Abstract
A diffusion barrier segregates the plasma membrane of the rod photoreceptor outer segment into 2 domains; one which is optimized for the conductance of ions in the phototransduction cascade and another for disk membrane synthesis. We propose the former to be named “phototransductive plasma membrane domain," and the latter to be named “disk morphogenic plasma membrane domain." Within the phototransductive plasma membrane, cGMP-gated channels are concentrated in striated membrane features, which are proximally located to the sites of active cGMP production within the disk membranes. For proper localization of cGMP-gated channel to the phototransductive plasma membrane, the glutamic acid-rich protein domain encoded in the β subunit plays a critical role. Quantitative study suggests that the disk morphogenic domain likely plays an important role in enriching rhodopsin prior to its sequestration into closed disk membranes. Thus, this and our previous studies provide new insight into the mechanism that spatially organizes the vertebrate phototransduction cascade.
Keywords: cyclic nucleotide gated channel, CNGA1, CNGB1, morphogenesis, photoreceptor, retina, rhodopsin
Abbreviations
- CNGA1
cyclic nucleotide gated channel α-1
- CNGB1
cyclic nucleotide gated channel β-1
- Dend2
Dendra2
- GARP
glutamic acid-rich protein
- GC
guanylate cyclase
- GCAP
guanylate cyclase activating protein
- GPCR
G protein-coupled receptor
- IS
inner segment
- OS
outer segment
- PDE6
phosphodiesterase 6
- Rho
rhodopsin
Introduction
cGMP-gated channel plays a crucial role in vertebrate vision. Within the rod outer segment (OS), cGMP concentration is regulated by rhodopsin and downstream phototransduction enzymes which are highly enriched in the photosensitive disk membranes.1 Changes in the cGMP concentration are sensed by cGMP-gated channels located on the OS plasma membrane.2 The synthesis and hydrolysis of cGMP is fine-tuned by calcium ions which are conducted by the cGMP gated channel.3 Accordingly, the lifetime of active state rhodopsin is regulated by rhodopsin phosphorylation, which is subject to regulation by calcium binding protein recoverin.4,5 The lifetime of rhodopsin is then correlated with the activity of phosphodiesterase which hydrolyzes cGMP to 5′-GMP. cGMP is synthesized by guanylate cyclase (GC) which is subject to regulation by another calcium binding protein — guanylate cyclase activating protein (GCAP).6,7 Furthermore, cGMP-gated channel is regulated by calmodulin and calcium ions.8 Thus, cGMP and calcium ions play critical roles in the regulation of cGMP-gated channels. We recently found that cGMP-gated channel is enriched to the striated structures of the OS plasma membrane, and it is excluded from the bottom of the OS.9 Currently it is unclear if such unique architectures of rod OS and the distribution of cGMP-gated channel have an impact on the performance of the phototransduction signaling cascade.
The OS membranes are divided into 2 major subdomains, plasma membrane and disk membrane. The disks contain the majority of phototransduction components that regulate cGMP concentration in a light dependent manner.10 The plasma membrane contains cGMP-gated channels which mediate the last step of the phototransduction cascade.2 The previous immunofluorescence and electron microscopy studies suggest that rhodopsin is present both in the disk and the plasma membranes.11 The high disk concentration of rhodopsin allows effective photon capture, as reduction in the rhodopsin contents can lead to compromised sensitivity of rod photoreceptors.12 Rhodopsin content has been compared and estimated in purified preparations of disk and plasma membranes. According to Molday et al.,13 rhodopsin constitutes ∼85% of total disk membranes, whereas, rhodopsin was estimated to constitute ∼50% of total plasma membrane proteins. This study, however, appears to have led to an overestimation of rhodopsin content as the plasma membrane preparation contained substantial amounts of disk membrane material such as peripherin/rds.13 The disparity in the disk and plasma membrane concentration would be generated by the cellular trafficking and compartmentalization mechanisms.
The cGMP-gated channel consists of one β (CNGB1) and 3 α (CNGA1) subunits 14,15 and is reported to interact with several other constituents of the rod OS. For example, cGMP-gated channel interacts with peripherin/rds,16 4.1G,17 and ankyrin G.18 While the role of channel-4.1G interactions is unclear, synchronized renewal of channel together with disk membranes is consistent with the interaction of channel with a disk rim component including peripherin/rds.9 Channel-ankyrin G interaction was proposed essential and sufficient for the trafficking of cGMP-gated channel to the rod OS plasma membrane. Consecutive isoleucine and leucine residues in the C-terminal tail region of CNGB1 are identified to be the site of the interaction between ankyrin G and cGMP-gated channel.18 Together with other cytoskeletal components, ankyrins often regulate the distribution of transmembrane proteins, including channels, on the plasma membrane.19 In rod photoreceptors, however, it is unclear if such ankyrin G binding is required for the confinement of cGMP-gated channel to the sub-plasma membrane locations.
To clarify the role of OS architecture and protein compartmentalization in cGMP signaling, we characterized the sub-disk membrane localizations of phototransduction enzymes, including GCs and calcium dependent regulator of phototransduction GCAP1, in Xenopus rod photoreceptors. Rhodopsin is a constituent of both disk and plasma membranes. To understand how the first step of the phototransduction cascade is spatially organized, we quantitatively studied the concentration of rhodopsin in disk and plasma membrane compartments. To elucidate the role of CNGB1 subunit in the sub-membrane assembly of cGMP-gated channels, we dissected the roles of amino acid sequences and regions that were reported to be critical for channel trafficking and distribution. Those regions include GARP domain, VXPX cilia targeting motif, and ankyrin G binding sequence. Those studies provide insight into the spatial distribution and compartmentalization of G protein-coupled receptor (GPCR) cascade, which culminates in the regulation of cGMP-gated channels.
