Abstract
Anchored protein kinase A (PKA) bound to A Kinase Anchoring Protein (AKAP) mediates effects of localized increases in cAMP in defined subcellular microdomains and retains the specificity in cAMP-PKA signaling to distinct extracellular stimuli. Gap junctions are pores between adjacent cells constituted by connexin proteins that provide means of communication and transfer of small molecules. While the PKA signaling is known to promote human trophoblast cell fusion, the gap junction communication through connexin 43 (Cx43) is a prerequisite for this process. We recently demonstrated that trophoblast fusion is regulated by ezrin, a known AKAP, which binds to Cx43 and delivers PKA in the vicinity gap junctions. We found that disruption of the ezrin-Cx43 interaction abolished PKA-dependent phosphorylation of Cx43 as well as gap junction communication and subsequently cell fusion. We propose that the PKA-ezrin-Cx43 macromolecular complex regulating gap junction communication constitutes a general mechanism to control opening of Cx43 gap junctions by phosphorylation in response to cAMP signaling in various cell types.
Keywords: cAMP, Cx43, ezrin, gap junction, PKA
Abbreviations
- PKA
cAMP-protein kinase A
- AKAP
A kinase anchoring protein
- AKB
A kinase binding domain
- Cx
connexin
- Ca2+
calcium
- cAMP
cyclic adenosine monophosphate
- IP3
inositol triphosphate
- Treg
regulatory T cell
- LH
luteinizing hormone
- hCG
human chorionic gonadotropin
- PKC
protein kinase C
- CK1
casein kinase 1
- MAPK
mitogen-activated protein kinase
- Src
proto-oncogene tyrosine-protein kinase
The cAMP-dependent protein kinase (PKA) signaling pathway is characterized in detail in a number of cell types and organ systems. Specificity in the activation of PKA in response to distinct extracellular stimuli is controlled by the intracellular compartmentalization and interaction of PKA with A kinase anchoring proteins (AKAPs).1 All AKAPs contain an A kinase binding domain (AKB) and a unique targeting domain localizing the PKA-AKAP complex to defined subcellular structures, membranes or organelles.2 Distinct subcellular targeting of PKA isozymes provides spatial regulation of different PKA signaling events by controlling the phosphorylation of specific substrates.3 In addition, AKAPs form supramolecular signaling complexes by scaffolding other kinases than PKA, protein phosphatases, phosphodiesterases and other proteins involved in signal transduction.4 Through this essential role in the spatial and temporal integration of effectors and substrates, AKAPs provide a high level of specificity and temporal regulation to the cAMP-PKA-signaling pathway.
In vertebrates, communication between adjacent cells occurs through gap junctions, which are composed of connexin hexamers in the cellular membrane forming hemiconnexons that align with similar structures on neighboring cell to form gap junction channels. These intercellular channels allow passage of ions, second messengers (e.g. cAMP, Ca2+, IP3) and other small molecules between cells. Connexin 43 (Cx43) is by far the most abundantly and widely expressed gap junction protein and its essential role is highlighted by the fact that Cx43 knockout mice die hours after birth and present malformations of the conotruncal region of the right ventricle.5 Gap junction intercellular communication through Cx43 is critically important in many cell processes including control of cell proliferation (e.g., metastatic process), embryonic development, cell differentiation and the coordinated contraction of heart and smooth muscle.6-8 Cx43 is expressed and form gap junction channels in cardiomyocytes, hepatocytes, placental trophoblasts and transiently between immune cells. In the heart, Cx43 gap junctions allows spread of the electrical signals from the sinoatrial node across the heart muscle by transmitting the sodium-mediated membrane depolarization signal to neighboring cells allowing the synchronous contraction of many cardiomyocytes at the same time in each heartbeat.9,10 In the immune system naturally occurring regulatory T cells (Tregs), a sub-population of T lymphocytes with suppressive properties, protect from autoimmune responses to self-antigens by inhibition of effector T cells. One of the suppressive mechanisms involve transfer of cAMP from naturally occurring regulatory T cells to effector T cells via gap junctions, presumably formed by Cx43 which is the connexin in T cells.11 In human placenta, cytotrophoblasts fuse to form the multinucleated syncytiotrophoblast involved in all the feto-maternal exchanges as well as in the placental hormonal functions. Passage of fusogenic signals through Cx43 gap junctions is required for trophoblastic cell fusion to proceed. The placental pregnancy hormone, human chorionic gonadotropin (hCG), increased during early stages of pregnancy, signals via G-protein coupled LH receptors leading to intracellular bursts of cAMP. Via PKA, hCG drives trophoblast fusion and differentiation to form syncytiotrophoblast.12,13 Finally, changes in gap junction communication involving Cx43 is characterized as the earliest alteration linked with malignant transformation in some cancer cells.14,15 Whereas the N-terminal two-thirds of the Cx43 sequence serve to form the pore, the C-terminal cytoplasmic part is more disordered and confers regulation of pore opening and conductivity.
