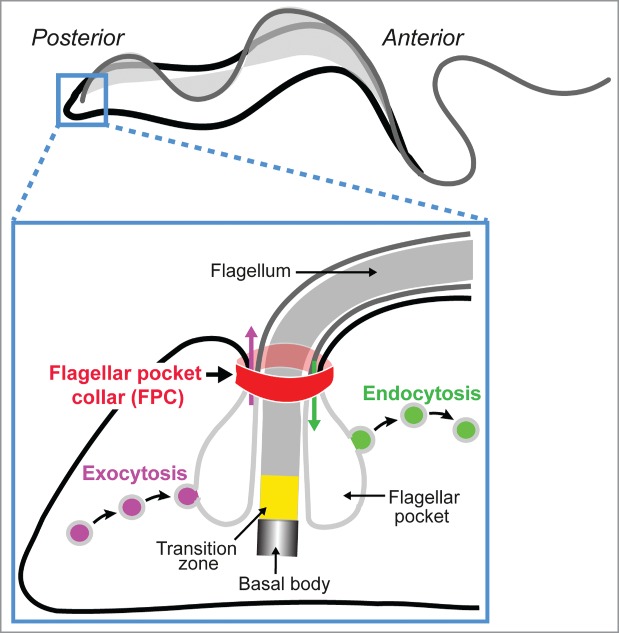
Schematic of the structure and function of the flagellar pocket in trypanosomes. The FP is a bulb-like invagination of the plasma membrane at the base of the flagellum at the cell posterior. Although it represents only 5% of the total cell surface, the FP is responsible for all endo- and exocytosis in the parasite. At the neck of the FP is a belt-like structure called the flagellar pocket collar (FPC). The plasma membrane is shown in black; membranes of the FP and the flagellum are shown in light and dark gray, respectively.
To date, the only known protein component of the FPC is BILBO1.5 Bioinformatic analysis indicates that there are 4 structural domains in the 67-kDa T. brucei BILBO1 (TbBILBO1), including a globular N-terminal domain, 2 central EF-hand motifs followed by a long coiled-coil domain, and a C-terminal leucine zipper (Fig. 2A). Recombinant TbBILBO1 by itself forms insoluble oligomers in vitro, which makes it intractable to any single conventional structural study method. We recently carried out a structural dissection of TbBILBO1 using integrative structural biology approaches including nuclear magnetic resonance (NMR) spectroscopy, electron microscopy (EM), and various biophysical methods. The high-resolution structure of the TbBILBO1 N-terminal domain revealed a variant ubiquitin-like fold with a conserved surface patch; mutagenesis of this patch caused cell death in vivo.6 We further showed that the coiled-coil domain forms an antiparallel dimer, and intermolecular interactions between adjacent leucine zippers allow TbBILBO1 to form extended filaments in vitro (Fig. 2B). These filaments were additionally shown to condense through lateral interactions into fibrous bundles, with the globular domains in register.7 How then might the filamentous oligomer of TbBILBO1 assemble at the FPC in the cell?
Figure 2.

Putative assembly mechanism of TbBILBO1 at the flagellar pocket collar. (A) Schematic depicting the arrangement of the 4 structural domains of TbBILBO1: N-terminal domain, EF hand motifs, coiled-coil domain, and leucine zipper. (B) A hypothetical model showing the possible assembly of TbBILBO1 at the FPC. Two monomeric TbBILBO1 molecules form an antiparallel dimer via their central coiled-coil domains, which further assemble into a long filamentous oligomer through the intermolecular interactions of the C-terminal leucine zippers. (C) A hypothetical model showing that the filament forms a solenoid in the FPC to encircle the flagellar pocket neck. An enlarged view of the boxed region shows the possible lateral interactions mediated by the EF-hand motifs (dashed arrows). The N-terminal domain might interact with an unknown FPC component or a regulatory protein (red arrows and hexagons) to facilitate FPC assembly. (D) An alternative model showing a horseshoe-like arrangement of TbBILBO1 filaments at the FPC with the microtubule quartet passing through the gap. A putative capping protein at the tip of the filaments is indicated by orange crescents. MtQ, microtubule quartet.
The assembly of TbBILBO1 at the FPC could occur in at least 3 different ways. The molecules could conceivably form a zigzag arrangement with the individual dimers running parallel to the long axis of the flagellum, although this seems unlikely given the lack of extreme flexibility between the dimers and the observed propensity of the threads to form long linear assemblies.7 Alternatively, they could wrap around the neck of the FP in a solenoid (Fig. 2C). A caveat here is that the FPC must permit passage of the specialized microtubule quartet that extends from the basal body region along the longitudinal axis of the cell toward the cell anterior.4 It could perhaps be accommodated by a local displacement of the solenoid. Although it is not yet known if TbBILBO1 is part of the electron-dense component of the FPC, if it is assumed to be, then a third model is possible. Here, laterally interacting threads of TbBILBO1 could wrap around the FP neck but be of insufficient length to completely encircle it. This model implies the existence of some kind of capping protein to limit filament growth (Fig. 2D). In this model, the capping proteins are adjacent to the microtubule quartet. Therefore, it is possible that the capping proteins may serve to link the FPC to the microtubule quartet. A possible means of distinguishing between these models would be to analyze whether the FPC becomes either thicker or wider upon overexpression of TbBILBO1.