Results and Discussions
Photoreceptor outer segment plasma membrane is divided into 2 subdomains
We propose the unique area of OS plasma membrane enriched with cGMP-gated channel to be named “phototransductive plasma membrane domain (or in short, phototransductive domain)," because the phototransduction cascade culminates in this plasma membrane region (Fig. 1A). As reported previously,9 cGMP-gated channel is observed in the sub-plasma membrane domain which encompass the tip and lateral portion of the OS, but is occluded from the sub-plasma membrane domain proximal to axoneme and at the bottom of the OS. Consistent with the distribution of cGMP-gated channels, cGMP binding sites, labeled by a fluorescent analog of cGMP, are located in the tip and lateral portion of the OS and they were poorly observed at the bottom of the OS.20 cGMP-gated channel is not observed at the bottom of the OS because it is directly trafficked to the lateral portion of OS plasma membrane, and because the lateral and bottom portions of the plasma membrane are clearly segregated by a diffusion barrier. Approximately 30% of cGMP-gated channel is mobile in the lateral OS plasma membrane.9 This mobile fraction did not enter the bottom of the OS plasma membrane, indicating that a diffusion barrier is present at the base of the OS (Fig. 1A) which prevents the components of the phototransductive plasma membrane domain from entering the area of disk membrane morphogenesis.
Figure 1.
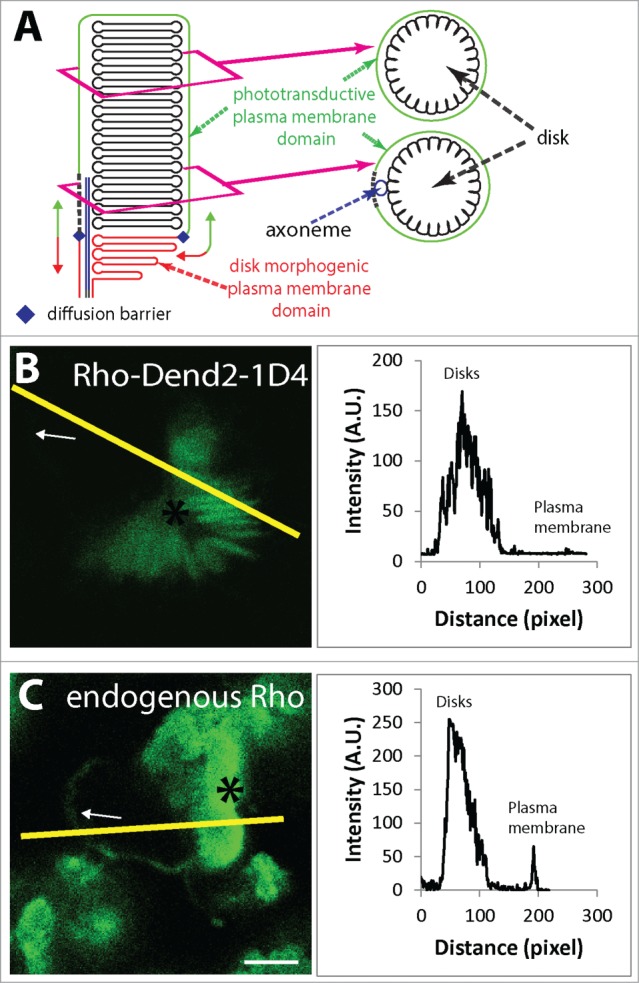
Diffusion barrier separates the outer segment plasma membrane. (A) Outer segment plasma membrane is divided by a diffusion barrier into 2 separate domains: phototransductive domain (green) and disk morphogenic domain (red). (B) Distribution of Rho-Dend2-1D4 in plasma membrane (arrow) and disks (asterisk) in hypotonically treated retinas. (C) Distribution of endogenous rhodopsin in plasma membrane (arrow) and disks (asterisk) in hypotonically treated retinas. Scale bar 5 μm.
Because of the diffusion barrier, the bottom of the OS constitutes another unique plasma membrane domain responsible for deriving disk components. We propose this bottom OS region to be named “disk morphogenic plasma membrane domain (or in short, disk morphogenic domain)” (Fig. 1A). It is important to note that there are 2 proposed models regarding the process of disk membrane morphogenesis (evagination/rim9,21 and endosome models22-24). In both models, disk membranes are synthesized at the base of the OS, and rhodopsin and other disk membrane constituents are proposed to originate from the OS plasma membrane. Rhodopsin is targeted to the disk morphogenic domain for ultimate incorporation into the disk membranes. For effective incorporation and enrichment of rhodopsin into disks, rhodopsin shall be prevented from entering the phototransductive plasma membrane. Current consensus is that rhodopsin carrier vesicles initially fuse to the distal inner segment (IS) plasma membrane. It is estimated that rhodopsin is carried through ciliary plasma membrane by intraflagellar trafficking, and reaches the plasma membrane at the bottom of the OS.25 This trafficking also involves Myosin VIIa motor protein, and deletion of Myosin VIIa leads to slowed intraflagellar trafficking and an increase in the steady state level of rhodopsin on the plasma membrane of the connecting cilia.26 Based on the evagination/rim formation model, evaginations are the precursor of disk membranes. Consistent with this model, disruption of F-actin leads to aberrant overgrowth of plasma membrane evagination at the base of the rod OS.27 In these evaginations, newly synthesized rhodopsin accumulated, but it did not enter significantly into the phototransductive plasma membrane region, indicating that rhodopsin in the disk membrane precursors is largely prevented from entering the phototransductive plasma membrane.9 Our study clearly demonstrates that the overgrown evaginations are not covered by the phototransductive plasma membrane. Therefore along with previous electron microscopy studies,27 our observations suggest that those are structures open to the extracellular environment.
