Abstract
We recently identified a novel role for podosomes in antigen sampling. Podosomes are dynamic cellular structures that consist of point-like concentrations of actin surrounded by integrins and adaptor proteins such as vinculin and talin. Podosomes establish cellular contact with the extracellular matrix (ECM) and facilitate cell migration via ECM degradation. In our recent paper, we studied podosomes of human dendritic cells (DCs), major antigen presenting cells (APC) that take-up, process, and present foreign antigen to naive T-cells. We employed gelatin-impregnated porous polycarbonate filters to demonstrate that the mechanosensitive podosomes of DCs selectively localize to regions of low-physical resistance such as the filter pores. After degradation of the gelatin, podosomes increasingly protrude into the lumen of these pores. These protrusive podosome-derived structures contain several endocytic and early endosomal markers such as clathrin, Rab5, and VAMP3, and, surprisingly, also contain C-type lectins, a type of pathogen recognition receptors (PRRs). Finally, we performed functional uptake experiments to demonstrate that these PRRs facilitate uptake of antigen from the opposite side of the filter. Our data provide mechanistic insight in how dendritic cells sample for antigen across epithelial barriers for instance from the lumen of the lung and gut.
Keywords: Podosome, Dendritic cell, Antigen sampling, C-type lectins, Receptor-mediated endocytosis, Pattern recognition receptors, Invadopodia, Actin, Leukocyte extravasation, Membrane trafficking
Abbreviations
- APC
antigen-presenting cell
- DC
dendritic cell
- ECM
extracellular matrix
- MHC
major histocompatibility complex
- MMP14 (MT1-MMP)
matrix metalloproteinase-14
- PRR
pathogen recognition receptor
Dendritic cells (DCs) are antigen-presenting cells (APCs) ubiquitously present in all parts of the human body and constantly sample for antigens via PRRs on their surface.1-3 DCs constitute the link between the innate and adaptive immune systems, because they are the only cells that can induce a primary immune response in naive T-lymphocytes.4 In order to perform this function, DCs have to migrate between their sites of origin (bone marrow), sites of sampling activity and lymph nodes where T-cells are activated by the antigens presented by major histocompatibility complex (MHC) molecules on the surface of the DCs. DCs are thus a very motile cell type, that travel inside the body not only passively within the blood stream, but can also “crawl” between cells thereby reaching almost any part of the body within a relatively short time. For this crawling, DCs need to adhere to the ECM and this adhesion is facilitated by podosomes.5-9
Podosomes are cellular structures that consist of dense actin cores surrounded by adaptor proteins such as vinculin, talin, and paxillin that connect the actin cytoskeleton to the membrane, regulatory proteins WASP, and Arp2/3 as well as integrins that allow cellular adhesion to the ECM.10-13 Podosomes are also points of local degradation of ECM, which is achieved by concentrated release of proteases such as MMP14 (also known as MT1-MMP).7,9,14-16 Podosomes are well-known to facilitate cell migration through endothelium, epithelium, and connective tissues.8,17,18 A more recently discovered fact is that podosomes are mechanosensitive and can sense the local stiffness of the substrate.19,20
In an elegant study, Gawden-Bone and coworkers demonstrated that when grown on porous polycarbonate filters, podosomes of dendritic cells can evolve into protrusive structures.16 Although these protrusive structures morphologically resemble invadopodia of cancer cells, they are more dynamic with shorter lifetimes and lower protrusion depths and, in contrast to invadopodia,7,21 they still depend on the protein WASP for their formation. Very similar to invadopodia, these protrusive podosome-derived structures turned out to contain stretches of tubulin which likely mediate trafficking of metalloproteinases to the protrusive tips for degradation of ECM.22,23 Indeed, in our recent paper24 we showed the presence of the metalloproteinase MMP14 at the tips of protrusive podosome-like structures of human monocyte-derived DCs by immunofluorescence. We recently confirmed this finding by overexpression of MMP14 tagged with the fluorescent protein mCherry25 (Fig. 1A). Here, we co-expressed the F-actin binding reporter protein LifeAct26 tagged to GFP to visualize the protrusion of the actin cores into the filter pores.
