Abstract
Perceptual skills can be improved through practice on a perceptual task, even in adulthood. Visual perceptual learning is known to be mostly specific to the trained retinal location, which is considered as evidence of neural plasticity in retinotopic early visual cortex. Recent findings demonstrate that transfer of learning to untrained locations can occur under some specific training procedures. Here, we evaluated whether exogenous attention facilitates transfer of perceptual learning to untrained locations, both adjacent to the trained locations (Experiment 1) and distant from them (Experiment 2). The results reveal that attention facilitates transfer of perceptual learning to untrained locations in both experiments, and that this transfer occurs both within and across visual hemifields. These findings show that training with exogenous attention is a powerful regime that is able to overcome the major limitation of location specificity.
Keywords: attention, perceptual learning, location specificity
Introduction
The brain is constantly bombarded with vast amounts of sensory information. To function effectively, the visual system must establish stability and selectively process the most important information. Mechanisms of learning and plasticity aid in this process, allowing us to adapt to new surroundings and efficiently process and evaluate stimuli that we regularly experience. Visual perceptual learning (VPL) is the improvement in performance in perceptual tasks with practice, which has been found to be highly specific to trained stimuli and task (Sasaki, Náñez, & Watanabe, 2009, 2012; Schoups, Vogels, & Orban, 1995; Shiu & Pashler, 1992; Watanabe et al., 2002). Performance improvements are mitigated or extinguished when posttraining sessions differ on certain task parameters that were stable during training, such as the stimulus retinal location (e.g., Ball & Sekuler, 1982; Berardi & Fiorentini, 1987; Crist, Kapadia, Westheimer, & Gilbert, 1997; Fahle, Edelman, & Poggio, 1995; Schoups et al., 1995; Shiu & Pashler, 1992), stimulus feature—orientation, contrast, motion direction (Ahissar & Hochestein, 1997; Berardi & Fiorentini, 1987; Fiorentini & Berardi, 1980, 1981; Watanabe & Náñez, 2001)—and even the eye used to perform the task (Karni & Sagi, 1991; for reviews, see Sagi, 2011; Watanabe & Sasaki, 2015).
Specificity and transfer in perceptual learning
One the one hand, location and feature specificity of VPL are often attributed to changes in primary visual cortex (V1; Ghose, Yu, & Maunsell, 2002; Gu et al., 2011; Seitz & Watanabe, 2005; Watanabe et al., 2002; Yotsumoto, Watanabe, & Sasaki, 2008; Zhang, Cong, Song, & Yu, 2013), as V1 neurons respond to precise retinal locations and primitive visual features. On the other hand, studies have implicated regions outside of the visual cortex, including both changes in connectivity between visual areas and “decision-making” areas (e.g., LIP), as well as changes within decision-making regions themselves (e.g., Chowdhury & DeAngelis, 2008; Jeter, Dosher, Liu, & Lu, 2010; Law & Gold, 2008). Notably, neurons in these higher-level areas are less selective for spatial locations and specific primitive visual features, and have larger receptive fields compared to early visual cortex. Models of perceptual learning have been proposed to explain specificity by a reweighting of sensory signals at the decision stage (Dosher, Jeter, Liu, & Lu, 2013; Jeter et al., 2010; Petrov, Dosher, & Lu, 2005).
A major clinical challenge is to devise more efficient training regimens that allow generalization of improvements during rehabilitation (Cavanaugh et al., in press; Das, Tadin, & Huxlin, 2014; Huxlin et al., 2009; McGraw, Webb, & Moore, 2009; Sahraie, 2007). Suitable perceptual learning (PL) training has been shown to improve visual performance in individuals with peripheral damage (Ahissar, Nahum, Nelken, & Hochstein, 2009), visual acuity in amblyopic adult patients (Levi, 2005; Levi & Li, 2009; Polat, Ma-Naim, Belkin, & Sagi, 2004), presbyopia (Polat et al., 2012), contrast sensitivity in cortically blind patients (Sahraie et al., 2006), and visual motion discrimination in patients with V1 damage (Cavanaugh et al., in press; Das et al., 2014; Huxlin et al., 2009). However, even with such interventions, the prognoses for these visual disorders remain poor. A greater understanding of the factors important to and mechanisms responsible for VPL generalization in the adult brain is crucial for creating effective visual rehabilitation protocols. Of particular interest is VPL location specificity and the potential for transfer to untrained locations, given that many vision disorders are characterized by functioning vision at some retinal locations and severe deficits at other locations.
Despite wide acceptance of specificity as a key aspect of VPL, some studies have shown that, with certain training procedures, PL generalizes to untrained locations, features, and tasks (Liu & Weinshall, 2000; Sasaki et al., 2009; Sowden, Rose, & Davies, 2002; Xiao et al., 2008; Zhang et al., 2013; T. Zhang et al., 2010). Location specificity is the subject of many reports of PL transfer. One of the most prominent training regimens reported to elicit transfer from trained to untrained retinal locations, known as “double training,” requires participants to perform a task with stimuli presented at the untrained retinal locations throughout training (Hung & Seitz, 2014; Wang, Zhang, Klein, Levi, & Yu, 2012, 2014; Xiao et al., 2008) or at some time before the posttest (Zhang et al., 2013; Zhang, Xiao, Klein, Levi, & Yu, 2010). A rule-based learning model has been proposed to account for these findings (Zhang, Cong, Klein, Levi, & Yu, 2014; Zhang, Zhang et al., 2010; Zhang et al., 2013). This model suggests that PL primarily involves learning rules for performing the task efficiently, and that specificity is a consequence of an inability to link signals from early visual cortex that represent untrained stimuli to the learned rule scheme. Additionally, the model predicts that exposure to untrained stimuli locations or features will result in transfer only if exposure occurs during or following training, because the rule scheme must be learned first. More recent studies have revealed that Vernier learning can be “piggybacked,” that is, transferred to an untrained location, when training on Vernier acuity is paired with orientation or motion-direction training at the same trained location (Hung & Seitz, 2014; Wang et al., 2014). This piggybacking paradigm is similar to double training, in that it requires learning of an additional task to promote location transfer. Ideally, a training regimen would allow for the transfer of learning to untrained retinal locations, distant from the trained location, with minimum effort from the observer; hence, without additional training either at the untrained locations or with other tasks. Here we provide evidence that attention enables this ideal training regimen.
Attention and perceptual learning
Selective attention, the process by which a small subset of sensory information is selected and prioritized for processing, is known to be critical for perception, learning, and memory. The role of selective attention in perceptual learning has been discussed for over two decades (for reviews see Ahissar & Hochstein, 2004; Goldstone, 1998; Lu, Liu, & Dosher, 2009; Roelfsema, Ooyen, & Watanabe, 2010; Seitz & Watanabe, 2009; Tsushima & Watanabe, 2009; Watanabe & Sasaki, 2015) but the link between these two systems is highly speculative and poorly understood. Attention's role in perceptual learning has often been inferred, and has been equated to task difficulty (Bartolucci & Smith, 2011; Huang & Watanabe, 2012), used interchangeably with conscious perception (Tsushima & Watanabe, 2009), used to describe the fact that observers perform a task with a specific stimulus (Meuwese et al., 2013; Paffen et al., 2008; Seitz & Watanabe, 2009; Watanabe et al., 2001; Watanabe & Sasaki, 2015), and has been inferred from neural activity in attention-related brain areas (Mukai et al., 2007; Tsushima, Sasaki, & Watanabe, 2006).
Visual attention can be covertly deployed (i.e., without accompanying eye movements) in a voluntary, conceptually driven manner (endogenous attention) or an involuntary, stimulus-driven fashion (exogenous). Both types of attention improve performance on a variety of tasks mediated by early visual processes (for reviews see Carrasco, 2011, 2014). Because attention serves as one of the most important mechanisms in gating what and how efficiently information is processed, a greater understanding of VPL requires an understanding of the effect of attention on VPL. Nonetheless, very few studies have directly manipulated attention to examine its effect; one deals with the decreased effects of object-based attention with training (Dosher, Han, & Lu, 2010), and the other two with spatial attention (Mukai, Bahadur, Kesavabhotla, & Ungerleider, 2011; Szpiro & Carrasco, in press). The Mukai et al. (2011) study revealed differential effects on pre- versus posttraining contrast thresholds between two groups of participants: one group trained with exogenous (involuntary, stimulus-driven) attentional cues, and the other trained with endogenous (voluntary, goal-driven) attentional cues (Mukai et al., 2011). Both cues resulted in better performance when the target appeared at the cued location, but only those trained with exogenous cues exhibited lower threshold after training. These results suggest that exogenous and endogenous attention may influence VPL via distinct mechanisms. Unfortunately, because all participants were trained with all cues of different validity (neutral, valid, and invalid) throughout all trials, the attention effect cannot be isolated. In the Szpiro and Carrasco (in press) study, observers who underwent training under an exogenous attention condition learned, but those who underwent training under a neutral condition did not. That study, the first to isolate the effects of exogenous attention during acquisition, revealed that attention can enable learning. However, none of these three studies was designed to assess location specificity.