In both the solenoid (Fig. 2C) and the horseshoe (Fig. 2D) models, the EF-hand motifs might play a regulatory role in the assembly and/or function of the FPC. In many EF-hand-containing proteins, calcium binding triggers conformational changes that can subsequently allow the EF-hand motif to interact with another region of the same protein or with a binding partner.8 We found that calcium-bound TbBILBO1 EF-hand motifs formed a compact globular structure whereas the apo form (without calcium) was loosely folded and structurally dynamic.7 It is conceivable that the TbBILBO1 EF-hand motifs, in response to calcium availability, change their structure to reversibly interact with another region of TbBILBO1 in a neighboring strand or even with an unknown FPC component (Fig. 2C, dashed arrows in the inset). Similarly, the essential N-terminal domain, which is spatially close to the filament junction, may bind an unknown component of the FPC or a key regulatory protein via its conserved surface patch (Fig. 2C, hexagons in the inset). The lateral association between neighboring filaments would then not only facilitate the formation of a condensed structure but might also serve to regulate the size of the FPC in a calcium-depending manner controlled by the EF-hand motifs.
Conclusion
Our studies reveal that the multidomain cytoskeletal protein TbBILBO1 assembles into long filaments via 2 types of helical structures – an unusually long antiparallel coiled coil and a leucine zipper at the C-terminus of the coiled coil, which also interacts in an antiparallel manner to bridge neighboring coiled coil dimers to form an extended filament. To condense into the compact structure of the FPC, TbIBLBO1 filaments could possibly associate via lateral interactions between neighboring structures as proposed in the solenoid and horseshoe-like models. If the horseshoe-like structure proves true, then it would be important to identify the capping protein and characterize the molecular mechanism of its function as a bridge between the microtubule quartet and the FPC. It is also important to identify the protein that binds to the conserved surface patch on the N-terminal domain of TbBILBO1. A high-resolution structure of this binary protein complex might provide guidance in designing small molecules and/or polypeptides with therapeutic properties.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by funding from the Max F. Perutz Laboratories (MFPL) and grant P24383-B21 from the Austrian Science Fund (FWF) to GD. KV was supported by an Österreichischer Austauschdienst (OeAD) graduate scholarship during 2009–2012.
References
- 1. Welburn SC, Maudlin I. Priorities for the elimination of sleeping sickness. Adv Parasitol 2012; 79: p. 299-337; PMID:22726645 [DOI] [PubMed] [Google Scholar]
- 2. Ralston KS, Kabututu ZP, Melehani JH, Oberholzer M, Hill KL. The Trypanosoma brucei flagellum: moving parasites in new directions. Annu Rev Microbiol 2009; 63: p. 335-62; PMID:19575562; http://dx.doi.org/ 10.1146/annurev.micro.091208.073353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Field MC. Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol, 2009. 7(11): p. 775-86; PMID:19806154; http://dx.doi.org/ 10.1038/nrmicro2221 [DOI] [PubMed] [Google Scholar]
- 4. Lacomble S, Vaughan S, Gadelha C, Morphew MK, Shaw MK, McIntosh JR, Gull K. Three-dimensional cellular architecture of the flagellar pocket and associated cytoskeleton in trypanosomes revealed by electron microscope tomography. J Cell Sci 2009; 122(Pt 8): p. 1081-90; PMID:19299460; http://dx.doi.org/ 10.1242/jcs.045740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonhivers M, Nowacki S, Landrein N, Robinson DR. Biogenesis of the trypanosome endo-exocytotic organelle is cytoskeleton mediated. PLoS Biol 2008; 6(5): p. e105; PMID:18462016; http://dx.doi.org/ 10.1371/journal.pbio.0060105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vidilaseris K, Morriswood B, Kontaxis G, Dong G. Structure of the TbBILBO1 protein N-terminal domain from Trypanosoma brucei reveals an essential requirement for a conserved surface patch. J Biol Chem 2014; 289(6): p. 3724-35; PMID:24362019; http://dx.doi.org/ 10.1074/jbc.M113.529032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vidilaseris K, Shimanovskaya E, Esson HJ, Morriswood B, Dong G. Assembly mechanism of Trypanosoma brucei BILBO1, a multidomain cytoskeletal protein. J Biol Chem 2014; 289(34):23870-81; PMID:25031322; http://dx.doi.org/ 10.1074/jbc.M114.554659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewit-Bentley A. Rety S. EF-hand calcium-binding proteins. Curr Opin Struct Biol 2000; 10(6): p. 637-43; PMID:11114499; http://dx.doi.org/ 10.1016/S0959-440X(00)00142-1 [DOI] [PubMed] [Google Scholar]