Figure 1.
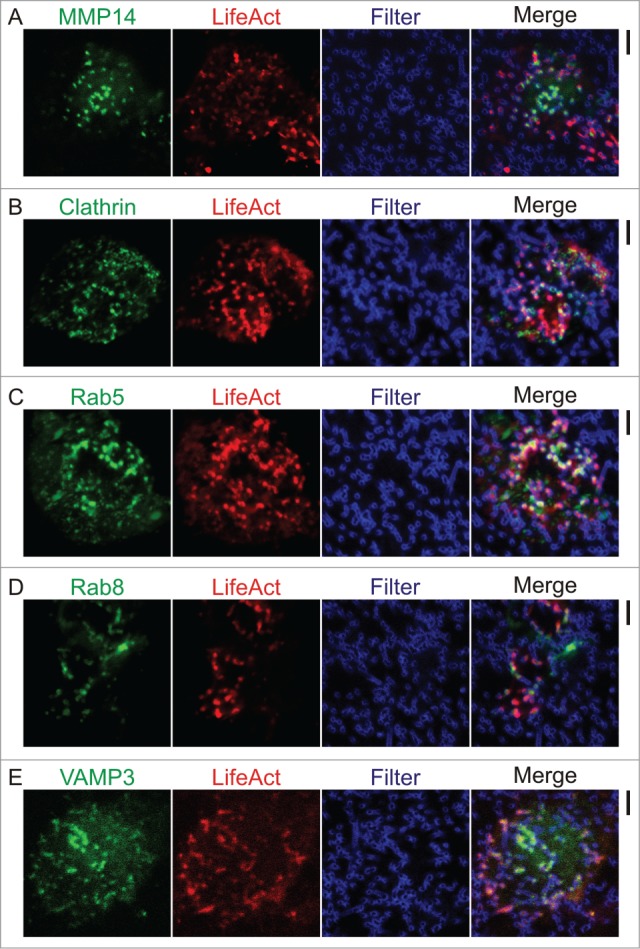
Membrane trafficking at protrusive podosomes-derived structures. Human monocyte derived DCs transiently expressing MMP14, clathrin, Rab5, Rab8, and VAMP3 tagged with fluorescent proteins. Confocal imaging of cells co-transfected with the F-actin reporter either LifeAct-RFP or LifeAct-GFP (red) and plasmids carrying the genes of interest (either GFP or mCherry-tagged: green) cultured on polycarbonate filters with 1 mm pore size and impregnated with gelatin labeled with Alexa fluor 633 (Filter; blue) at least for 1 h prior to imaging. (A) MMP14-mCherry and LifeAct-GFP. (B) Clathrin-GFP and LifeAct-RFP. (C) Rab5-GFP and LifeAct-RFP. (D) Rab8-GFP and LifeAct-RFP. (E) VAMP3-GFP and LifeAct-RFP. Transfections and imaging were performed as described.24 Scale bars represent 5 μm.
Not only is MMP14 released at the tips of protrusive podosome-like structures, but endocytosis also occurs at these spots and Gawden-Bone et al. already demonstrated uptake of degraded gelatin.16 In agreement with this notion that when podosomes become invasive, membrane trafficking is increased at these sites, we showed the presence of several endocytic and endosomal markers at these protrusive structures. We first established the presence of clathrin at protrusive podosome-like structures by immunofluorescence24 and recently confirmed this by overexpression of GFP-tagged clathrin (Fig. 1B). Several overexpressed GFP-tagged versions of other markers for endocytic activity also located to these protrusive podosome-evolved structures: Rab5, Rab8, and VAMP3 (Fig. 1C–E).24 Localization was specific for these proteins, as free GFP and GFP-tagged VAMP7 did not or only little protrude into the filter pores. Rab5 and Rab8 are members of the Rab family of small GTPases, and are well-known regulators of intracellular membrane trafficking of early and recycling endosomes.27-29 VAMP3 is a soluble NSF attachment protein receptor (SNARE) that catalyzes membrane fusion of early endosomes30,31 and is also involved in the extension of pseudopods in phagocytosis.32 Although Rab5, Rab8, and VAMP3 clearly localized in vesicles in the cytosol, our resolution was insufficient to discern a vesicular localization of these proteins in protrusive podosome-derived structures. Nevertheless, our results show that protrusions are sites of membrane trafficking and corroborate with the study of Gawden-Bone et al.16
Since Gawden-Bone et al.16 and we24 demonstrated that endocytosis occurs at protrusive podosome-like structures and since the main function of DCs is antigen presentation, it seemed logical to suggest that these protrusive structures might be involved in endocytosis of antigens. Indeed, protrusive podosome-evolved structures contain various PRRs that can recognize foreign antigens and our immunofluorescence data showed the localization of several members from the C-type lectin family: DC-SIGN (CD209; Fig. 2A), DCIR (CLEC4A), dectin-1 (CLEC7A), and the mannose receptor (CD206).24 Further investigation by transmission electron microscopy and immunogold labeling confirmed that DC-SIGN and CD206 were present on the protrusive structures (Fig. 2B–D for CD206).