Even in light of the scarcity of empirical evidence, several papers have relied on hypotheses regarding the role of attention on VPL (Ahissar & Hochstein, 2004; Dolan et al., 1997; Gilbert, Sigman, & Crist, 2001; Sasaki et al., 2009, 2012; Wang et al., 2014; Watanabe & Nañez, 2001; Watanabe & Sasaki, 2015; Xiao et al., 2008; Yotsumoto & Watanabe, 2008). For example, attention is considered a gate for PL (Ahissar & Hochstein, 2004; Roelfsema et al., 2010; Sasaki et al., 2010), and to have important implications for the emergence of transfer versus specificity (Fahle, 2009; Sasaki et al., 2012; Mukai et al., 2007; Shiu & Pashler, 1992; Wang et al., 2014; Watanabe & Sasaki, 2015; Yotsumoto & Watanabe, 2008; Zhang et al., 2013; Zhang, Xiao et al., 2010).
Here, we investigate the effect of attention on PL. In two experiments we manipulated attention; the participants were trained with either neutral cues or valid exogenous cues. We chose to manipulate exogenous attention because it is automatic and requires no additional cognitive effort from the observer. To maximize the effect of attention at the trained location, we used only valid cues, which direct attention to the location of the upcoming target, and not invalid cues, which direct attention to a nontarget location. We note that cue validity does not affect the magnitude of either the benefit or the cost of exogenous attention (Giordano, McElree, & Carrasco 2009; see Carrasco, 2011, 2014 for reviews). Specifically, we investigated the effect of attention on location specificity for both adjacent locations (Experiment 1) and distant locations (Experiment 2). In both experiments, participants were trained on a visual orientation discrimination task at two locations, and tested at those trained locations as well as at untrained locations. In Experiment 1, we investigated the effect of attentional training on degree of specificity of VPL to untrained locations that were adjacent to the trained locations. In Experiment 2, we assessed the effect of attention on transfer of VPL to distant untrained locations, either within the same hemifield or between hemifields. On one hand, if learning transfer were constrained to the hemifield of the trained locations, it would suggest that attention's influence on transfer is mediated by changes in early visual regions. On the other hand, if learning transferred to untrained locations in a different visual hemifield, it would indicate that attention's influence is not merely mediated by early visual areas, and suggest the involvement of higher level areas in the mechanism of attention-induced transfer.
Experiment 1
Methods
Participants
Thirteen New York University undergraduate observers between the ages of 19 and 22 (five male, eight female) participated in this experiment. All observers had normal or corrected-to-normal vision and were naïve about the purpose of the study. None of the observers had participated in an orientation discrimination task prior to participation in this study.
Apparatus
Stimuli were displayed on a gamma-corrected P260 IBM 21 in. Multiscan color monitor in a dark room. A video attenuator drove the green gun of the monitor to increase rendering precision at low contrast levels from 8 bits to 12 bits (Pelli & Zhang, 1991). The background luminance was set to the middle of the monitor range, 18 cd/m2. Participants viewed the screen from 114 cm away, using a chin rest to stabilize head position.
Stimuli and procedure
Before the training sessions began, all observers completed a practice session of 200 trials to learn the experimental routine. Solid circles replaced Gabor patches during this session so that observers did not learn the actual task and were not exposed to the to-be-trained stimuli. Rather than reporting the tilt, observers reported whether the target circle was light or dark. Immediately following the practice session, Gabor contrast thresholds were measured using a modified QUEST staircase procedure (Watson & Pelli, 1983) with a 75% performance criterion and β of 3.5 for 60-trial runs in which observers performed an orientation discrimination task identical to the neutral condition at the trained locations (details below; Figure 1). The average threshold contrast was 15%. Each observer began the Training phase three days after the practice phase and the Testing phase one week following the start of the training phase. Stimulus contrast remained the same throughout all training and testing sessions.
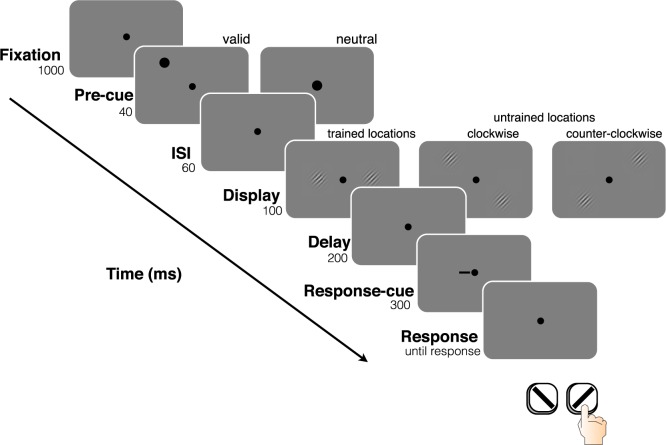
Figure 1.
Trial sequence for Experiment 1.
Each trial began with the presentation of a fixation point (black circle, subtending 0.1° of visual angle) presented at the center of the screen. The fixation point remained on the screen for the duration of each trial. After 1000 ms of only the fixation point on the screen, a precue (black circle, subtending 4°) appeared for 40 ms. The precue could either be neutral (presented directly over the fixation point) or valid (presented 1.5° above the upcoming target Gabor). Following a brief interstimulus interval (ISI, 60 ms), two Gabor patches (4 c/° sinusoidal grating in a Gaussian envelope; subtending 2°) appeared simultaneously for 100 ms on either side of fixation, along the horizontal meridian at 4° eccentricity. Each Gabor was oriented either 4° clockwise or 4° counterclockwise (polar angle) relative to vertical (the orientation of both Gabors was generated randomly and independently). Following another ISI of 200 ms, a response cue (0.5° horizontal line) appeared adjacent to the fixation cross, pointing to the location (either right or left) of the Gabor target. Observers reported the orientation (clockwise or counterclockwise) of the Gabor indicated by the cue, and auditory feedback was provided informing the observer of the accuracy of the response (Figure 1).
Participants completed nine blocks of 60 trials each for 5 consecutive days (Training phase), followed by a 2-day break period, and then another 5 consecutive days of experimental sessions (Testing phase) with the same number of block and trials-per-block (nine blocks of 60 trials). During the Testing phase, the Gabor patches could appear at three different pairs of locations (all at 4° eccentricity): (a) At the trained locations; (b) At the “clockwise testing locations,” with one Gabor on the left side of fixation 2° above the horizontal meridian, and the other on the right side of fixation 2° below the horizontal meridian; and (c) At the “counterclockwise testing locations,” with one Gabor on the left side of fixation 2° below the horizontal meridian, and the other on the right side of fixation 2° above the horizontal meridian. Therefore, each untrained location was just adjacent to one of the trained locations. On testing days, three blocks were performed at the trained locations, three at the counterclockwise untrained locations, and three at the clockwise untrained locations. For the analyses presented here, the “clockwise untrained” and “counterclockwise untrained” were combined as they did not differ, and these are referred to as the “untrained locations” (Figure 2).
Figure 2.
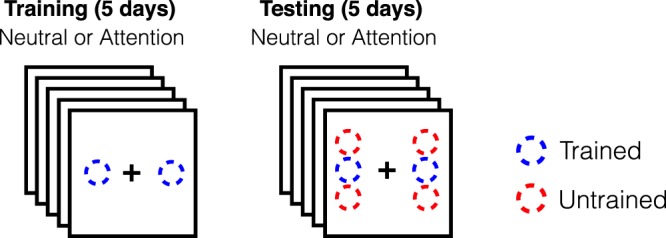
Training and testing schedule for Experiment 1.
