Gap junction hemichannels (connexons) contain 6 subunits, termed connexins (Fig. 1). The complete intercellular channel is formed by the docking of 2 hemichannels. Many studies have examined the properties of expressed channels composed uniformly of individual connexins (homomeric, homotypic channels) and have shown that they differ in properties including unitary conductance, permeability-selectivity, and gating.
Figure 1.
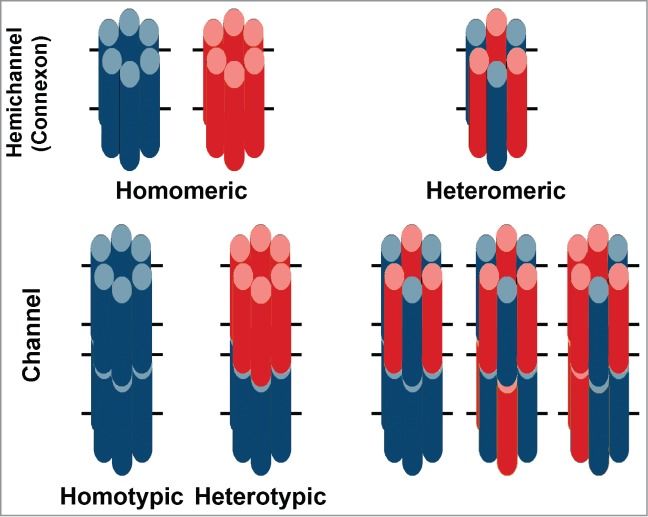
Diagrams illustrating the hemichannels and intercellular channels formed from a single connexin or 2 different connexins. Six identical connexin subunits (blue or red) can oligomerize to form a homomeric hemichannel (connexon). Two hemichannels containing the same connexin dock with each other to form a homotypic channel. Two hemichannels composed of 2 different connexins form a heterotypic channel. Two co-expressed connexins may oligomerize with each other to form a heteromeric connexon. Heteromeric connexons can dock with homomeric or heteromeric connexons to form a large variety of different channels; only a few configurations are illustrated.
Gap junctions can also be formed by the mixing of different connexins. The most common case occurs in a cell that co-expresses multiple connexins, allowing the formation of heteromeric hemichannels and their docking to make a large variety of hetero-oligomeric channels (Fig. 1).
A special case of connexin mixing occurs when a cell expressing one connexin isoform encounters a cell expressing a different connexin, allowing the potential formation of a heterotypic channel (Fig. 1). Expression studies have cataloged the members of the connexin family that are compatible or incompatible as partners for heterotypic gap junction channel formation and defined sequence motifs that influence compatibility (reviewed by Koval et al.1).
Among these studies, conflicting data have been presented regarding the abilities (or inabilities) of connexin40 (Cx40) and connexin43 (Cx43) to form heterotypic gap junction channels.2-4 The current study by Lin et al.5 provides very persuasive data supporting the functionality of Cx40-Cx43 heterotypic channels. Moreover, their characterization demonstrates that of these mixed channels have asymmetric transjunctional voltage gating properties and altered channel conductances. Interestingly, the ability of Cx40 and Cx43 to form heterotypic channels (when paired with each other) was greatly reduced compared to their formation of homotypic channels (when paired with themselves).
The formation of Cx40-Cx43 heterotypic channels is of more than academic interest. Studies of connexin distribution (like those by Davis et al.6) have shown that there are anatomic locations where a cell expressing predominantly Cx40 (like the cells of the His-Purkinje system) communicates with one expressing predominantly Cx43 (like a ventricular myocyte). Heterotypic gap junction channel formation might well contribute to the delays and diminution of conduction that occur at such sites. It initially seems paradoxical that the formation of Cx40-Cx43 heterotypic channel might hinder, rather than facilitate coupling between these cells. However, in some cases, partial uncoupling leads to an improvement in impulse conduction.7 Therefore, some authors8 have speculated that these heterotypic junctions might ensure safe conduction by diminishing coupling at sites (like the Purkinje-myocyte interface) that have a mismatch between a small current source and a large recipient (sink).
References
- 1. Koval M, Molina SA, Burt JM. Mix and match: investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett 2014; 588:1193-204; PMID:24561196; http://dx.doi.org/ 10.1016/j.febslet.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruzzone R, Haefliger JA, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell 1993; 4:7-20; PMID:8382974; http://dx.doi.org/ 10.1091/mbc.4.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol 1995;129:805-17; PMID:7537274; http://dx.doi.org/ 10.1083/jcb.129.3.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valiunas V, Weingart R, Brink PR. Formation of heterotypic gap junction channels by connexins 40 and 43. Circ Res 2000; 86:E42-9; PMID:10666425 [DOI] [PubMed] [Google Scholar]
- 5. Lin X, Xu Q, Veenstra RD. Functional formation of heterotypic gap junction channels by connexins-40 and -43. Channels 2014; 8:433-43; PMID:25483586; http://dx.doi.org/ 10.4161/19336950.2014.949188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis LM, Kanter HL, Beyer EC, Saffitz JE. Distinct gap junction phenotypes in cardiac tissues with disparate conduction properties. J Am Coll Cardiol 1994; 24:1124-32. [DOI] [PubMed] [Google Scholar]
- 7. Rohr S, Kucera JP, Fast VG, Kleber AG. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science 1997; 275:841-4; PMID:9012353; http://dx.doi.org/ 10.1126/science.275.5301.841 [DOI] [PubMed] [Google Scholar]
- 8. Saffitz JE, Schuessler RB. Connexin-40, bundle-branch block, and propagation at the purkinje- myocyte junction. Circ Res 2000; 87:835-6; PMID:11073876; http://dx.doi.org/ 10.1161/01.RES.87.10.835 [DOI] [PubMed] [Google Scholar]