The diffusion barrier may also allow the disparity of rhodopsin concentrations observed between the disk and phototransductive plasma membrane. To visualize the disk and plasma membrane localized rhodopsin-Dendra2 fusion protein (Rho-Dend2-1D4), the OS was ruptured by low osmotic pressure. This rupturing leads to disk membranes opening like a “fan” around a whirled plasma membrane structure,13 and allowed us to spatially discriminate disk (Fig. 1B asterisk) and plasma membrane (Fig. 1B arrow) domains.
By using fluorescence derived from Rho-Dend2-1D4, we estimated the concentration ratio of rhodopsin localized in disk and plasma membranes. It was noted that the majority of plasma membrane in this preparation did not contain detectable amounts of Rho-Dend2-1D4. If we overestimate the plasma membrane contents of rhodopsin, by intentionally selecting only the ruptured OS which contained rhodopsin in the plasma membrane, the rhodopsin concentration in the plasma membrane was 7.62 ± 5.15% (n = 20) of the concentration in the disk membranes. This observation is inconsistent with the previously estimated high concentration of rhodopsin in the plasma membrane, the concentration as high as 50% of what's seen in the disks.13
The above estimations were made using Rho-Dend2-1D4 heterologously expressed in Xenopus rods. To estimate the concentration ratio of endogenous rhodopsin, rod OSs were hypotonically ruptured, chemically fixed, and then stained with 11D5 antibody which was raised against the C-terminal tail region of rhodopsin28 (Fig. 1C). As the samples had to be processed through chemical fixation and permeabilized by detergent, the disk structures were compromised, but this indirect immunofluorescence method allowed the comparison of disk (Fig. 1C, asterisk) and plasma membrane (Fig. 1C, arrow) localized rhodopsin. Level of endogenous rhodopsin plasma membrane was 26 ± 10% (n = 14) of the concentration in the disk membrane. Thus, regardless of the methods introduced, the plasma membrane content of rhodopsin is lower than the previous estimation (50% of disk contents). The difference between the previous and our estimations may be possibly derived from a species dependent difference, as this study was conducted for Xenopus laevis and the previous study was for bovine. However, it is also likely that previous study overestimated the rhodopsin plasma membrane contents due to contamination of the plasma membrane preparation by the disk membrane contents as demonstrated by the quantification of a disk specific component peripherin/rds. Within the plasma membrane preparation, peripherin/rds content was between 15–25% of the amount found in the disk membranes. Thus if 25% of disk membrane contaminant was subtracted from the estimated levels in plasma membrane, rhodopsin plasma membrane concentration would be as low as 25%, the value closer to 7.62 ± 5.14% and 26 ± 10% in our study. Overall, the discrepancy in the rhodopsin concentration and high disk content of rhodopsin is consistent with an idea that the diffusion barrier prevents disk membrane components from entering the phototransductive plasma membrane domain. Specific targeting of disk membrane proteins to the disk morphogenic domain allows high concentration of disk constituents (Fig. 1A).
Compartmentalization of cGMP and Ca2+ signaling into restricted volumes of the photoreceptor outer segment
Within the phototransductive plasma membrane, cGMP-gated channel is concentrated in striated features proximal to the calyceal processes (see Nemet et al.9). This localization pattern is consistent with the distribution of cGMP binding sites, which were studied by a fluorescent cGMP analog and were concentrated on striated features.20 Striated features were also labeled previously by anti-spectrin antibody, which is known to cross react to cGMP-gated channel.29 While this past study did not discriminate spectrin and cGMP-gated channel, the result is consistent with cGMP-gated channel localizing to the striated plasma membrane features. The striated features are juxtaposed to the disk membrane incisures, and shall be of functional significance. cGMP concentration profile is determined by the rate of its hydrolysis and synthesis. For example, phosphodiesterase 6 (PDE6), an enzyme that catalyzes hydrolysis of cGMP to 5′-GMP, is localized to the disk rim and incisure regions in frog photoreceptors.30 While PDE6 is localized uniformly around the circumference of the disk, the involuted structure of the incisures accommodates more enzyme per area of proximal phototransductive plasma membrane. Thus the disk incisures may allow quick hydrolysis of cGMP in the limited cytoplasmic volumes that are proximately located to the striated features.
GCs are the enzymes responsible for cGMP synthesis from GTP. There are 2 types of GCs, GC1 and GC2, expressed in cone and rod photoreceptors. Deletions of GC1 gene lead to primarily cone dystrophy, whereas deletions of both GC1 and GC2 lead to cone and rod dystrophy.31,32 Given the important role of GCs in rods, we tested the localization of endogenous GC1 and GC2 using a polyclonal antibody raised against a peptide sequence which is well conserved both in GC1 and GC2. GCs also localize primarily to the rim and incisure regions of rod disk membranes (Fig. 2A). This observation is consistent with the previously reported localization of murine GC1 fused to EGFP, heterologously expressed in Xenopus laevis rod photoreceptors.33 GCs are regulated by Ca2+ and Ca2+ binding protein GCAP. GCAP1 also localized primarily to the rim and incisures (Fig. 2B) where it constitutively binds to GC1 and GC2 and regulates their activity. Calcium ions, which regulate the activity of GCs, are conducted by cGMP-channel. Thus cGMP-gated channels and GCs regulate each other by a Ca2+-cGMP mediated feedback loop, which can be regulated more quickly in the cytoplasmic area surrounded by incisure and striated features. Striated localization of cGMP-gated channels in the phototransductive plasma membrane would allow the spatial confinement of the feedback mechanism so that channels can quickly respond to the changes in the cGMP concentration, and can provide rapid feedback to GC for timely reestablishment of cGMP level after completion of phototransduction (Fig. 2C).