Mutations in Cx43 have been linked to several diseases, including oculodentodigital dysplasia (ODDD), atrioseptal defects, arrhythmias and ischemia/reperfusion injury.6 More than 70 mutations in the Cx43 open reading frame have been characterized and linked to ODDD. These mutations leading mostly to substitutions in the amino acid sequence localize in the N-terminal two-thirds of the Cx43 peptide sequence and are mainly associated with a loss of intercellular communication function. To our knowledge only a single mutation in the C-terminal sequence of the Cx43 has been described (S364P), which suggests that mutations in this region, especially if leaving the pore constitutively open, could be lethal in the early stages of the development.16 Taken together, this suggests some selection pressure to conserve the C-terminal part of the Cx43 amino acid sequence intact in order to avoid impaired regulation of gap junction communication. As abnormal Cx43 gap junction communication between neighboring cells contributes to the development of a set of diseases there is interest to develop therapeutic approaches (i.e., gene therapy, peptidomimetics and small molecules) to modulate Cx43 gap junction communication to counteract pathologies associated with an impaired Cx43 function. Interestingly, it has been described that a down regulation of Cx43 or attenuation of gap junction communication in keratinocyte enhances wound closure.17 Furthermore, it has been demonstrated that gap junction blockers prevent migraine occurrence.18 Conversely, in cardiomyocytes a reduced Cx43 gap junction communication (both electrical and chemical) triggers arrhythmias after myocardial ischemia. It has been shown that compounds that trigger Cx43 intercellular communication prevent the risk of arrhythmias by increasing the conduction velocity.19 In certain cancers, loss of Cx43 associates with progression and Cx43 has been defined as a tumor suppressor. Enhancement or restoration of Cx43 intercellular communication restrains tumor cell growth and potentiates tumor sensitivity to therapy.17 Abnormal Cx43 junction communication has also been linked to immunological disorders, placental complications, hearing loss and gastrointestinal diseases.17,20
The C-terminal part of Cx43 appears to be the region that becomes phosphorylated. Several kinases are shown to phosphorylate Cx43 on various specific sites with different effects on Cx43 behavior. For instance, PKA stimulates assembly of gap junction proteins at the cell membrane as well as intercellular communication, whereas PKC abrogates both assembly and communication through gap junctions. CK1 phosphorylation is shown to promote cell communication, while the effects of Src and MAPK appear to inhibit Cx43 channel gating by affecting channel closure.21 Interestingly, a serine to proline conversion at S364, one of the known PKA phosphorylation sites, was first identified as a mutation in a subset of patients with visceral atrial heterotaxia. Transfected cells expressing the mutation display altered gap junction properties in response to increased PKA activity.21 Moreover, transgenic mice expressing a heart-specific, C-terminally truncated Cx43 mutant display constitutively open channels with increased gap junction permeability, altered electrophysiological properties (susceptibility to arrhythmias) and larger damaged areas in the heart following myocardial infarction than wild type littermates.22
We examined whether the hCG- and cAMP-stimulated cell fusion in primary cultures of human placental cytotrophoblasts required subcellular anchoring of PKA to AKAPs and how this could regulate cell fusion. We found that cell fusion is regulated by ezrin, a known AKAP. We proceeded to examine where in the cell fusion process ezrin was working and found that it confers hCG and cAMP regulation on the opening of gap junctions formed by Cx43.12 Upon local increases in the pool of cAMP, PKA bound to ezrin is activated and phosphorylates Cx43. The phosphorylation of Cx43 by PKA at a specific site(s) in the region between amino acid 362 and 379 promotes the opening of the gap junction and allows the passage of signal molecules (Fig. 1). These PKA phosphorylation sites are key regulators of Cx43 gating. We proceeded to identify a contact site between Cx43 and ezrin, map its molecular contacts and decipher the minimal amino acid sequence required for binding of ezrin to Cx43: 362−RPSSRASSRASSRPRPDD−379. Interestingly, this sequence overlaps with the PKA phosphorylation sites in the C-terminal part of Cx43.23,24 The immediate vicinity between the AKAP docking site and the PKA phosphorylation sites in Cx43 highlights the importance of the spatial organization of available PKA activity to regulate gap junction communication with the necessary kinetics. Furthermore, we show that disruption of the Cx43-ezrin interaction not only abolish Cx43 phosphorylation but also inhibit gap junction flux of tracers.