Figure 2.
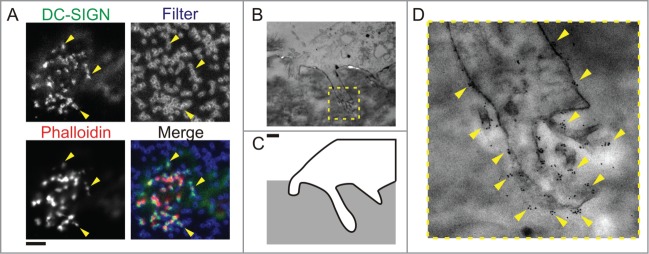
C-type lectins locate to protrusive podosomes-derived structures. (A) Confocal images of human dendritic cells cultured on polycarbonate membrane filters with pore sizes of 1 μm. Actin was stained with phalloidin-Alexa-Fluor-546 (Phalloidin, red), DC-SIGN was labeled by specific primary antibody and secondary antibody conjugated to Alexa-Fluor-488 (DC-SIGN, green). Yellow arrow heads indicate the positioning of protrusion and filter pore. (B–D) Transmission electron microscopy of protrusive podosome-like structures. (B) Electron micrographs of human dendritic cells cultured on polycarbonate filter with 1 μm pore size, impregnated with gelatin and immunogold labeled for CD206. (C) An outline of part of the cell with the protrusion shown in the micrograph in panel (B) (yellow dashed box). (D) Magnification of the protrusion indicated by the yellow box in panel (B). Yellow arrowheads mark positions of clusters of gold-beads which indicate the localization of CD206. Scale bar represents 5 μm (A) and 1 μm (B–D).
We then established that the PRRs residing at the protrusive podosome-like structures indeed were capable to sample for foreign antigen.24 We designed a functional assay where DCs were cultured on gelatin-impregnated filters and where fluorescently labeled mock antigen specific for either DC-SIGN or CD206 was present at the opposite side of the filter.24 With this assay, the ability of dendritic cells to endocytose antigens exclusively via protrusive podosome-like structures could be tested. Control experiments were performed: 1) without mock antigen, 2) with filters containing too narrow pores for podosome protrusion (< 1 μm), 3) competitive blockage of PRRs with mannose, 4) with the endocytic inhibitor Pitstop II, and 5) with the WASP inhibitor wiskostatin. These functional uptake experiments demonstrated the exclusive role of protrusive podosome-evolved structures for uptake of antigen through the filter pores.24 We were also able to show that antigen which was taken up via protrusive podosome-like structures could be actively processed in the cell and loaded onto MCH class II to finally be presented to T-cells.24
Our study directly demonstrates a novel role for podosomes in antigen sampling (Fig. 3). Podosomes sense for spots of low physical resistance of the substrate where they exert mechanical forces and actively degrade the ECM. This process results in remodeling of the ECM and podosomes become increasingly invasive and start protruding in the extracellular environment.16,19,20,24,33 At some point, when the pores in the extracellular environment reach beyond a threshold size of about 3 μm, these protrusive podosomes-derived structures facilitate migration of dendritic cells through ECM and across endo- and epithelial barriers.16,17,24,34,35 It is increasingly well established that podosomes thereby help DCs to rapidly migrate for instance to sites of infection in the body.36,37 In our study, we showed that protrusive podosome-derived structures are sampling stations that actively probe for antigen which would otherwise not be accessible because of its deeper localization within the substrate or across epithelial/endothelial membranes.24 In these cases, PRRs locating to the protrusive tips of podosomes-derived structures can trigger receptor-mediated uptake and this propels subsequent antigen degradation, MHC class II presentation and finally T-cell activation.24 Our findings provide a mechanistic understanding for the well-known capability of DCs to probe for antigens across epithelial barriers, for instance in the lumen of the gut, lungs and small intestine.34,35,38-44 Thereby, our data constitute a novel way of how DCs sample for antigen in the human body and this aids our understanding of the immune system.