Figure 2.
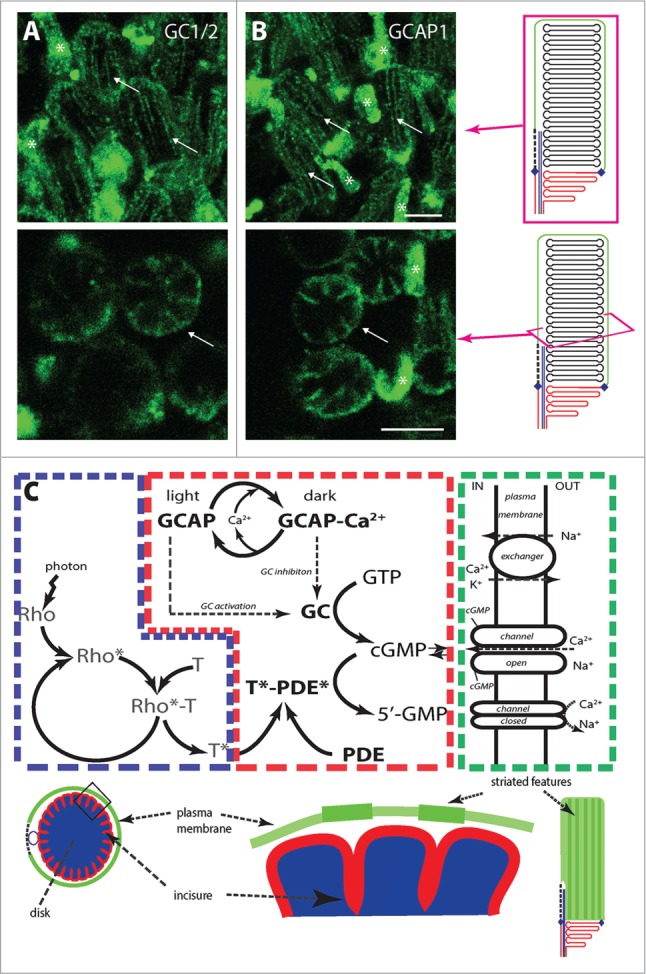
Compartmentalization of cGMP and Ca2+ signaling into restricted volumes of the photoreceptor outer segment. Guanylate cyclase 1 and 2 (GC1/2) (A) and guanylate cyclase activating protein 1 (GCAP1) (B) localize primarily to the incisures and rim region of rod disk membranes. Arrows: rods; asterisks: cone cells. (C) Simplified phototransduction cascade in rod OS and localization of its components in disks (blue), disk rims and incisures (red), and OS plasma membrane (green). Rho: rhodopsin, T: transducin, PDE: phosphodiesterase, GC: guanylate cyclase, GCAP: guanylate cyclase activating protein, channel: cGMP gated channel. Images in upper panels are maximum projections of optical slices and single optical slices in lower panel. Scale bar 5 μm.
Dissection of CNGB1 regions which dictate the trafficking and localization of cGMP-gated channels
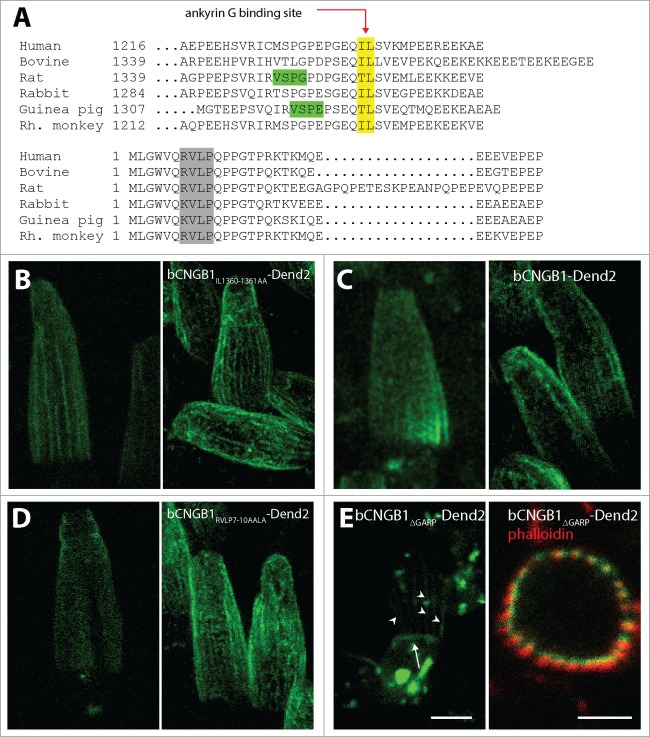
Functional cGMP-gated channel is a heterotetramer consisting of 1 β and 3 α subunits,14,15 and this heterotetramerization is essential for the trafficking to the OS,34 which is a part of modified primary cilium. CNGB1 stays in the cytosol when expressed alone and heterologously in cultured cells or oocytes.35,36 Co-expression of β with α subunits allows for heterotetramer formation and plasma membrane localization. While CNGA1 can be targeted to the plasma membrane in cultured cells,35,36 it failed to localize to the OS in dog and mouse models lacking functional CNGB1.34,37,38 Therefore, CNGA1 itself does not contain sufficient information for the OS targeting in rod photoreceptor cells in vivo. Location of the OS targeting signal in CNGB1 is under active debate, and accordingly 2 hypotheses were previously tested. One hypothesis assumes that cGMP-gated channel does not contain any trafficking signals, and that interaction of CNGB1 subunit with ankyrin G is essential and sufficient for OS targeting of cGMP-gated channels because ankyrin G is targeted to the OS and brings the CNG channel along with it.18 Another hypothesis, which was tested for rat CNGB1 channel, is that CNGB1 channel contains the VXPX motif 39 (also compatible with RVXP motif 40) at the C-terminal tail region, and trafficked to the cilia similar to rhodopsin which also contains the VXPX signal. This idea was tested using ciliated cells in culture.35 We obtained evidence that these 2 hypotheses are not well supported for rod cGMP-gated channel. First of all, bovine CNGB1, for which ankyrin G binding motif was disrupted (Fig. 3A, yellow), was properly trafficked to the rod OS (Fig. 3B) similar to the WT CNGB1 (Fig. 3C). Thus, ankyrin G binding motif is non-essential for OS targeting of CNGB1. Second, the VXPX motif, which was previously proposed to be essential for trafficking rat CNGB1, is not preserved across the vertebrate species including human and bovine (Fig. 3A, green). Thus it is unlikely that the VXPX motif, which was only found in rodent CNGB1, play any roles in the cilia targeting of CNG channel in other species. While there is no conserved VXPX motif in the C-terminal tail region, N terminal region of CNGB1 contains R7VXP sequence which is also compatible with VXPX motif (Fig. 3A, gray). Previously, this RVXP sequence was proposed to be essential for cilia targeting of polycystin2.40 However, we found that this R7VXP sequence is also non-essential, as replacement of R7VLP sequence to AALA did not affect the OS targeting of bCNGB1-Dend2 (Fig. 3D).