Figure 1.
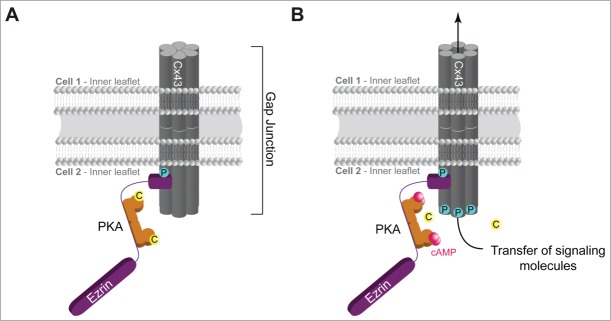
Cx43 gap junction communication is controlled by PKA anchoring through ezrin. (A) Schematic depiction of a resting state gap junction in trophoblast with Cx43 and a compartmentalized pool of PKA anchored to ezrin which again is bound to Cx43. (B) Elevated intracellular cAMP levels lead to activation of PKA and subsequent spatiotemporally controlled phosphorylation of Cx43 which promotes the communication through the gap junction. C, catalytic subunit of PKA; P for phosphorylation; pink dots, molecules of cAMP.
Cx43 shares some of the same phosphorylation sites with other member of the connexins α-group (Cx31.9, Cx37, Cx40, Cx43, Cx45, Cx46, Cx46.6 Cx50 and Cx59) but with the exception of Cx46, do not appear to share the essential residue correctly positioned to support the anchoring of ezrin (R370).
Based on its important physiological role it has been of interest to design pharmacological tools to module Cx43 synthesis, assembly, stabilization or degradation.25 Some molecules that regulate Cx43 gap junction formation are currently tested in clinical trials.17 Taking into account that Cx43 is by far the most abundant and ubiquitously represented, additional drug design need to be performed to optimize selectivity, tissue specificity and off-target effects.
We suggest that the PKA-ezrin-Cx43 macromolecular complex controlling the gap junction communication constitutes a general mechanism to regulate opening of Cx43 gap junctions by phosphorylation and in response to cAMP. Furthermore, targeting the Cx43-ezrin interaction site may provide a new method to block opening of Cx43 gap junctions. Use of Cx43-ezrin interaction inhibitors (such as peptides, peptidomimetics and small molecules) could inhibit opening of gap junctions for example in cardiomyocytes, regulatory T cells or human placental trophoblasts.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Pascale Gerbaud and Danièle Evain-Brion for helpful comments and input on various parts of the work.
Funding
Our work is funded by INSERM, the Norwegian Cancer Society, Research Council of Norway, and the K.G Jebsen Foundation.
References
- 1.Pidoux G, Tasken K. Specificity and spatial dynamics of PKA signaling organized by A kinase anchoring proteins. J Mol Endocrinol 2010; 44:271-84; PMID:20150326; http://dx.doi.org/ 10.1677/JME-10-0010 [DOI] [PubMed] [Google Scholar]
- 2.Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. J Biol Chem 1992; 267:13376-82; PMID:1618839 [PubMed] [Google Scholar]
- 3.Carlson CR, Lygren B, Berge T, Hoshi N, Wong W, Tasken K, Scott JD. Delineation of type I protein kinase A-selective signaling events using an RI anchoring disruptor. J Biol Chem 2006; 281:21535-45; PMID:16728392; http://dx.doi.org/ 10.1074/jbc.M603223200 [DOI] [PubMed] [Google Scholar]
- 4.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. Embo J 2001; 20:1921-30; PMID:11296225; http://dx.doi.org/ 10.1093/emboj/20.8.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC. Cardiac malformation in neonatal mice lacking connexin43. Sci 1995; 267:1831-4; http://dx.doi.org/ 10.1126/science.7892609 [DOI] [PubMed] [Google Scholar]
- 6.Solan JL, Lampe PD. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS letters 2014; 588:1423-9; PMID:24508467; http://dx.doi.org/ 10.1016/j.febslet.2014.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruzzone R, White T, Paul D. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem 1996; 15:1-27; PMID:8665925; http://dx.doi.org/ 10.1111/j.1432-1033.1996.0001q.x [DOI] [PubMed] [Google Scholar]
- 8.Saez J, Berthoud V, Moreno A, Spray D. Gap junctions. Multiplicity of controls in differentiated and undifferentiated cells and possible functional implications. Adv Second Messenger Phosphoprotein Res 1993; 27:163-98; PMID:8380327 [PubMed] [Google Scholar]
- 9.Beauchamp P, Choby C, Desplantez T, de Peyer K, Green K, Yamada KA, Weingart R, Saffitz JE, Kléber AG. Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice. Cir Res 2004; 95:170-8; PMID:15192022; http://dx.doi.org/ 10.1161/01.RES.0000134923.05174.2f [DOI] [PubMed] [Google Scholar]