Before running the experiment, we confirmed that performance did not differ for the to-be trained and the to-be tested locations (Figure 3 shows data for two observers who did not participate in the experiment).
Figure 3.
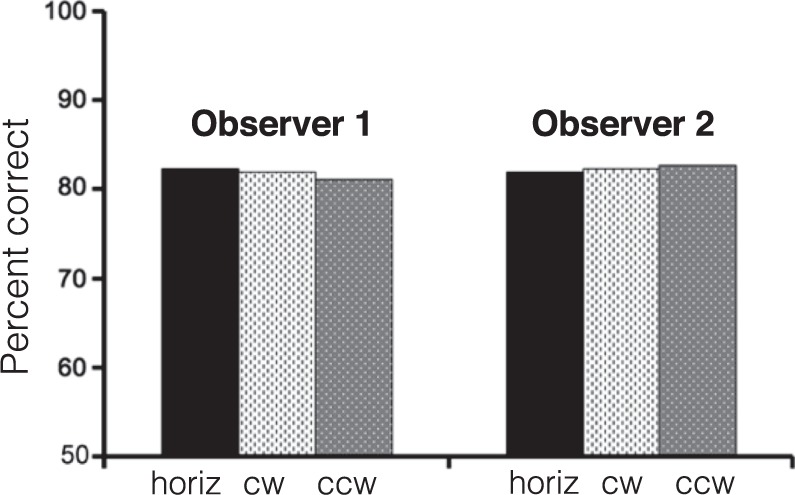
Results from a control experiment. Two observers completed one session with stimuli at each of the locations that would be tested in Experiment 1. There is virtually no difference in performance between any of the to-be-trained locations (horiz) and to-be-tested locations (cw and ccw).
Participants were divided into three experimental groups, which differed only in the type of precue presented on each trial. The Neutral/Neutral group (n = 4) was presented with only neutral cues for all sessions; the Attention/Attention group (n = 4) was presented with only valid cues for all sessions; the Attention/Neutral (n = 5) was presented with valid cues on all training sessions (days 1–5), and neutral cues on all test sessions (days 1–5). Our critical comparison, within and between groups, is performance at the trained and untrained locations, especially on the first day of testing, in which participants have been trained at the task at the trained locations but not at the untrained locations. The Testing phase consisted of five sessions with stimuli at the trained and untrained locations to assess whether learning at the untrained locations would mirror that at the trained location in the Neural and Attention groups. It could be the case that on the first day of testing, learning is location specific for the Neutral group, but by the second day of testing, performance would be the same at the trained and untrained locations. Conversely, superior performance at the trained than at the untrained locations could remain through these five days, suggesting independent learning at these locations.
Results
Each participant's performance was quantified as the percent correct in each condition on each session. Figure 4 indicates the performance across each experimental session for Neutral/Neutral (4A & 4D), Attention/Attention (4B & 4E), and Attention/Neutral (4C & 4F) groups. We conducted a mixed analysis of variance (ANOVA; within-subject factors, location and session; between-subjects factor, group). First, we confirmed that learning occurred during training. There was a significant improvement across groups comparing session 1 versus session 5, F(1, 10) = 18.49, p < 0.002, and no interaction with group, F(2, 10) = 1.9, p > 0.1. Second, we evaluated whether learning was maintained after 3 days; that is, whether there was a significant difference between the last day of training and the first day of testing for the trained location. Performance at the trained locations was similar between session 5 and session 6 for all groups; session main effect, F(1, 10) = 1.92, p > 0.1, and session X group interaction, F(2, 10) = 2.39, p > 0.1.
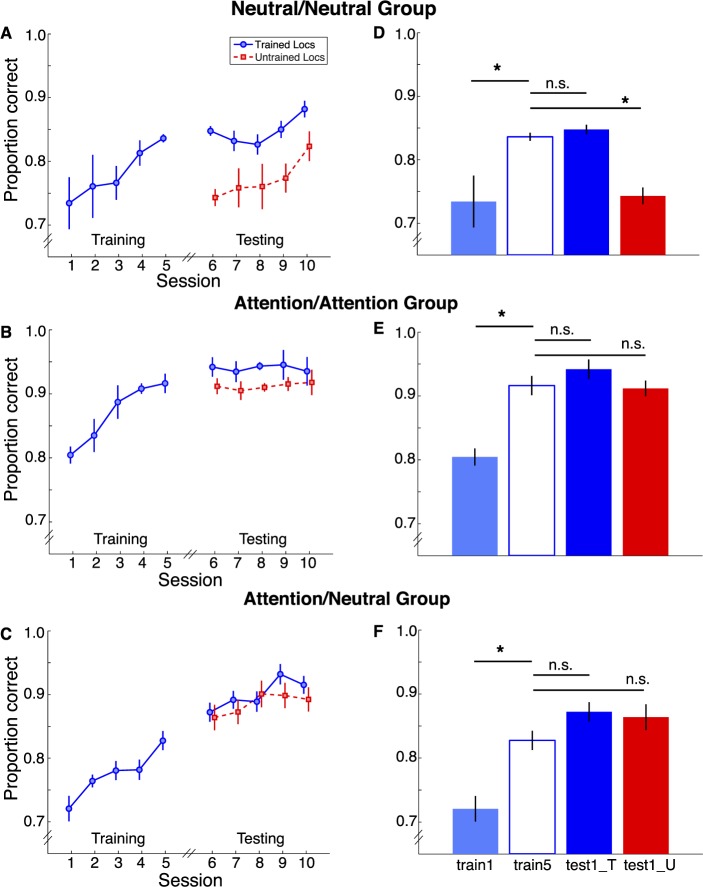
Figure 4.
Results from Experiment 1. Percent correct across all sessions is plotted for the Neutral/Neutral group (A), Attention/Attention group (B), and Attention/Neutral group at the trained locations (blue) and untrained locations (red). Performance on the first day of training (train1, light blue), the fifth day of training (train5, hollow dark blue), the first day of posttraining with stimuli at the trained locations (test1_T, solid dark blue) and at the untrained locations (test1_U, red) are plotted for the Neutral/Neutral (D), Attention/Attention (E), and Attention/Neutral (F) groups.
Third, we evaluated whether learning was specific to the trained locations; that is, whether there was a significant difference between the last day of training and the first day of testing for the untrained location. There was a significant session X group interaction, F(2, 10) = 5.94. p < 0.02. For the Neutral/Neutral group, there was a significant drop in performance, t(3) = 6.16, p < 0.01, but for the Attention/Attention group, t(3) = .19, p > 0.1, and the Attention/Neutral group, t(3) = .3, p > 0.1, performance did not drop. Interestingly, performance improved gradually between each testing session in the Neutral group, in a similar fashion as at the trained location during training, suggesting that learning at the trained and untrained locations were independent. In contrast, in both Attention groups, performance at the untrained locations was the same as performance at the trained locations, and this advantage remained throughout testing, indicating that training with valid peripheral cues resulted in stable location transfer.
We analyzed reaction time (RT) as a secondary measure to rule out any speed–accuracy trade-off. In short, observers became more accurate and faster during training in all conditions across training days, indicating no speed accuracy trade-off. The benefit to performance persisted at the trained locations during the Testing phase. It was specific for the trained locations when observers trained with the neutral condition, but generalized across locations for the two conditions in which observers trained with exogenous attention. These results show that attention generalizes learning to adjacent locations.
Experiment 2
The results from Experiment 1 indicate that training with exogenous attentional cues causes learning to transfer to adjacent untrained locations, whereas with neutral cues, there is only learning at the trained locations and none at the untrained locations. This is true regardless of the attention manipulation during testing, as we found transfer both when testing with attention (the Attention/Attention group) and with neutral cues (Attention/Neutral group).
Can attention facilitate longer-distance transfer? To further investigate the influence of attention on PL and location transfer, in this experiment we trained participants at two locations, each in one quadrant of the visual field (e.g., upper left and lower left), and tested them at those locations and at two untrained locations in the remaining quadrants (e.g., upper right and lower right). This allowed us to address whether or not attention facilitates transfer of VPL to untrained locations that are distant from the trained locations. Additionally, this design allowed us to address whether or not attention facilitates PL transfer to retinal locations in a separate visual hemifield from the trained retinal location. To differentiate retinal distance from cortical distance, half of the participants were trained at locations within a single visual hemifield, whereas the remaining participants were trained at locations in both hemifields. Findings of VPL being constrained to the hemifield of the trained locations would suggest that attention's influence on transfer is mediated by changes in early visual regions. Conversely, if transfer occurs to untrained locations in a different visual hemifield, this would indicate that attention's influence on is not mediated by early visual areas alone, suggesting the involvement of higher level areas in the mechanism of attention-induced transfer.