Figure 3.
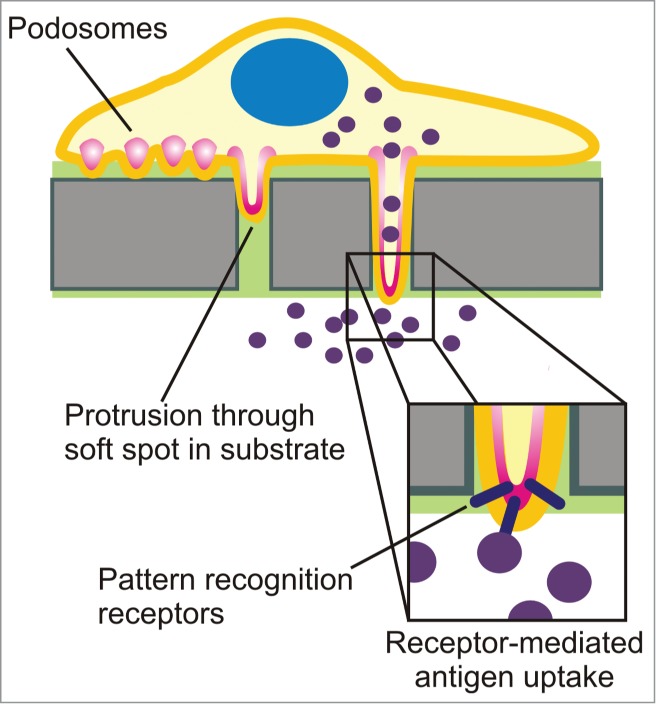
Schematic representation of the protrusive podosome-derived structures of dendritic cells and their role in receptor-mediated antigen uptake by PRRs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Philippe Chavrier and Emilie Lagoutte (Institut Curie Research Center, Paris, France) for the gift of the construct coding for mCherry-labeled MMP14. GvdB is funded by a fellowship from the Radboud University Medical Centre and is the recipient of a Starting Grant from the European Research Council under the European Union's Seventh Framework Programme [grant agreement number 336479].