Figure 3.
GARP region of CNGB1 is required for CNG channel localization to the OS PM. (A) Partial protein sequences of rod CNGB1. Ankyrin G binding residues (IL, yellow) at C-terminus are highly conserved among species while VXPX motif is not (green); however, (R)VXPX motif at N-terminus is highly conserved among species (gray). (B) CNGB1 with mutated ankyrin G binding site (bCNGB1IL1360-1361AA-Dend2) properly localized to the rod OS similar to the WT (bCNGB1-Dend2) (C). (D) R7VXP sequence is also non-essential for the channel trafficking to the OS, as replacement of R7VLP sequence to A7ALA (bCNGB1-RVLP7-10AALA-Dend2) did not affect channel localization to the OS. (E) Trafficking signal is located in the CNGB1 GARP domain, as removal of GARP domain (bCNGB1-ΔGARP-Dend2) led to its accumulation in the IS and localization to the apical membrane of the IS (arrow) as well as in the calyceal processes (arrowheads and co-localization with F-actin (phalloidin) right panel). In (B–E), left panels are live and unfixed retinas, right panels are fixed retinas stained with Dend2 antibody and (E) phalloidin (F-actin). Scale bar 5 μm.
While none of these trafficking relevant domains were essential for the OS localization of CNGB1, we found that GARP domain plays a critical role in the OS localization of CNGB1. The majority of bCNGB1-Dend2 deficient in the GARP domain (bCNGB1ΔGARP-Dend2) localized mainly to rod IS. A minority of bCNGB1ΔGARP-Dend2 localized to the apical plasma membrane of the IS (Fig. 3E, arrow), as well as in the calyceal processes extending from the IS plasma membrane (Fig. 3E left panel arrowheads and right panel). This observation suggests that the absence of OS localization is not due to misfolding of CNGB1, as the plasma membrane localization indicates that CNGB1 passed through the quality control mechanism in the ER. The GARP requirement is unique to rod cGMP-gated channel, because olfactory cGMP-gated channel, which lacks the GARP domain, can reach the olfactory sensory cilia.35,41 Therefore the cilia targeting mechanism of cGMP-gated channel appear to vary among different sensory cells.
Summary
In this manuscript, we extended the discussion about the 2 major aspects of membrane biology involving cGMP-gated channel. First, we focused on the compartmentalization of cGMP-gated channel into the specific plasma membrane compartment. cGMP-gated channel is localized to unique phototransductive plasma membrane domain, which is segregated from the disk morphogenic plasma membrane domain by a diffusion barrier. The same diffusion barrier allows rhodopsin to concentrate in the disk membranes. Within the phototransductive plasma membrane, cGMP-gated channels are further enriched in striated plasma membrane features. Such segregation and enrichment would allow effective operation of the phototransduction cascade. The molecular identity of the diffusion barrier, and identity of the proteins tethering cGMP-gated channel to the striated features remain to be elucidated. Regarding the tethering of cGMP-gated channel, interesting developments were made for elucidating the interactive partners of cGMP-channels in the rod OSs.17
Second, we focused on the trafficking mechanism of cGMP-gated channel. The role of ankyrin G in the cGMP-gated channel biology shall be revisited. Previous study suggested that disruption of the ankyrin G binding motif leads to defective OS trafficking of human CNGB1 and retinitis pigmentosa.18 The same mutation in bovine CNGB1 did not lead to the OS targeting defect. Thus, there is a possibility that a species dependent difference affected the property of cGMP-gated channel mutants, for example the protein stability. Unstable nature of human cGMP-gated channel might have led to its IS mislocalization,18 and cause retinitis pigmentosa.42 By analogy, rhodopsin is known to demonstrate dramatic species dependency in structural stability, where bovine rhodopsin mutant is more stable than the corresponding human rhodopsin mutant.43 Instability of human CNGB1 may possibly be caused by compromised ankyrin G-CNGB1 interaction prior to entering the secretory pathway. Our finding that GARP domain is essential for cGMP-gated channel trafficking is largely consistent with recent developments elucidating the trafficking signal of CNGB1.44 Complementary to our study in which the entire GARP region was deleted, it appears that a partial deletion of the GARP region does not appear to affect the localization of CNGB1.45 Thus, further studies of cGMP-gated channel will lead to a discovery of a novel cilia trafficking signal.