Additionally, in Experiment 2, all pre- and posttests had only neutral cues. By keeping pre- and posttests the same across observers in all groups, the only difference between groups was the type of precue during the three days of training, either with neutral precues or valid precues. This design allowed us to isolate the effect of attentional training on VPL transfer itself, as the critical measurement of transfer or specificity arises from sessions (the pre- and posttests) in which attention is not manipulated for either group. The inclusion of a pretest for untrained locations also allowed us to assess performance at untrained locations relative to themselves between the pre- and the posttest.
Methods
Participants
Thirty-two participants (21 females, 11 males; mean age 22) participated in an orientation discrimination task for five consecutive sessions, one session per day (except two participants who had a 1-day gap between two sessions and completed the study within 6 days). All participants had normal or corrected-to-normal vision, were naïve to the purposes of the study, and had not participated in an orientation discrimination task prior to participation in this study.
Apparatus
Stimuli were generated using Matlab (MathWorks, Natick, MA) and PsychToolbox (Brainard, 1997; Pelli, 1997), and were displayed on a 21-in. CRT Monitor (1280 × 960 at 85 Hz). Eye position was monitored using an EyeLink CL infrared eye-tracker (SR research, Kanata, Ontario, Canada).
Stimuli and procedure
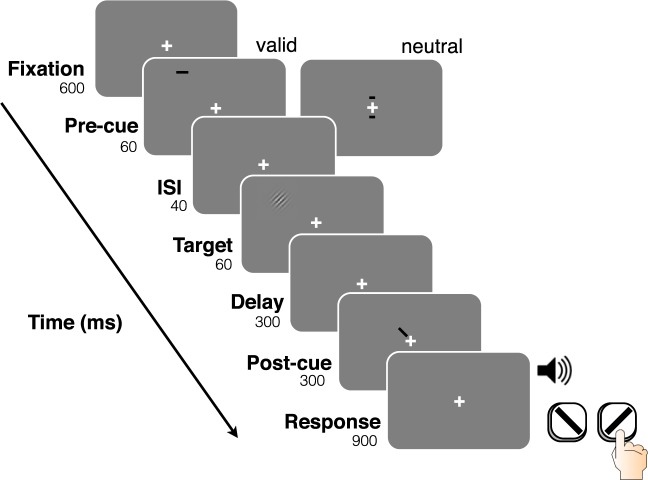
Stimuli were presented on a gray background. Figure 5 shows a trial sequence. Each trial stared with the presentation of a white fixation cross (0.4° × 0.4°, degrees of visual angle) for 600 ms. A precue was then presented for 60 ms. The precue was either Neutral, two 0.2° × 0.1° black lines above and below the fixation cross (0.9° from fixation), or Peripheral, one 0.4° × 0.1° black line 1.55° from the location of the upcoming target (above for a target in the upper visual field, below for a target in the lower visual field). Following a 40-ms interstimulus interval (ISI), one Gabor patch (4 c/° sinusoidal grating in a Gaussian envelope; subtending 2°) was presented for 60 ms at one of four intercardinal (equidistant from horizontal and vertical meridian) isoeccentric locations 5° from fixation (center-to-center). Following a 300 ms ISI, to eliminate location uncertainty, a postcue (black line 0.75° in length) was presented for 300 ms 0.65° from fixation, pointing toward the location where the target had just been presented. After the postcue disappeared, a brief tone indicated that the participant could respond. Participants were required to report the target orientation, either clockwise or counterclockwise relative to vertical, within 900 ms. Auditory feedback was provided after each trial informing the participants of the accuracy of each response, and text feedback was provided at the end of each block informing participants of their percent correct on that block. Target contrast varied from 2%–64%, with a total of eight contrast levels (2%, 4%, 8%, 12%, 16%, 24%, 32%, and 64%), each occurring on an equal number of trials per block in a random order. Participants were required to fixate at the center of the cross before the trial began, and stimulus presentation was contingent on maintaining fixation. If participants broke fixation at any point between the beginning of the trial until the beginning of the response window, the trial would end immediately, the fixation cross would turn red for 300 ms, and a trial with identical parameters (stimulus location, contrast, and tilt) would be added to the end of the block, ensuring the successful completion of all trials within the block without an eye movement.
Figure 5.
Trial sequence for Experiment 2.
Figure 6 illustrates the training schedule. On the first session, the pretest, all participants completed 60–100 trials of practice to familiarize themselves with the procedure, to reduce procedural learning during the experimental blocks, and to accurately measure baseline performance on the task. Practice trials were also used to determine the target orientation, relative to vertical, that would result in performance between 75% and 80% accuracy at 64% contrast. Most participants reached this level of performance with stimuli at around 4° tilt from vertical (mean 3.97°, SD 2.52). The pretest consisted of two blocks of 480 trials each (30 trials at each location at each contrast, clockwise and counterclockwise stimuli counterbalanced), with a Neutral precue on each trial. Within a single block, the target appeared at one of two locations located diagonally from each other (top left and bottom right in one block, top right and bottom left in the other block). There were short breaks between blocks and halfway through each block (240 trials). The next three sessions were training sessions, in which two blocks of 640 trials each were completed (40 trials at each location at each of eight contrast levels), with short breaks between each block and after every quarter-block (160 trials).
Figure 6.
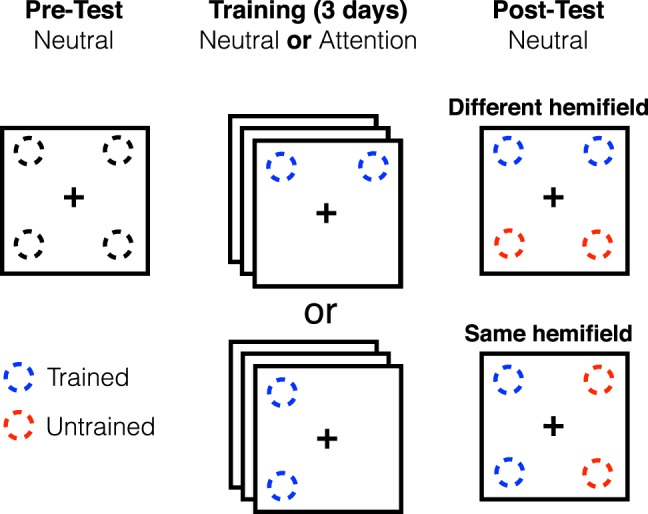
Training and testing schedule for Experiment 2.
Half of the participants were in the Neutral training group, in which the precue was neutral on all trials during training sessions. The other half of participants were in the Attention training group, in which precues during training were valid peripheral cues adjacent to the target location. Participants in each of these groups were divided in two subgroups based on the target location during training. Participants in the Same-Hemifield training group were trained with targets at two locations in the same visual hemifield (top and bottom right or top and bottom left) during training, leaving the two locations in the other hemispheres untrained. Those in the Different-Hemifield training group were trained with stimuli presented at locations in different visual hemifields, but on the same side of the horizontal meridian (top left and top right or bottom left and bottom right), such that the two untrained locations were in the same visual hemifield, but the other side of the horizontal meridian as the trained locations (Figure 6). As a result, there were four training groups: Neutral Different-Hemifield, Attention Different-Hemifield, Neutral Same-Hemifield, and Attention Same-Hemifield, with eight different participants per group. Posttests were identical in structure to pretests, with neutral precues on each trial and targets at locations diagonal from each other on any given block (on opposite sides of vertical and horizontal meridians). Notably, on every block in the pre- and posttests, one target location was to-be-trained or trained, whereas the other was untrained.