References
- 1. Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev 2002; 82:97-130; PMID:11773610 [DOI] [PubMed] [Google Scholar]
- 2. McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol 2005; 17:18-24; PMID:15653305; http://dx.doi.org/10.1016/j.coi.2004.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaisho T, Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol 2000; 20:393-405; PMID:11145217 [PubMed] [Google Scholar]
- 4. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52; PMID:9521319; http://dx.doi.org/10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 5. Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol 2004; 5:647-57; PMID:15366708; http://dx.doi.org/10.1038/nrm1436 [DOI] [PubMed] [Google Scholar]
- 6. Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol 2008; 20:235-41; PMID:18337078; http://dx.doi.org/10.1016/j.ceb.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 7. Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol 2011; 27:185-211; PMID:21801014; http://dx.doi.org/10.1146/annurev-cellbio-092910-154216 [DOI] [PubMed] [Google Scholar]
- 8. Schachtner H, Calaminus SD, Thomas SG, Machesky LM. Podosomes in adhesion, migration, mechanosensing and matrix remodeling. Cytoskeleton (Hoboken) 2013; 70:572-89; PMID:23804547; http://dx.doi.org/10.1002/cm.21119 [DOI] [PubMed] [Google Scholar]
- 9. Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol 2007; 17:107-17; PMID:17275303; http://dx.doi.org/10.1016/j.tcb.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 10. van den Dries K, Meddens MB, de Keijzer S, Shekhar S, Subramaniam V, Figdor CG, Cambi A. Interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations. Nat Commun 2013; 4:1412; PMID:23361003; http://dx.doi.org/10.1038/ncom-ms2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A 1999; 96:9648-53; PMID:10449748; http://dx.doi.org/10.1073/pnas.96.17.9648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood 2001; 98:1142-9; PMID:11493463; http://dx.doi.org/10.1182/blood.V98.4.1142 [DOI] [PubMed] [Google Scholar]
- 13. Dovas A, Gevrey JC, Grossi A, Park H, Abou-Kheir W, Cox D. Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J Cell Sci 2009; 122:3873-82; PMID:19808890; http://dx.doi.org/10.1242/jcs.051755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Helden SF, Krooshoop DJ, Broers KC, Raymakers RA, Figdor CG, van Leeuwen FN. A critical role for prostaglandin E2 in podosome dissolution and induction of high-speed migration during dendritic cell maturation. J Immunol 2006; 177:1567-74; PMID:16849464; http://dx.doi.org/10.4049/jimmun-ol.177.3.1567 [DOI] [PubMed] [Google Scholar]
- 15. Matías-Román S, Gálvez BG, Genís L, Yáñez-Mó M, de la Rosa G, Sánchez-Mateos P, Sánchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is involved in migration of human monocytes and is regulated through their interaction with fibronectin or endothelium. Blood 2005; 105:3956-64; PMID:15665118; http://dx.doi.org/10.1182/blood-2004-06-2382 [DOI] [PubMed] [Google Scholar]
- 16. Gawden-Bone C, Zhou Z, King E, Prescott A, Watts C, Lucocq J. Dendritic cell podosomes are protrusive and invade the extracellular matrix using metalloproteinase MMP-14. J Cell Sci 2010; 123:1427-37; PMID:20356925; http://dx.doi.org/10.1242/jcs.056515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity 2007; 26:784-97; PMID:17570692; http://dx.doi.org/10.1016/j.immu-ni.2007.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calle Y, Carragher NO, Thrasher AJ, Jones GE. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J Cell Sci 2006; 119:2375-85; PMID:16723743; http://dx.doi.org/10.1242/jcs.02939 [DOI] [PubMed] [Google Scholar]
- 19. Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N. Self-organized podosomes are dynamic mechanosensors. Curr Biol 2008; 18:1288-94; PMID:18760605; http://dx.doi.org/10.1016/j.cub.2008.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van den Dries K, van Helden SF, te Riet J, Diez-Ahedo R, Manzo C, Oud MM, van Leeuwen FN, Brock R, Garcia-Parajo MF, Cambi A, et al. . Geometry sensing by dendritic cells dictates spatial organization and PGE(2)-induced dissolution of podosomes. Cell Mol Life Sci 2012; 69:1889-901; PMID:22204022; http://dx.doi.org/10.1007/s00018-011-0908-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis 2009; 26:289-98; PMID:18600304; http://dx.doi.org/10.1007/s10585-008-9190-2 [DOI] [PubMed] [Google Scholar]
- 22. Wiesner C, Faix J, Himmel M, Bentzien F, Linder S. KIF5B and KIF3A/KIF3B kinesins drive MT1-MMP surface exposure, CD44 shedding, and extracellular matrix degradation in primary macrophages. Blood 2010; 116:1559-69; PMID:20505159; http://dx.doi.org/10.1182/blood-2009-12-257089 [DOI] [PubMed] [Google Scholar]