Methods
Constructs
Full-length bovine cyclic nucleotide-gated channel β-1 (bCNGB1) cDNA was a generous gift from Dr. Andrew F.X. Goldberg (Eye Research Institute, Oakland University, Rochester, MI) and Dr. Robert Molday (department of Biochemistry and Molecular Biology, University of British Columbia, Vancouver, Canada). The coding region of bCNGB1 was cloned into a TOPO vector which contained the Xenopus rhodopsin promoter upstream and Dendra 2 (Clontech Laboratories) downstream of the cloning site (bCNGB1-Dend2). Mutations and deletions of bCNGB1 were generated by standard cloning methods. Human rhodopsin was fused to Dend2 and 1D4 epitope (Rho-Dend2–1D4) as described previously.46
Generation of transgenic Xenopus laevis
All animal experiments were carried out in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and based on the protocol approved by the institutional animal care and use committee at Case Western Reserve University. Transgenic Xenopus laevis expressing bCNGB1-Dend2, and Rho-Dend2-1D4 were generated as previously described9,46 by using the intra-cytoplasmic sperm injection method.47 In all experiments, tadpoles were 12–16 d old (stage range 46–4748). Unless otherwise specified, a minimum of 4 animals were used in each experiment. Albino Xenopus laevis was used for all the procedures.
Immunofluorescence microscopy
For retinal flat mounts, the neural retina was excised from each eye in modified Wolf medium (55% MEM; Invitrogen; 31% Earle's sodium-free BBS, 10% FBS, 30 mM NaHCO3, 700 mg/l D-glucose). Rod OS was ruptured by exposing retinas for 5 min to a hypotonic buffer (2 mM HEPES, 2 mM EGTA, pH 7.4). Rupturing enabled spatial separation of disk membranes from the OS plasma membrane for microscopy observation. Neural retinas were fixed in methanol/DMSO (80:20; v/v) for 15 min at −20°C or in 4% w/v formaldehyde in PBS for 1 h at room temperature and processed for immunofluorescence. Hypotonically-treated retinas were fixed in 4% w/v formaldehyde containing 0.01% w/v glutaraldehyde in PBS for 1 h at room temperature. Fixed retinas were blocked in 1.5% normal goat serum diluted in PBS with 0.1% (v/v) Triton X-100 (PBST) for 1 h, incubated with primary antibody overnight, and then secondary antibody for 1 h at room temperature. Antibodies against GCAP1 (UW83, raised against bovine GCAP149,50), GC1/2 (UW85, raised against GTFRMRHMPEVPVRIRIG peptide) were generous gifts from Dr. Krzysztof Palczewski at Case Western Reserve University. Monoclonal anti-rhodopsin 11D5 was a kind gift from Dr. Dusanka Deretic at University of New Mexico Health Sciences Center.28 Rabbit polyclonal antibody against Dendra2 (Dendra2-138) was raised against the entire peptide sequence of Dend2.46 Donkey anti-rabbit Alexa Fluor 488 (ImmunoResearch Laboratories, Cat# 711-545-152) was used as secondary antibody. For visualizing F-actin, Alexa Fluor 546-conjugated phalloidin (Invitrogen), at a concentration of 22 nM, was incubated with retinas overnight at room temperature in PBS with 0.1% (v/v) Triton X-100.
Confocal microscopy
Living, fixed, or hypotonically-treated retinas were imaged using the HCX PL APO CS 40.0 x 1.25 oil UV objective of a Leica TCS SP2 laser scanning confocal microscope as previously described.9
Images analysis
Intensity profiles for Rho-Dend2-1D4 and endogenous rhodopsin were determined in a single optical section by using ImageJ software. Some x-y panel images of the OS cross-sections were processed by 2D blind deconvolution using AutoDeblur and AutoVisualize 9.3 (MediaCybernetics).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Mr. Richard Lee for excellent technical assistance in the preparation of this paper and for reading the manuscript. We also thank Mr. Christopher M Strauch for reading the manuscript prior to submission and for his helpful suggestions. We would like to thank Dr. Andrew F.X. Goldberg from Oakland University and Dr. Robert Molday from University of British Columbia for full-length bovine CNGB1 cDNA, Dr. Krzysztof Palczewski from Case Western Reserve University for providing GCAP1, GC1/2 polyclonal antibodies and Dr. Dusanka Deretic from University of New Mexico Health Sciences Center for providing 11D5 antibody.
Funding
This work was supported by the US. National Institutes of Health grants EY020826 and EY011373.