Results
Figure 7A shows the results for a representative observer from the Neutral group. For each observer, performance was assessed separately for the pretest and posttest at trained locations and untrained locations. Performance was evaluated as percent correct at each stimulus contrast. The data were fit to a Weibull function:
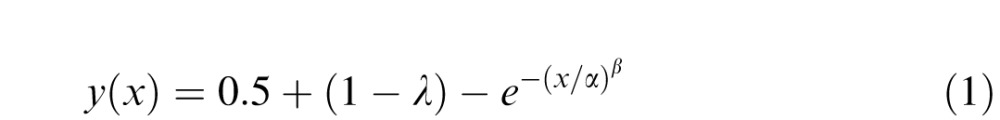 |
using a maximum likelihood criterion, where y(x) represents the performance as a function of contrast (x), λ is 1 minus the asymptotic performance at high contrast values, α is the contrast at which the observer achieves 63.21% of the asymptotic performance, and β determines the slope of the psychometric function. We report the difference between the asymptotic performance, calculated as 1 − λ, on the prettest at all locations and the posttest at trained and untrained locations separately. We used this metric based on previous reports that, without location uncertainty, exogenous attention mediates performance via response gain, that is, an increase in the asymptote of the psychometric curve (Herrmann, Montaser-Kouhsari, Carrasco, & Heeger, 2010; Ling & Carrasco, 2006; Pestilli, Ling, & Carrasco 2009; Pestilli, Viera, & Carrasco, 2007). Because participants varied in the asymptotic performance, and because initial high values for asymptote would tend to be paired with small changes between the pre- and posttests, we applied an arcsine square root
 |
transformation on these asymptotic performance values for each subject. This transformation applies the following calculation on each value.
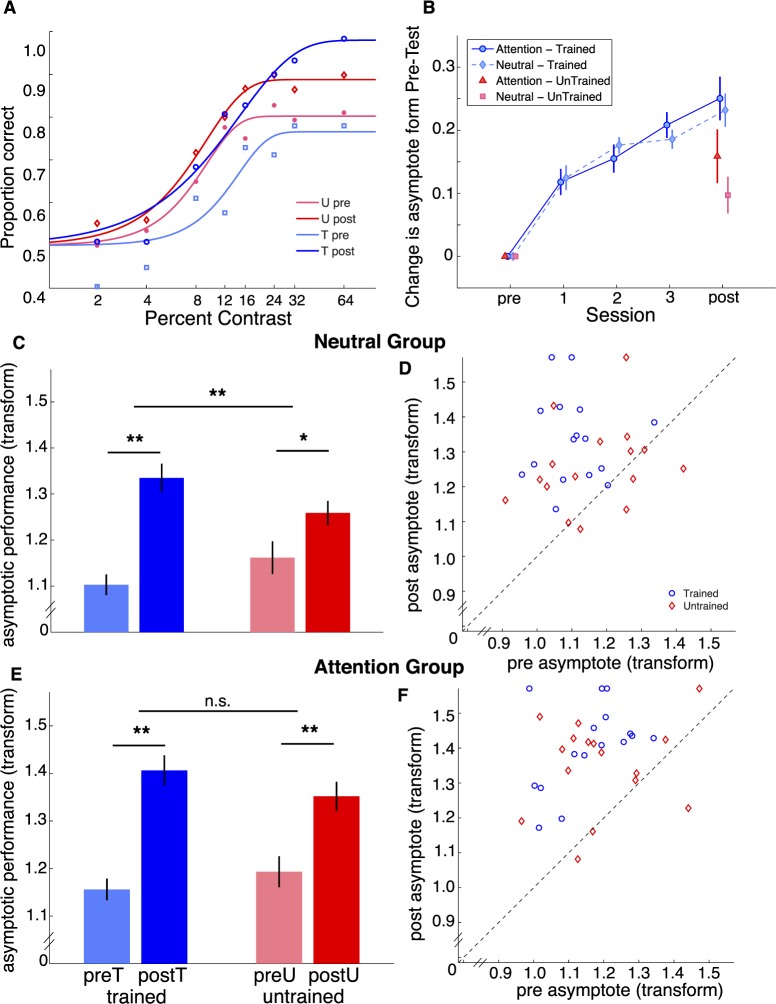
Figure 7.
Results from Experiment 2. (A) Fitted curves to an example observer for pretest at trained (light blue) and untrained (light red) locations, and posttest for trained (dark blue) and untrained (dark red) locations. All other plots show asymptotic performance (1 − λ) after arcsine square root transformation. (B) Mean difference relative to the pretest for the Neutral group (light blue and red) and Attention group (dark blue and red). (C) Values for the pretest and posttest at trained and untrained locations for the Neutral group and (E) Attention group; scatter plots with pretest asymptote on the x-axis and posttest asymptote on the y-axis for the Neutral (D) and Attention (F) groups.
In both groups, no significant differences between trained and untrained locations were found in α , β, c50 (value of x where the y value reaches 50% of the asymptote), average percent correct, or reaction time. Figure 7B shows the mean asymptote for the Neutral and Attention groups for each session, averaged across Hemifield groups. Importantly, there was no significant interaction of cue type (neutral or valid), trained hemifield (same or different), and the difference in asymptote at trained and untrained locations, F(1, 28) = 1.563, p > 0.1. Therefore, we analyzed the combined results of Neutral Different-Hemifield group and Neutral Same-Hemifield group, as well as the combined results of the Attention Different-Hemifield group and Attention Same-Hemifield group. Mean asymptotic performance for the trained and untrained location on the pre- and posttests are shown in Figure 7C and 7E for the Neutral and Attention groups, respectively. Individual observers' asymptotic performance on the pre- and posttests for the trained and untrained locations are shown in Figure 7D and 7F for the Neutral and Attention groups, respectively. In the Neutral group performance improved for everyone but one observer at the trained locations and for 10 out of 16 observers at the untrained locations. In the Attention group, performance improved for everyone at the trained locations and for 13 out of 16 observers at the untrained locations. Moreover, the shift in performance (distance from the unit line) in both trained and untrained locations for observers in the Attention group was more pronounced than the shift for the Neutral group.
We conducted a mixed analysis of variance (ANOVA; within-subject factors, location and session; between-subjects factor, group). First, we confirmed that learning occurred during training. There was a significant improvement for both groups when comparing pretest and posttest, F(1, 30) = 94.29, p < 0.0001, and a marginal interaction with group, F(1, 30) = 3.95, p = 0.056, which seems to be due to a slightly greater effect for the Attention group than the Neutral group. There was a significant location (trained vs. untrained) X session (pretest vs. posttest interaction, F(1, 30) = 13.51, p < 0.001). Additionally, the difference between the improvement at the trained and untrained locations was significantly greater in the Neutral group compared to the Attention group, F(1, 30) = 4.79; p < 0.036. Both Neutral and Attention groups show significant increase in asymptote in the posttest compared to the pretest at the trained locations, t(15) = 5.91, p < 0.001; t(15) = 8.23, p < 0.001, respectively. In the Neutral group, there was a significant increase in asymptote at the untrained locations, t(15) = 2.45, p < 0.027, but this difference was significantly smaller than that at the trained location, F(1, 15) = 17.07, p < 0.001. The Attention group showed strong increases in asymptote at the untrained locations, t(15) = 3.59, p < 0.003, and there was no significant difference with the difference at the trained location, F(1, 15) = 3.08, p = 0.1.
In sum, both groups had significant learning, and whereas there was complete transfer to the untrained locations for the Attention group, there was only partial transfer in the Neutral group, as performance increased more at the trained than the untrained locations.
Discussion
We find that attention facilitated transfer to untrained locations in two experiments. In Experiment 1, we tested untrained adjacent locations. For the Neutral group, consistent with previous studies (e.g., Ball & Sekuler, 1982; Berardi & Fiorentini, 1987; Crist et al., 1997; Fahle et al., 1995; Schoups et al., 1995; Shiu & Pashler, 1992; Sowden et al., 2002), learning was location specific, even though the untrained locations we tested were only 2° from the trained locations and that difference remained throughout 5 days of testing. However, with exogenous attention, training generalized across locations, regardless of whether they were tested with a neutral condition or with attention. These results show that attention generalizes learning to adjacent locations. In Experiment 2, we tested distant locations to examine the extent to which attention can facilitate transfer and whether transfer is limited by cortical versus retinal distance. The results indicate that exogenous attention facilitates perceptual learning at retinal locations distant from the trained locations. In the Neutral group, participants showed some location specificity; more learning at the trained locations than untrained locations. In contrast, in the Attention group, participants showed similar learning at trained and untrained locations (i.e., transfer of VPL across locations). Additionally, location transfer occurs both within and across hemifields. These findings suggest that attention's influence on VPL is not mediated only by early visual areas, as attention facilitates the transfer from trained retinal locations to untrained locations even in a separate visual hemisphere. Critically, the findings that attention-induced transfer is not constrained to only within-hemifield transfer underscores the great potential for attentional manipulations to generalize learning.
