Abstract
The small GTPase Rab5 has been frequently studied in the context of intracellular trafficking, but evidence obtained more recently has implicated Rab5 as a critical regulator of cell adhesion, migration and invasion in both normal and tumor cells. These recent findings showing that Rab5 promotes Rac1 activation and focal adhesion dynamics have highlighted the question as to what the upstream regulators of Rab5 activity might be and how these are connected to cell migration. The efforts to shed light on this issue identified in metastatic cancer cells a novel Caveolin‑1/p85α/Rab5/Tiam1/Rac1 signaling axis relevant to cancer cell migration and invasion. In this addendum, we highlight aspects concerning Rab5 regulation in this context.
Keywords: Caveolin-1, cell migration, Rab5, invasion, p85α, Rac1
Abbreviations
- CAV1
Caveolin-1
- GAP
GTPase activating protein
- GEF
guanine exchange factor
- PI3K
phospahtidylinositol-3-kinase
Rab5 in Cell Migration
Rab proteins are small GTPases that control intracellular trafficking, by acting as molecular switches that cycle between an inactive GDP-bound and an active GTP-bound state. More than 60 members of this family have been identified and classified according to sequence, function and subcellular localization.1,2 Among these proteins, Rab5 is of particular interest, because it coordinates early endosome fusion, dynamics and transport, as well as vesicle formation.1 Moreover, recent observations extend the functional repertoire of Rab5 beyond its cannonical role in membrane trafficking (reviewed in3). Specifically, in the context of cell migration, Rab5 was shown to promote Rac1-GTP loading, remodeling of the actin cytoskeleton and formation of lamellipodia.4-7 In addition, Rab5 associates in a complex with integrin β1, increasing integrin recycling and sustaining cell migration.7,8 Consistent with these observations, our more recent findings indicate that Rab5 is required for focal adhesion disassembly, tumor cell migration and invasion,9 and, therefore, that Rab5 is a critical player in cell migration.10,11 Thus, to advance further in understanding the non-canonical functions of Rab5, the identification of putative Rab5 regulators became essential. As a small GTPase, Rab5 function (i.e. GTP-loading) is tightly controlled by guanine exchange factors (GEFs) and GTPase activating proteins (GAPs). In this context, the regulatory subunit of Class I phospahtidylinositol-3-kinase (PI3K), p85α, became a particularly interesting candidate, because it is implicated not only in cell migration via the regulation of PI3K, but also as a Rab5‑GAP.12,13
The Caveolin-1/p85α/Rab5/Tiam1/Rac1 Axis
Rab5 activation is followed by the recruitment of Tiam1, a Rac1-GEF, to early endosomes, leading to Rac1 activation, localized actin remodeling and cell migration.5 However, the identity of regulators and upstream elements implicated in this signaling axis remained poorly understood. In this respect, we recently identified Caveolin-1 (CAV1), a membrane-bound protein, as a critical regulator of Rab5 activity in metastatic cancer cells.14 CAV1 was previously known to stimulate Rac1 activity and focal adhesion turnover in metastatic melanoma and breast cancer cells,15,16 but the precise connection between CAV1 and Rac1 remained unknown. Our recent work14 showed that CAV1 promotes Rab5 activation, leading to Rac1 activation, migration and invasion in 3 different metastatic lines, B16-F10 mouse melanoma, HT-29(US) human colon adenocarcinoma and MDA-MB-231 breast cancer cells. Mechanistically, CAV1 increased the amount of Tiam1 at Rab5‑positive early endosomes and, accordingly, Tiam1 was shown to be necessary to link the CAV1‑Rab5 axis to enhanced Rac1 activation, cell migration and invasion.
The search for molecules implicated in CAV1‑dependent activation of Rab5 led to the identification of p85α, which was shown to associate in a complex with CAV1. Sequestration of p85α by CAV1 precluded Rab5-GTP hydrolysis, leading to increased Rab5 activation, as well as migration and invasion in metastatic cancer cells.14 Intriguingly, this sequestration mechanism is reminiscent of that previously described for Caspase‑8‑dependent activation of Rab5 in neuroblastoma cells.17 In that case, Caspase‑8 phosphorylation on Y380 created an SH2‑binding site that recruited p85α, preventing Rab5 inactivation.17-19 Whether this mechanism is valid for other proteins that connect to Rab5 and favoring migration as well as invasion, remains to be elucidated.