- 23. Cornfine S, Himmel M, Kopp P, El Azzouzi K, Wiesner C, Krüger M, Rudel T, Linder S. The kinesin KIF9 and reggie/flotillin proteins regulate matrix degradation by macrophage podosomes. Mol Biol Cell 2011; 22:202-15; PMID:21119006; http://dx.doi.org/10.1091/mbc.E10-05-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baranov MV, Ter Beest M, Reinieren-Beeren I, Cambi A, Figdor CG, van den Bogaart G. Podosomes of dendritic cells facilitate antigen sampling. J Cell Sci 2014; 127:1052-64; PMID:24424029; http://dx.doi.org/10.1242/jcs.141226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D’Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol 2008; 181:985-98; PMID:18541705; http://dx.doi.org/10.1083/jcb.200709076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, et al. . Lifeact: a versatile marker to visualize F-actin. Nat Methods 2008; 5:605-7; PMID:18536722; http://dx.doi.org/10.1038/nmeth.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci 2000; 113:183-92; PMID:10633070 [DOI] [PubMed] [Google Scholar]
- 28. Barr FA. Review series: Rab GTPases and membrane identity: causal or inconsequential? J Cell Biol 2013; 202:191-9; PMID:23878272; http://dx.doi.org/10.1083/jcb.201306010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91:119-49; PMID:21248164; http://dx.doi.org/10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Südhof TC. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature 1993; 364:346-9; PMID:8332193; http://dx.doi.org/10.1038/364346a0 [DOI] [PubMed] [Google Scholar]
- 31. Daro E, van der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci U S A 1996; 93:9559-64; PMID:8790369; http://dx.doi.org/10.1073/pnas.93.18.9559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bajno L, Peng XR, Schreiber AD, Moore HP, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol 2000; 149:697-706; PMID:10791982; http://dx.doi.org/10.1083/jcb.149.3.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Labernadie A, Thibault C, Vieu C, Maridonneau-Parini I, Charrière GM. Dynamics of podosome stiffness revealed by atomic force microscopy. Proc Natl Acad Sci U S A 2010; 107:21016-21; PMID:21081699; http://dx.doi.org/10.1073/pnas.1007835107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001; 2:361-7; PMID:11276208; http://dx.doi.org/10.1038/86373 [DOI] [PubMed] [Google Scholar]
- 35. Strisciuglio C, Duijvestein M, Verhaar AP, Vos AC, van den Brink GR, Hommes DW, Wildenberg ME. Impaired autophagy leads to abnormal dendritic cell-epithelial cell interactions. J Crohns Colitis 2013; 7:534-41; PMID:22981596; http://dx.doi.org/10.1016/j.crohns.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 36. Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 2011; 6:323-44; PMID:21073340; http://dx.doi.org/10.1146/annurev-pathol-011110-130224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vestweber D. Novel insights into leukocyte extravasation. Curr Opin Hematol 2012; 19:212-7; PMID:22395664; http://dx.doi.org/10.1097/MOH.0b013e3283523e78 [DOI] [PubMed] [Google Scholar]
- 38. Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, et al. . Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013; 342:447-53; PMID:24072822; http://dx.doi.org/10.1126/science.1237910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. . CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005; 307:254-8; PMID:15653504; http://dx.doi.org/10.1126/science.1102901 [DOI] [PubMed] [Google Scholar]
- 40. Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med 2006; 203:2841-52; PMID:17145958; http://dx.doi.org/10.1084/jem.20061884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, Jung S. Transepithelial pathogen uptake into the small intestinal lamina propria. J Immunol 2006; 176:2465-9; PMID:16456006; http://dx.doi.org/10.4049/jimmunol.176.4.2465 [DOI] [PubMed] [Google Scholar]
- 42. Lelouard H, Fallet M, de Bovis B, Méresse S, Gorvel JP. Peyer's patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology 2012; 142:592-601, e3; PMID:22155637; http://dx.doi.org/10.1053/j.gastro.2011.11.039 [DOI] [PubMed] [Google Scholar]
- 43. Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, Huang X, Krummel MF. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med 2012; 209:1183-99; PMID:22585735; http://dx.doi.org/10.1084/jem.20112667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, Furtado GC, Lira SA, Shakhar G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 2013; 38:581-95; PMID:23395676; http://dx.doi.org/10.1016/j.immuni.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]