Previous studies have reported location transfer of PL given particular training regimens. One prominent example is the procedure known as “double training,” in which participants are trained in one task at one location, and another task at another location (Xiao et al., 2008; Zhang, Xiao et al., 2010), or at the trained location, in the case of piggybacking (Hung & Seitz, 2014; Wang et al., 2012, 2014). This effect is observed when both tasks use the same stimuli (e.g., Gabors) as well as different stimuli (e.g., Gabors and Vernier acuity lines). Although these findings are important for understanding how and under what circumstances location transfer arises, these procedures are limited in their application to improving visual performance and perception. An important constraint is the requirement that participants train on a second task, which lengthens training, thus requiring additional time and effort from participants.
For clinical rehabilitation purposes, an ideal training regimen would allow for the transfer of learning to retinal locations distant from the trained location without the reliance on additional training on a separate task. Here, we introduce a training procedure that does just that: training on a single task with exogenous cues facilitates transfer to an untrained location that was not presented with any stimuli during training, let alone received directed attention or was utilized to perform a task. Furthermore, the results from Experiment 1 show that attention facilitates transfer to locations at which no stimulus ever appeared until after training was completed. Given our findings of attention-facilitated location transfer, the exogenous attention procedure is a powerful tool for improving visual perception and performance across the visual field that may have crucial clinical applications, especially for those with deficits in early vision.
It may be surprising that attention facilitated the transfer of learning across hemispheres, given that both exogenous attention and VPL are known to modulate activity in early visual areas, where neurons are most selective for precise retinal locations and where the left and right cortical hemispheres respond to stimulation to the right and left visual hemifields, respectively. Still, because VPL has been associated with changes in both low- and high-level areas, our findings of across-hemisphere transfer may be incorporated into our understanding of VPL and VPL transfer. However, none of the current PL models predicts these results. For example, the rule-based learning hypothesis (Zhang et al., 2014; Zhang et al., 2013; Zhang, Zhang et al., 2010) cannot account for our findings, as location transfer occurred without exposure to the stimulus at untrained locations during training (Experiments 1 and 2), and even without any exposure at the untrained locations before testing (Experiment 1). Even though, in principle, the learned rules could be applicable to any location, no study supporting this hypothesis has shown transfer without exposure to the untrained locations or features. Moreover, the authors have proposed that some stimulation to the untrained locations is necessary as transfer arises when connections between decision-making areas and sensory areas are reactivated, and unstimulated locations are proposed to be suppressed, thus preventing transfer (Zhang, Zhang et al., 2010). Because exogenous spatial attention decreases activity at unattended locations (e.g., Herrmann et al., 2010; Pestilli & Carrasco, 2005; Pestilli et al., 2007), the rule-based learning hypothesis would predict specificity, not transfer, in the Attention groups of the current experiments, and therefore cannot account for the findings of attention-induced transfer reported here. Indeed, the same group of authors has recently stated limitations to their hypothesis, specifically in terms of location specificity versus transfer in Vernier acuity tasks (Wang et al., 2014).
Our findings seem somewhat consistent with another prominent explanation of PL, Reverse Hierarchy Theory (RHT; Ahissar & Hochstein, 2004; Ahissar et al., 2009), which postulates that VPL is a top-down guided process that begins with higher-level regions, but progresses to lower-level sensory regions with greater task difficulty. According to RHT, prolonged training, which is required with difficult tasks when sensory signals are weak and noisy, results in location-specific learning associated with plasticity in early visual regions. Thus, one possible explanation of the present attention-induced transfer could be that attention improves the signal-to-noise ratio throughout training, increasing stimulus visibility and making task performance less difficult during training, thereby resulting in less specificity and more location transfer. The fact that our finding of VPL transfer was revealed in asymptote may be explained by the possibility that higher contrasts would be less likely to rely on plasticity in early visual areas.
Could the difference in improvement in the Attention and Neutral groups be due to the fact that the precue in the attention group reduced spatial uncertainty? This alternative is unlikely as observers in both groups were always presented with stimuli only at one of two constant locations, and a response cue following the target eliminated location uncertainty before observers provided a response.
Results from Experiments 1 and 2 reveal a notable difference: the Neutral/Neutral group in Experiment 1 showed full specificity (i.e., no learning in the untrained locations), whereas the Neutral group in Experiment 2 showed partial transfer (i.e., some learning in the untrained locations but significantly less than in the trained locations). Some of the differences between the experimental design of both experiments could account for these results:
Length of training. Longer training phases have been reportedly paired with greater degrees of specificity (Jeter et al., 2010). Observers in Experiment 1 were trained for 5 days, but only for 3 days in Experiment 2.
Variability in task difficulty within each session. A study using double training of a hyperacuity task reported that training with a single, long staircase, in which the majority of trials are near threshold, results in specificity. Conversely, training with multiple short staircases, which had greater variability in the difficulty within each session, resulted in the location transfer reported with double training (Hung & Seitz, 2014; but see discussion of Wang et al., 2014). In Experiment 1, observers were presented with stimuli of a single contrast at a predetermined threshold, whereas in Experiment 2 observers were presented with stimuli of eight different contrast levels randomly intermixed and spanning a wide range.
Pretest at untrained locations. Zhang, Xiao et al. (2010) reported that the inclusion of a pretest at an untrained location facilitated transfer of learning from the fovea to the periphery. In Experiment 1, there was no pretest at the to-be-untrained locations and participants were not exposed to stimuli at these locations until after training, whereas in Experiment 2, participants were pretested at the to-be-untrained locations.
In sum, these and other factors could explain the differences in the degree of specificity in the neutral condition. In any case, regardless of the total or partial specificity of the neutral condition, our findings with respect to the influence of attention on VPL and its generalizability are clear and consistent in both experiments: training with exogenous attention reduced location specificity and facilitated transfer, whether by eliminating full specificity (Experiment 1) or converting partial transfer into full transfer (Experiment 2).
We found differences in individual variability between groups in Experiment 2: observers in the Attention training group exhibited transfer consistently, whereas observers in the Neutral group showed greater variability. This finding is consistent with a study showing individual differences in conditions in which specificity is canonically predicted. Zhang et al. (2013) reported that half of the participants in the same experimental group showed transfer, whereas the other half showed specificity. Using electroencephalography, the authors reported that, on trials with stimuli at the untrained location, the transfer group and the specificity group exhibited differences in the components P1 (100 ms after stimulus onset) and N1 (145–200 ms) of event-related potentials (ERPs). These findings suggest that individual differences correlate with neurophysiological factors and may contribute to the emergence of either specificity or transfer. Attention may reduce individual variability and ensure consistent transfer across observers.
The transfer brought about by attention is reliable. We have replicated it and it occurs regardless of whether one (Experiment 2) or two stimuli (Experiment 1) were trained at a time, and for different tasks, including acuity (Tortarolo, Barbot, & Carrasco, 2014). In addition, we have found that exogenous attention during training enables learning, when the same amount of training with a neutral precue does not result in learning (Szpiro & Carrasco, in press). We do not know the mechanisms underlying the effects on learning and transfer brought about by attention; we are currently investigating them. However, because task and stimulus were identical within each experiment we can rule out a number of possible factors that are known to affect VPL, including task relevance, task difficulty, precision, length of training, double training, piggybacking, and adaptation.
Attention has been often invoked as a key mechanism in determining the magnitude of PL and instances of specificity or transfer, yet the experiments reported here are the first to explicitly manipulate and isolate the effects of attention on training and location specificity while holding all other aspects of the training and testing procedures constant across all observers. The findings reported in the current study, including that attention generalizes VPL within- and across-visual hemifields, underscore the potential for exogenous attention to greatly improve outcomes of rehabilitation. Further investigation of the phenomenon and underlying mechanisms of attention-induced transfer will be highly important for advancing our understanding of plasticity in the adult brain in normal and clinical populations.
Supplementary Material
Acknowledgments
This study was supported by NIH R01 EY016200 to MC. We thank Lauren Baideme who collected and analyzed data for two conditions in Experiment 1. Experiment 1 was reported in Carrasco, Giordano, & Baideme (2009). Experiment 2 was reported in Donovan, Szpiro, & Carrasco (2014).