References
- 1. Arshavsky VY, Lamb TD, Pugh EN Jr. G proteins and phototransduction. Annu Rev Physiol 2002; 64:153-87; PMID:11826267; http://dx.doi.org/ 10.1146/annurev.physiol.64.082701.102229 [DOI] [PubMed] [Google Scholar]
- 2. Cook NJ, Molday LL, Reid D, Kaupp UB, Molday RS. The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J Biol Chem 1989; 264:6996-9; PMID:2468664 [PubMed] [Google Scholar]
- 3. Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca(2+)-binding proteins in the retina. Trends Neurosci 1996; 19:547-54; PMID:8961484; http://dx.doi.org/ 10.1016/S0166-2236(96)10059-X [DOI] [PubMed] [Google Scholar]
- 4. Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature 1993; 362:855-7; PMID:8386803; http://dx.doi.org/ 10.1038/362855a0 [DOI] [PubMed] [Google Scholar]
- 5. Kawamura S, Hisatomi O, Kayada S, Tokunaga F, Kuo CH. Recoverin has S-modulin activity in frog rods. J Biol Chem 1993; 268:14579-82; PMID:8392055 [PubMed] [Google Scholar]
- 6. Gorczyca WA, Gray-Keller MP, Detwiler PB, Palczewski K. Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc Natl Acad Sci U S A 1994; 91:4014-8; PMID:7909609; http://dx.doi.org/ 10.1073/pnas.91.9.4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palczewski K, Subbaraya I, Gorczyca WA, Helekar BS, Ruiz CC, Ohguro H, Huang J, Zhao X, Crabb JW, Johnson RS, et al. . Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron 1994; 13:395-404; PMID:7520254; http://dx.doi.org/ 10.1016/0896-6273(94)90355-7 [DOI] [PubMed] [Google Scholar]
- 8. Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 1993; 361:76-9; PMID:7678445; http://dx.doi.org/ 10.1038/361076a0 [DOI] [PubMed] [Google Scholar]
- 9. Nemet I, Tian G, Imanishi Y. Submembrane assembly and renewal of rod photoreceptor cGMP-gated channel: insight into the actin-dependent process of outer segment morphogenesis. J Neurosci 2014; 34:8164-74; PMID:24920621; http://dx.doi.org/ 10.1523/JNEUROSCI.1282-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vision Res 2008; 48:2052-61; PMID:18456304; http://dx.doi.org/ 10.1016/j.visres.2008.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nir I, Papermaster DS. Differential distribution of opsin in the plasma membrane of frog photoreceptors: an immunocytochemical study. Invest Ophthalmol Visual Sci 1983; 24:868-78; PMID:6223003 [PubMed] [Google Scholar]
- 12. Makino CL, Wen XH, Michaud NA, Covington HI, DiBenedetto E, Hamm HE, Lem J, Caruso G. Rhodopsin expression level affects rod outer segment morphology and photoresponse kinetics. PLoS One 2012; 7:e37832; PMID:22662234; http://dx.doi.org/ 10.1371/journal.pone.0037832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molday RS, Molday LL. Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J Cell Biol 1987; 105:2589-601; PMID:2447095; http://dx.doi.org/ 10.1083/jcb.105.6.2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 2002; 36:891-6; PMID:12467592; http://dx.doi.org/ 10.1016/S0896-6273(02)01099-1 [DOI] [PubMed] [Google Scholar]
- 15. Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature 2002; 420:193-8; PMID:12432397; http://dx.doi.org/ 10.1038/nature01201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poetsch A, Molday LL, Molday RS. The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem 2001; 276:48009-16; PMID:11641407 [DOI] [PubMed] [Google Scholar]
- 17. Cheng CL, Molday RS. Interaction of 4.1G and cGMP-gated channels in rod photoreceptor outer segments. J Cell Sci 2013; 126:5725-34; PMID:24144699; http://dx.doi.org/ 10.1242/jcs.137679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kizhatil K, Baker SA, Arshavsky VY, Bennett V. Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science 2009; 323:1614-7; PMID:19299621; http://dx.doi.org/ 10.1126/science.1169789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Matteis MA, Morrow JS. The role of ankyrin and spectrin in membrane transport and domain formation. Curr Opin Cell Biol 1998; 10:542-9; PMID:9719877; http://dx.doi.org/ 10.1016/S0955-0674(98)80071-9 [DOI] [PubMed] [Google Scholar]
- 20. Caretta A, Saibil H. Visualization of cyclic nucleotide binding sites in the vertebrate retina by fluorescence microscopy. J Cell Biol 1989; 108:1517-22; PMID:2538481; http://dx.doi.org/ 10.1083/jcb.108.4.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol 1980; 190:501-8; PMID:6771304; http://dx.doi.org/ 10.1002/cne.901900307 [DOI] [PubMed] [Google Scholar]
- 22. Obata S, Usukura J. Morphogenesis of the photoreceptor outer segment during postnatal development in the mouse (BALB/c) retina. Cell Tissue Res 1992; 269:39-48; PMID:1423483; http://dx.doi.org/ 10.1007/BF00384724 [DOI] [PubMed] [Google Scholar]
- 23. Miyaguchi K, Hashimoto PH. Evidence for the transport of opsin in the connecting cilium and basal rod outer segment in rat retina: rapid-freeze, deep-etch and horseradish peroxidase labelling studies. J Neurocytol 1992; 21:449-57; PMID:1383431; http://dx.doi.org/ 10.1007/BF01191508 [DOI] [PubMed] [Google Scholar]
- 24. Chuang JZ, Zhao Y, Sung CH. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell 2007; 130:535-47; PMID:17693260; http://dx.doi.org/ 10.1016/j.cell.2007.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol 2002; 157:103-13; PMID:11916979; http://dx.doi.org/ 10.1083/jcb.200107108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu X, Udovichenko IP, Brown SD, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci 1999; 19:6267-74; PMID:10414956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams DS, Linberg KA, Vaughan DK, Fariss RN, Fisher SK. Disruption of microfilament organization and deregulation of disk membrane morphogenesis by cytochalasin D in rod and cone photoreceptors. J Comp Neurol 1988; 272:161-76; PMID:3397406; http://dx.doi.org/ 10.1002/cne.902720202 [DOI] [PubMed] [Google Scholar]