Concluding Remarks
Recent studies support the notion that Rab5 is a key regulator not only of intracellular trafficking, but also of additional non-canonical functions, such as cell migration and invasion. Because enhanced migratory and invasive capacity represent hallmarks characteristic of malignant tumor cells, the identification of mechanisms leading to deregulation of Rab5 function is currently of great interest. The recently described Caveolin-1/p85α/Rab5/Tiam1/Rac1 signaling axis sheds light on how this may occur, especially since the pathway was found to be constitutively activated (Fig. 1) in the metastatic cancer cell lines analyzed. However, more experiments are required to demonstrate the functional importance in animal models in vivo and also in clinically relevant settings. Indeed, the future for this novel signaling axis looks bright given that it brings together a number of proteins that are commonly deregulated in cancer and other human pathologies.
Figure 1.
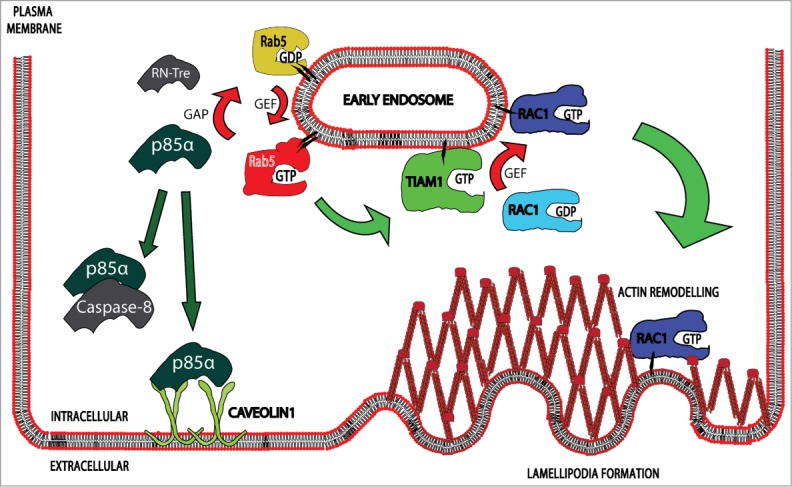
The figure depicts key signaling proteins implicated in CAV1 and Rab5 dependent cell migration. CAV1 promotes Rab5-GTP loading by sequestering p85α, which is a Rab5-GAP, leading to Rab5-dependent recruitment of Tiam1, a Rac1-GEF, to early endosomes. This cascade of events is followed by the activation of Rac1, leading to local remodeling of the actin cytoskeleton, cell migration and invasion. This mechanism is reminiscent of that previously described for Caspase‑8, which is included in the scheme to the left.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Funding
This work was supported by Fondecyt 1140907 and 11100287 (VT); CONICYT-FONDAP 15130011, Anillo ACT 1111, Fondecyt 1130250 (AFGQ); Conicyt PhD Student Fellowship (JD, PS).