Commercial relationships: none.
Corresponding author: Marisa Carrasco.
Email: marisa.carrasco@nyu.edu.
Address: Department of Psychology and Neural Science, New York University, New York, NY, USA.
Contributor Information
Ian Donovan, Email: ian.donovan@nyu.edu.
Sarit Szpiro, Email: sarit.szpiro@nyu.edu.
Marisa Carrasco, Email: marisa.carrasco@nyu.edu.
References
- Ahissar M.,, Hochestein S. (1997). Task difficulty and the specificity of perceptual learning. Nature, 387, 401–406. [DOI] [PubMed] [Google Scholar]
- Ahissar M.,, Hochstein S. (2004). The reverse hierarchy theory of visual perceptual learning. Trends in Cognitive Sciences, 8 (10), 457–464. [DOI] [PubMed] [Google Scholar]
- Ahissar M.,, Nahum M.,, Nelken I.,, Hochstein S. (2009). Reverse hierarchies and sensory learning. Philosophical Transactions of the Royal Society B: Biological Sciences, 364 (1515), 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K.,, Sekuler R. (1982). A specific and enduring improvement in visual motion discrimination. Science, 218 (4573), 697–698. [DOI] [PubMed] [Google Scholar]
- Bartolucci M.,, Smith A. T. (2011). Attentional modulation in visual cortex is modified during perceptual learning. Neuropsychologia, 49, 3898–3907. [DOI] [PubMed] [Google Scholar]
- Berardi N.,, Fiorentini A. (1987). Interhemispheric transfer of visual information in humans: Spatial characteristics. Journal of Physiology, 384, 633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10 (4), 433–436. [PubMed] [Google Scholar]
- Carrasco M. (2011). Visual attention: The past 25 years. Vision Research, 51, 1484–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. (2014). Spatial covert attention: Perceptual modulation. Kastner S., Nobre A. C. (Eds.) The Oxford handbook of attention (pp 183–230). New York: Oxford University Press. [Google Scholar]
- Carrasco M.,, Giordano A. M.,, Baideme L. (2009). Covert attention generalizes perceptual learning. Journal of Vision, 9 (8): 857 [Abstract] [Google Scholar]
- Cavanaugh M. R.,, Zhang R.,, Melnick M. D.,, Das A.,, Roberts M.,, Tadin D.,, Carrasco M.,, Huxlin K. R. (2015). Visual recovery in cortical blindness is limited by high internal noise. Journal of Vision, 15 (10): 11, 1–18, doi:10.1167/15.10.9. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S. A.,, DeAngelis G. C. (2008). Fine discrimination training alters the causal contribution of macaque area MT to depth perception. Neuron, 60, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist R. E.,, Kapadia M. K.,, Westheimer G.,, Gilbert C. D. (1997). Perceptual learning of spatial localization: Specificity for orientation, position, and context. Journal of Neurophysiology, 78, 2889–2894. [DOI] [PubMed] [Google Scholar]
- Das A.,, Tadin D.,, Huxlin K. R. (2014). Beyond blindsight: Properties of visual relearning in cortically blind fields. The Journal of Neuroscience, 34 (35), 11652–11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan R. J.,, Fink G. R.,, Rolls E.,, Booth M.,, Holmes A.,, Frackowiak R. S. J.,, Friston K. J. (1997). How the brain learns to see objects and faces in an impoverished context. Nature, 389 (6651), 596–599. [DOI] [PubMed] [Google Scholar]
- Donovan I.,, Szpiro S. F. A.,, Carrasco M. (2014). Exogenous attention facilitates perceptual learning transfer within and across visual hemifields. Journal of Vision, 14 (10):. [Abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher B.,, Han S.,, Lu Z.-L. (2010). Perceptual learning and attention: Reduction of object attention limitations with practice. Vision Research, 50, 402–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher B.,, Jeter P.,, Liu J.,, Lu Z.-L. (2013). An integrated reweighting theory of perceptual learning. Proceedings of the National Academy of Sciences, USA, 110 (33), 13678–13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M. (2009). Perceptual learning and sensomotor flexibility: Cortical plasticity under attentional control? Philosophical Transactions of the Royal Society B: Biological Sciences, 364 (1515), 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M.,, Edelman S.,, Poggio T. (1995). Fast perceptual learning in hyperacuity. Vision Research, 35, 3003–3013. [DOI] [PubMed] [Google Scholar]
- Fiorentini A.,, Berardi N. (1980). Perceptual learning specific for orientation and spatial frequency, Nature, 287, 43–44. [DOI] [PubMed] [Google Scholar]
- Fiorentini A.,, Berardi N. (1981). Learning in grating waveform discrimination: Specificity for orientation and spatial frequency. Vision Research, 21, 1149–1158. [DOI] [PubMed] [Google Scholar]
- Ghose G.,, Yu T.,, Maunsell J. H. R. (2002). Physiological correlates of perceptual learning in monkey V1 and V2. Journal of Neurophysiology, 87, 1867–1888. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D.,, Sigman M.,, Crist R. E. (2001). The neural basis of perceptual learning. Neuron, 31, 681–697. [DOI] [PubMed] [Google Scholar]
- Giordano A. M.,, McElree B.,, Carrasco M. (2009). On the automaticity and flexibility of covert attention: A speed–accuracy trade-off analysis. Journal of Vision, 9 (3): 11 1–10, doi:10.1167/9.3.30. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone R. L. (1998). Perceptual learning. Annual Review of Psychology, 49, 585–612. [DOI] [PubMed] [Google Scholar]
- Gu Y.,, Liu S.,, Fetsch C.R.,, Yang Y.,, Folk S.,, Sunkara A.,, Angelaki D.E. (2011). Perceptual learning reduces interneuronal correlations in macaque visual cortex. Neuron, 71, 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K., Montaser-Kouhsari L.,, Carrasco M.,, Heeger D. J. (2010). When size matters: Attention affects performance by contrast or response gain. Nature Neuroscience, 13 (12), 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.-R.,, Watanabe T. (2012). Task attention facilitates learning of task-irrelevant stimuli. PLoS ONE, 7 (4), e35946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung S.-C.,, Seitz A. R. (2014). Prolonged training at threshold promotes robust retinotopic specificity in perceptual learning. The Journal of Neuroscience, 34 (25), 8423–8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxlin K. R.,, Martin T.,, Kelly K.,, Riley M.,, Friedman D. I.,, Burgin W. S.,, Hayhoe M. (2009). Perceptual relearning of complex visual motion after V1 damage in humans. The Journal of Neuroscience, 29 (13), 3981–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter P. E.,, Dosher B. A.,, Liu S.-H.,, Lu Z.-L. (2010). Specificity of perceptual learning increases with increased training. Vision Research, 50, 1928–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A.,, Sagi D. (1991). Where practice makes perfect in texture discrimination: Evidence for primary visual cortex plasticity. Proceedings of the National Academy of Sciences, USA, 88, 4966–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C. T.,, Gold J. I. (2008). Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nature Neuroscience, 11, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M. (2005). Perceptual learning in adults with amblyopia: A reevaluation of critical periods in human vision. Developmental Psychobiology, 46 (3), 222–232. [DOI] [PubMed] [Google Scholar]
- Levi D. M.,, Li R. W. (2009). Perceptual learning as a potential treatment for amblyopia: A mini-review. Vision Research, 49, 2535–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S.,, Carrasco M. (2006). When sustained attention impairs perception. Nature Neuroscience, 9, 1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.,, Weinshall D. (2000). Mechanisms of generalization in perceptual learning. Vision Research, 40, 97–109. [DOI] [PubMed] [Google Scholar]
- Lu Z.-L.,, Liu J.,, Dosher B. A. (2010). Modeling perceptual learning in external noise with Hebbian reweighting. Vision Research, 50, 375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw P. V.,, Webb B. S.,, Moore D. R. (2009). Introduction. Sensory learning: From neural mechanisms to rehabilitation. Philosophical Transactions of the Royal Society B: Biological Sciences, 364 (1515), 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwese J. D.,, Post R. A.,, Scholte H. S.,, Lamme V. A. (2013). Does perceptual learning require consciousness or attention? Journal of Cognitive Neuroscience, 25, 1579–1596. [DOI] [PubMed] [Google Scholar]