- 10.Darrow BJ, Fast VG, Kleber AG, Beyer EC, Saffitz JE. Functional and structural assessment of intercellular communication. Increased conduction velocity and enhanced connexin expression in dibutyryl cAMP-treated cultured cardiac myocytes. Circ Res 1996; 79:174-83; PMID:8755993; http://dx.doi.org/ 10.1161/01.RES.79.2.174 [DOI] [PubMed] [Google Scholar]
- 11.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, et al.. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med 2007; 204:1303-10; PMID:17502663; http://dx.doi.org/ 10.1084/jem.20062129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pidoux G, Gerbaud P, Dompierre J, Lygren B, Solstad T, Evain-Brion D, Taskén K. A PKA-ezrin-connexin 43 signaling complex controls gap junction communication and thereby trophoblast cell fusion. J Cell Sci 2014; 127:4172-85; PMID:25052094; http://dx.doi.org/ 10.1242/jcs.149609 [DOI] [PubMed] [Google Scholar]
- 13.Keryer G, Alsat E, Tasken K, Evain-Brion D. Cyclic AMP-dependent protein kinases and human trophoblast cell differentiation in vitro. J cell Sci 1998; 111:995-1004; PMID:9490643 [DOI] [PubMed] [Google Scholar]
- 14.Mehta PP, Bertram JS, Loewenstein WR. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell 1986; 44:187-96; PMID:2416473; http://dx.doi.org/ 10.1016/0092-8674(86)90497-6 [DOI] [PubMed] [Google Scholar]
- 15.Mehta PP, Lokeshwar BL, Schiller PC, Bendix MV, Ostenson RC, Howard GA, Roos BA. Gap-junctional communication in normal and neoplastic prostate epithelial cells and its regulation by cAMP. Mol Carcinog 1996; 15:18-32; PMID:8561862; http://dx.doi.org/ 10.1002/(SICI)1098-2744(199601)15:1%3c18::AID-MC4%3e3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- 16.Laird DW. Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett 2014; 588:1339-48; PMID:24434540; http://dx.doi.org/ 10.1016/j.febslet.2013.12.022 [DOI] [PubMed] [Google Scholar]
- 17.Grek CL, Rhett JM, Ghatnekar GS. Cardiac to cancer: connecting connexins to clinical opportunity. FEBS Lett 2014; 588:1349-64; PMID:24607540; http://dx.doi.org/ 10.1016/j.febslet.2014.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durham PL, Garrett FG. Neurological mechanisms of migraine: potential of the gap-junction modulator tonabersat in prevention of migraine. Cephalalgia 2009; 29 Suppl 2:1-6; PMID:19723120; http://dx.doi.org/ 10.1111/j.1468-2982.2009.01976.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vuyst E, Boengler K, Antoons G, Sipido KR, Schulz R, Leybaert L. Pharmacological modulation of connexin-formed channels in cardiac pathophysiology. Br J Pharmacol 2011; 163:469-83; PMID:21265827; http://dx.doi.org/ 10.1111/j.1476-5381.2011.01244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair RR, Jain M, Singh K. Reduced expression of gap junction gene connexin 43 in recurrent early pregnancy loss patients. Placenta 2011; 32:619-21; PMID:21669459; http://dx.doi.org/ 10.1016/j.placenta.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 21.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 2004; 36:1171-86; PMID:15109565; http://dx.doi.org/ 10.1016/S1357-2725(03)00264-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishman GI, Moreno AP, Spray DC, Leinwand LA. Functional analysis of human cardiac gap junction channel mutants. Proc Natl Acad Sci U S A 1991; 88:3525-9; PMID:1850831; http://dx.doi.org/ 10.1073/pnas.88.9.3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J 2009; 419:261-72; PMID:19309313; http://dx.doi.org/ 10.1042/BJ20082319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG. Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. J Cell Biol 2001; 155:1307-18; PMID:11756479; http://dx.doi.org/ 10.1083/jcb.200102017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyer EC, Berthoud VM. Gap junction synthesis and degradation as therapeutic targets. Curr Drug Targets 2002; 3:409-16; PMID:12448693; http://dx.doi.org/ 10.2174/1389450023347245 [DOI] [PubMed] [Google Scholar]