References
- 1. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; http://dx.doi.org/ 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- 2. Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol 2001; 2:REVIEWS 3007; PMID:11387043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mendoza P, Diaz J, Torres VA. On the role of Rab5 in cell migration. Curr Mol Med 2014; 14:235-45; PMID:24467205; http://dx.doi.org/ 10.2174/1566524014666140128111347 [DOI] [PubMed] [Google Scholar]
- 4. Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature 2004; 429:309-14; PMID:15152255; http://dx.doi.org/ 10.1038/nature02542 [DOI] [PubMed] [Google Scholar]
- 5. Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 2008; 134:135-47; PMID:18614017; http://dx.doi.org/ 10.1016/j.cell.2008.05.034 [DOI] [PubMed] [Google Scholar]
- 6. Spaargaren M, Bos JL. Rab5 induces Rac-independent lamellipodia formation and cell migration. Mol Biol Cell 1999; 10:3239-50; PMID:10512863; http://dx.doi.org/ 10.1091/mbc.10.10.3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Torres VA, Mielgo A, Barbero S, Hsiao R, Wilkins JA, Stupack DG. Rab5 mediates caspase-8-promoted cell motility and metastasis. Mol Biol Cell 2010; 21:369-76; PMID:19923319; http://dx.doi.org/ 10.1091/mbc.E09-09-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol 2006; 173:767-80; PMID:16754960; http://dx.doi.org/ 10.1083/jcb.200509019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendoza P, Ortiz R, Diaz J, Quest AF, Leyton L, Stupack D, Torres VA. Rab5 activation promotes focal adhesion disassembly, migration and invasiveness in tumor cells. J Cell Sci 2013; 126:3835-47; PMID:23813952; http://dx.doi.org/ 10.1242/jcs.119727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendoza P, Diaz J, Silva P, Torres VA. Rab5 activation as a tumor cell migration switch. Small GTPases 2014; 5(1); PMID:24691381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Torres VA. Rab'ing tumor cell migration and invasion: Focal adhesion disassembly driven by Rab5. Cell Adh Migr 2014; 8; 84-7; PMID:24727246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chamberlain MD, Berry TR, Pastor MC, Anderson DH. The p85alpha subunit of phosphatidylinositol 3'-kinase binds to and stimulates the GTPase activity of Rab proteins. J Biol Chem 2004; 279:48607-14; PMID:15377662; http://dx.doi.org/ 10.1074/jbc.M409769200 [DOI] [PubMed] [Google Scholar]
- 13. Chamberlain MD, Chan T, Oberg JC, Hawrysh AD, James KM, Saxena A,Xiang J, Anderson DH. Disrupted RabGAP function of the p85 subunit of phosphatidylinositol 3-kinase results in cell transformation. J Biol Chem 2008; 283:15861-8; PMID:18387942; http://dx.doi.org/ 10.1074/jbc.M800941200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diaz J, Mendoza P, Ortiz R, Diaz N, Leyton L, Stupack D, Quest AF, Torres VA. Rab5 is required in metastatic cancer cells for caveolin-1-enhanced Rac1 activation, migration and invasion. J Cell Sci 2014; 127:2401-6; PMID:24659799; http://dx.doi.org/ 10.1242/jcs.141689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lobos-Gonzalez L, Aguilar L, Diaz J, Diaz N, Urra H, Torres VA, Silva V, Fitzpatrick C, Lladser A, Hoek KS,. et al. E-cadherin determines caveolin-1 tumor suppression or metastasis enhancing function in melanoma cells. Pigment Cell Melanoma Res 2013; 26:555-70; PMID:23470013; http://dx.doi.org/ 10.1111/pcmr.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Urra H, Torres VA, Ortiz RJ, Lobos L, Diaz MI, Diaz N, Härtel S, Leyton L, Quest AF. Caveolin-1-enhanced motility and focal adhesion turnover require tyrosine-14 but not accumulation to the rear in metastatic cancer cells. PLoS One 2012; 7:e33085; PMID:22505999; http://dx.doi.org/ 10.1371/journal.pone.0033085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torres VA, Mielgo A, Barila D, Anderson DH, Stupack D. Caspase 8 promotes peripheral localization and activation of Rab5. J Biol Chem 2008; 283:36280-9; PMID:18974049; http://dx.doi.org/ 10.1074/jbc.M805878200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barbero S, Barila D, Mielgo A, Stagni V, Clair K, Stupack D. Identification of a critical tyrosine residue in caspase 8 that promotes cell migration. J Biol Chem 2008; 283:13031-4; PMID:18216014; http://dx.doi.org/ 10.1074/jbc.M800549200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Senft J, Helfer B, Frisch SM. Caspase-8 interacts with the p85 subunit of phosphatidylinositol 3-kinase to regulate cell adhesion and motility. Cancer Res 2007; 67:11505-9; PMID:18089778; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5755 [DOI] [PubMed] [Google Scholar]