- Mukai I.,, Bahadur K.,, Kesavabhotla K.,, Ungerleider L. G. (2011). Exogenous and endogenous attention during perceptual learning differentially affect post-training target thresholds. Journal of Vision, 11 (1): 11 1–15, doi:10.1167/11.1.25. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai I.,, Kim D.,, Fukunaga M.,, Japee S.,, Marrett S.,, Ungerleider L. G. (2007). Activations in visual and attention-related areas predict and correlate with the degree of perceptual learning. The Journal of Neuroscience, 27, 11401–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffen C. L.,, Verstraten F. A.,, Vidnyanszky Z. (2008). Attention-based perceptual learning increases binocular rivalry suppression of irrelevant visual features. Journal of Vision, 8 (4): 11, 1–11, doi:10.1167/8.4.25. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10, 437–442. [PubMed] [Google Scholar]
- Pelli D. G.,, Zhang L. (1991). Accurate control of contrast on microcomputer displays. Vision Research, 31, 1337–1350. [DOI] [PubMed] [Google Scholar]
- Pestilli F.,, Carrasco M. (2005). Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Research, 45, 1867–1875. [DOI] [PubMed] [Google Scholar]
- Pestilli F.,, Ling S.,, Carrasco M. (2009). A population-coding model of attention's influence on contrast response: Estimating neural effects from psychophysical data. Vision Research, 49, 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestilli F.,, Viera G.,, Carrasco M. (2007). How do attention and adaptation affect contrast sensitivity? Journal of Vision, 7 (7): 11 1–12, doi:10.1167/7.7.9. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov A. A.,, Dosher B. A.,, Lu Z. L. (2005). The dynamics of perceptual learning: An incremental reweighting model. Psychological Review, 112, 715–743. [DOI] [PubMed] [Google Scholar]
- Polat U.,, Ma-Naim T.,, Belkin M.,, Sagi D. (2004). Improving vision in adult amblyopia by perceptual learning. Proceedings of the National Academy of Sciences, USA, 101 (17), 6692–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U.,, Shor C.,, Tong J. L.,, Zoet A.,, Lev M.,, Yehezkel O.,, Levi D. M. (2012). Training the brain to overcome the effect of aging on the human eye. Scientific Reports, 2:278, 1–6. [DOI] [PMC free article] [PubMed]
- Roelfsema P. R.,, Ooyen A.V.,, Watanabe T. (2010). Perceptual learning rules based on reinforcers and attention. Trends in Cognitive Science, 14 (2), 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi D. (2011). Perceptual learning in Vision Research. Vision Research, 51 (13), 1552–1566. [DOI] [PubMed] [Google Scholar]
- Sahraie A. (2007). Induced visual sensitivity changes in chronic hemianopia. Current Opinion in Neurobiology, 20, 661–666. [DOI] [PubMed] [Google Scholar]
- Sahraie A.,, Trevethan C. T.,, MacLeod M. J.,, Murray A. D.,, Olson J. A.,, Weiskrantz L. (2006). Increased sensitivity after repeated stimulation of residual spatial channels in blindsight. Proceedings of the National Academy of Sciences, USA, 103 (40), 14971–14976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y.,, Gold J.,, Watanabe T. (2010). Perceptual learning: cortical changes when cats learn a new trick. Current Biology, 20 (13), R557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y.,, Náñez J. E.,, Watanabe T. (2009). Advances in visual perceptual learning and plasticity. Nature Reviews Neuroscience, 11 (1), 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y.,, Náñez J. E.,, Watanabe T. (2012). Recent progress in perceptual learning research. Wiley Interdisciplinary Reviews: Cognitive Science, 3 (3), 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups A. A.,, Vogels R.,, Orban G. A. (1995). Human perceptual learning in identifying the oblique orientation: Retinotopy, orientation specificity and monocularity. Journal of Physiology, 483 (3), 797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz A.,, Watanabe T. (2005). A unified model for perceptual learning. Trends in Cognitive Sciences, 9 (7), 329–334. [DOI] [PubMed] [Google Scholar]
- Seitz A. R.,, Watanabe T. (2009). The phenomenon of task-irrelevant perceptual learning. Vision Research, 49 (21), 2604–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu L.-P.,, Pashler H. (1992). Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Perception & Psychophysics, 52 (5), 582–588. [DOI] [PubMed] [Google Scholar]
- Sowden P. T.,, Rose D.,, Davies I. R. L. (2002). Perceptual learning of luminance contrast detection: Specific for spatial frequency and retinal location but not orientation. Vision Research, 42, 1249–1258. [DOI] [PubMed] [Google Scholar]
- Szpiro S. F. A.,, Carrasco M. (in press). Exogenous attention enables visual perceptual learning. Psychological Science. [DOI] [PMC free article] [PubMed]
- Tortarolo C.,, Barbot A.,, Carrasco M. (2014). Spatial attention generalizes perceptual learning to untrained locations in an acuity task. Journal of Vision, 14 (10): 11 doi:10.1167/14.10.1166. [Abstract] [Google Scholar]
- Tsushima Y.,, Sasaki Y.,, Watanabe T. (2006). Greater disruption due to failure of inhibitory control on an ambiguous distractor. Science, 314, 1786–1788. [DOI] [PubMed] [Google Scholar]
- Tsushima Y.,, Watanabe T. (2009). Roles of attention in perceptual learning from perspectives of psychophysics and animal learning. Learning & Behavior, 37 (2), 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.,, Zhang J. Y.,, Klein S. A.,, Levi D. M.,, Yu C. (2012). Task relevancy and demand modulate double-training enabled transfer of perceptual learning. Vision Research, 61, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.,, Zhang J. Y.,, Klein S. A.,, Levi D. M.,, Yu C. (2014). Vernier perceptual learning transfers to completely untrained retinal locations after double training: A “piggybacking” effect. Journal of Vision, 14 (13): 11 1–10, doi:10.1167/14.13.12. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T.,, Náñez J. E. (2001). Perceptual learning without perception. Nature, 413, 884–889. [DOI] [PubMed] [Google Scholar]
- Watanabe T.,, Náñez J. E.,, Koyama S.,, Mukai I.,, Liederman J.,, Sasaki Y. (2002). Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nature Neuroscience, 5 (10), 1003–1009. [DOI] [PubMed] [Google Scholar]
- Watanabe T.,, Sasaki Y. (2015). Perceptual learning: Toward a comprehensive theory. Annual Review of Psychology, 66, 197–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T.,, Sasaki Y.,, Nañez J. (2001). Perceptual learning without perception. Nature, 413, 844–848. [DOI] [PubMed] [Google Scholar]
- Watson A. B.,, Pelli D. G. (1983). QUEST: A Bayesian adaptive psychometric method. Perception & Psychophysics, 33 (2), 113–120. [DOI] [PubMed] [Google Scholar]
- Xiao L.-Q.,, Zhang J.-Y.,, Wang R.,, Klein S. A.,, Levi D. M.,, Yu C. (2008). Complete transfer of perceptual learning across retinal locations enabled by double training. Current Biology, 18 (24), 1922–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y.,, Watanabe T. (2008). Defining a link between perceptual learning and attention. PLoS Biology, 6 (8), 1623–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y.,, Watanabe T.,, Sasaki Y. (2008). Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron, 57 (6), 827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Y.,, Cong L. J.,, Klein S. A.,, Levi D. M.,, Yu C. (2014). Perceptual learning improves adult amblyopic vision through rule-based cognitive compensation. Investigative Ophthalmology & Visual Science, 55, 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. L.,, Cong L. J.,, Song Y.,, Yu C. (2013). ERP P1-N1 changes associated with Vernier perceptual learning and its location specificity and transfer. Journal of Vision, 13 (4): 11 1–13, doi:10.1167/13.4.19. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Zhang T.,, Xiao L.-Q.,, Klein S. A.,, Levi D. M.,, Yu C. (2010). Decoupling location specificity from perceptual learning of orientation discrimination. Vision Research, 50 (4), 368–374. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y.,, Zhang G. L.,, Xiao L. Q.,, Klein S. A.,, Levi D. M.,, Yu C. (2010). Rule-based learning explains visual perceptual learning and its specificity and transfer. Journal of Neuroscience, 30 (37), 12323–12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.