- 28. Deretic D, Papermaster DS. Polarized sorting of rhodopsin on post-Golgi membranes in frog retinal photoreceptor cells. J Cell Biol 1991; 113:1281-93; PMID:1828467; http://dx.doi.org/ 10.1083/jcb.113.6.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eckmiller MS. Diverse localization of cyclic nucleotide gated channels in the outer segments of rods and cones. Retinal Degenerative Dis Exp Ther: Springer, 1999:449-60; http://dx.doi.org/ 10.1007/978-0-585-33172-0_42 [DOI] [Google Scholar]
- 30. Muradov H, Boyd KK, Haeri M, Kerov V, Knox BE, Artemyev NO. Characterization of human cone phosphodiesterase-6 ectopically expressed in Xenopus laevis rods. J Biol Chem 2009; 284:32662-9; PMID:19801642; http://dx.doi.org/ 10.1074/jbc.M109.049916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang RB, Robinson SW, Xiong WH, Yau KW, Birch DG, Garbers DL. Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J Neurosci 1999; 19:5889-97; PMID:10407028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem 2007; 282:8837-47; PMID:17255100; http://dx.doi.org/ 10.1074/jbc.M610369200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karan S, Tam BM, Moritz OL, Baehr W. Targeting of mouse guanylate cyclase 1 (Gucy2e) to Xenopus laevis rod outer segments. Vision Res 2011; 51:2304-11; PMID:21945483; http://dx.doi.org/ 10.1016/j.visres.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huttl S, Michalakis S, Seeliger M, Luo DG, Acar N, Geiger H, Hudl K, Mader R, Haverkamp S, Moser M, et al. . Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J Neurosci 2005; 25:130-8; PMID:15634774; http://dx.doi.org/ 10.1523/JNEUROSCI.3764-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol 2006; 16:1211-6; PMID:16782012; http://dx.doi.org/ 10.1016/j.cub.2006.04.034 [DOI] [PubMed] [Google Scholar]
- 36. Trudeau MC, Zagotta WN. An intersubunit interaction regulates trafficking of rod cyclic nucleotide-gated channels and is disrupted in an inherited form of blindness. Neuron 2002; 34:197-207; PMID:11970862; http://dx.doi.org/ 10.1016/S0896-6273(02)00647-5 [DOI] [PubMed] [Google Scholar]
- 37. Winkler PA, Ekenstedt KJ, Occelli LM, Frattaroli AV, Bartoe JT, Venta PJ, Petersen-Jones SM. A large animal model for CNGB1 autosomal recessive retinitis pigmentosa. PLoS One 2013; 8:e72229; PMID:23977260; http://dx.doi.org/ 10.1371/journal.pone.0072229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahonen SJ, Arumilli M, Lohi H. A CNGB1 frameshift mutation in Papillon and Phalene dogs with progressive retinal atrophy. PLoS One 2013; 8:e72122; PMID:24015210; http://dx.doi.org/ 10.1371/journal.pone.0072122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci USA 2005; 102:3301-6; PMID:15728366; http://dx.doi.org/ 10.1073/pnas.0500095102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 2006; 119:1383-95; PMID:16537653; http://dx.doi.org/ 10.1242/jcs.02818 [DOI] [PubMed] [Google Scholar]
- 41. Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev 2002; 82:769-824; PMID:12087135 [DOI] [PubMed] [Google Scholar]
- 42. Kondo H, Qin M, Mizota A, Kondo M, Hayashi H, Hayashi K, Oshima K, Tahira T, Hayashi K. A homozygosity-based search for mutations in patients with autosomal recessive retinitis pigmentosa, using microsatellite markers. Invest Ophthalmol Visual Sci 2004; 45:4433-9; PMID:15557452; http://dx.doi.org/ 10.1167/iovs.04-0544 [DOI] [PubMed] [Google Scholar]
- 43. Tam BM, Moritz OL. The role of rhodopsin glycosylation in protein folding, trafficking, and light-sensitive retinal degeneration. J Neurosci 2009; 29:15145-54; PMID:19955366; http://dx.doi.org/ 10.1523/JNEUROSCI.4259-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pearring JN, Arshavsky Vy. Outer segment targeting of the CNG Channel in Xenopus rod photoreceptors. ARVO 2014. abstract. Invest Ophthalmol Vis Sci 2014;55: E-Abstract 2976; PMID:19299621; http://dx.doi.org/ 10.1126/science.1169789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pittler SJ, McKeown AS, Kraft TW, Zhang Y. Rod photoreceptor expression of an N-terminal truncated Cngb1a {beta}-subunit in Cngb1-X1 knockout mice rescues structure and function. Invest Ophthalmol Vis Sci 2012; 53:1627 [Google Scholar]
- 46. Lodowski KH, Lee R, Ropelewski P, Nemet I, Tian G, Imanishi Y. Signals governing the trafficking and mistrafficking of a ciliary GPCR, rhodopsin. J Neurosci 2013; 33:13621-38; PMID:23966685; http://dx.doi.org/ 10.1523/JNEUROSCI.1520-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sparrow DB, Latinkic B, Mohun TJ. A simplified method of generating transgenic Xenopus. Nucleic Acids Res 2000; 28:E12; PMID:10648800; http://dx.doi.org/ 10.1093/nar/28.4.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nieuwkoop PD, Faber J. Normal Table for Xenopus laevis (Daudin), Amsterdam: North-Holland, 282 pp. 1967. [Google Scholar]
- 49. Gorczyca WA, Polans AS, Surgucheva IG, Subbaraya I, Baehr W, Palczewski K. Guanylyl cyclase activating protein. A calcium-sensitive regulator of phototransduction. J Biol Chem 1995; 270:22029-36; PMID:7665624; http://dx.doi.org/ 10.1074/jbc.270.37.22029 [DOI] [PubMed] [Google Scholar]
- 50. Solessio E, Mani SS, Cuenca N, Engbretson GA, Barlow RB, Knox BE. Developmental regulation of calcium-dependent feedback in Xenopus rods. J Gen Physiol 2004; 124:569-85; PMID:15504902; http://dx.doi.org/ 10.1085/jgp.200409162 [DOI] [PMC free article] [PubMed] [Google Scholar]