Abstract
TRPC4 is important regulators of electrical excitability in gastrointestinal myocytes, pancreatic β-cells and neurons. Much is known regarding the assembly and function of these channels including TRPC1 as a homotetramer or a heteromultimer and the roles that their interacting proteins play in controlling these events. Further, they are one of the best-studied targets of G protein-coupled receptors and growth factors in general and Gαi/o and Gαq protein coupled receptor or epidermal growth factor and leptin in particular. However, our understanding of the roles of small G proteins and leptin on TRPC4 channels is still rudimentary. We discuss potential roles for Rasd1 small G protein and leptin in channel activation in addition to their known role in cellular signaling.
Introduction
Canonical transient receptor potential 4 (TRPC4) channels are calcium-permeable, nonselective cation channels that are widely distributed in mammalian cells. TRPC4 channels are activated by Gαq/11-phospholipase C (PLC) pathway or directly activated by Gαi/o proteins.1-3 Rasd1 is a protein that is encoded by the RASD1 gene. It is also known as Dexras1 (Dexamethasone-induced Ras-related protein 1) or Ags1 (Activators of G-protein signaling 1). It belongs to the Ras superfamily of small GTPase.4 Our focus here will be on the roles of small G proteins Rasd1 in the activation of TRPC4 channels. Also, the roles of glucocorticoids and leptin via TRPC4 in insulin secretin will be discussed.
TRPC4 Channels
The TRPC subfamily consists of 7 proteins designated as TRPC1 to 7, which can be further divided into 4 subgroups based on their sequence homology and functional similarities: (1) TRPC1, (2) TRPC4 and TRPC5, (3) TRPC3, TRPC6 and TRPC7, and (4) TRPC2.5,6 The activation of TRPC channels involves Gαq/11 proteins and PLC. Molecules downstream of PLC, such as IP3 and DAG, and PIP2 hydrolysis have been suggested as activators and as an activation mechanism for TRPC channels, respectively.2 TRPC4 channel consist of 6 transmembrane domains, intracellularly located amino and carboxyl termini and a putative pore-forming region.7 Within the TRPC family, TRPC4 and TRPC5 represent a structurally distinct subgroup that is characterized by its ability to form homo- and heteromultimeric channels with each other as well as with TRPC1.8 TRPC4α and TRPC4β are the most abundantly expressed and functionally characterized. TRPC4β channel lacks a domain of 84 amino acids in the C-terminal region containing a putative binding site for calmodulin (CaM) binding and inositol 1, 4, 5-triphosphate (IP3) receptors. The deleted regions of TRPC4 is also responsible for the channel activity by PI(4, 5)P2.7 TRPC4 has the 4 short amino acid sequence motifs (M1-M4) that show typical features of TRP. M1 motif is discovered upstream of TM1 and is conserved in this form throughout the TRPC subfamily. M2 motif located within the cytosolic loop between TM4 and TM5. M3 motif is part of TM6 of all TRPCs, and TRPVs. M4 motif is a highly conserved in this form throughout the TRPC family. This channel contains a coiled-coil domain, 4 ankyrin-like repeats, and predicted multimerization domain in the N-terminus as potential protein-protein interaction motifs.7
TRPC4 is expressed in diverse organs and cell types including the dendrites and soma of various types of neurons, smooth muscles, the cardiovascular system, including endothelial and cardiac cells, skeletal muscle cells, the myometrium, the kidney, and immune cells. TRPC4 channels differ extremely in their permeability and pharmacological modulation, other biophysical properties, and mode of activation depending on the cellular environment. Activation of TRPC4 channels by agonists induced Ca2+ entry directly or indirectly via depolarization and activation of voltage-gated Ca2+ channels. TRPC4 channel was reported that this channel, as well as phospholipase C β1 and β2 interact with the first PDZ domain of NHERF, regulatory factor of the Na+/H+ exchanger.9 The C-terminal PDZ (Dlg, Zo-1, PSD-95) motif of TRPC4 has been implicated in the control of channels surface expression and localization.10 The PDZ deletion motif (T-R-L) of TRPC4 dramatically reduced the plasma membrane levels of the channel, which led the authors to suggest that the interaction between hTRPC4 and NHERF is required for the retention and stabilization of hTRPC4 channels in the cell membrane.10 Caveolin-1 (Cav-1) is associated with dynamic protein complex consisting of TRPC4, TRPC1 and IP3Rs. The loss of Cav-1 blunted the localization of TRPC4 and agonist-stimulated complex formation.11 TRPC4 channel has been proposed to be activated by Gαq/phospholipase C signaling, release of intracellular Ca2+stores, or vesicular translocation to the membrane. Gαq-PLC signaling mediated activation of PLC increased IP3 that binds to the IP3 receptor located on the endoplasmic reticulum and released intracellular Ca2+.12 A conformational change of TRPC channel bound to IP3 receptors, which bind to the C-terminal end of the TRPC channels via the IP3/calmodulin receptor binding domain.12 Among TRP channels, TRPC4 channels are unique in that they activated by trivalent cations Gd3+ and La3+ independently of GPCR. Previously, we reported that Gαi proteins are potent activators of the TRPC4β channel. Gαi2 protein strongly increased TRPC4β current. Mutation and modeling demonstrated that the K715 and R716 regions are important for the interaction between Gαi2 and TRPC4β channel. Thus, we suggest that the regions 700–720 containing the K715 and R716 regions are important for the interaction between Gαi2 and TRPC4β channel. Gβγ subunits are not obligatory for the action of TRPC4β channels.
The diversity of TRPC4 channels interfere with the development of specific agonists or antagonists. Recently, ML204 was identified as a blocker of both recombinant and endogenous TRPC4 channel that lacks activity on most voltage-gated channels and other TRPs.14 Selectivity for block of TRPC4 channels was examined in fluorescent and electrophysiological experiments against closely related TRPC channels and more distantly related TRPV, TRPA and TRPM channels, and against non-TRP ion channels.14 ML204 afforded good selectivity (fold19-) against TRPC6 channels and more modest selectivity against TRPC3 and TRPC5 (fold9-) channels. Little or no block of TRPV, TRPA, TRPM or voltage-gated ion channels was observed. ML204 exhibited properties useful for a variety of in vitro investigations.14 ML204 decreased the increase in intracellular Ca2+ mediated by TRPC4β after activation of μ-opioid receptors.
Recently, receptor-operated or/and store-operated mechanisms contribute to the activation of TRPC4 channels is especially important for the activation mechanism of epidermal growth factor (EGF) on TRPC4. TRPC4 mediated Ca2+ signaling pathway evoked by EGF was identified specifically in sub-confluent, proliferating clusters of human microvascular endothelial cells.15 There are direct/indirect mechanisms that EGFR activates TRPC. Direct mechanism is that 2 tyrosine residues of the C-terminus in human TRPC4, Tyr-959 and Tyr-972 were phosphorylated following EGF receptor (EGFR) stimulation of COS-7 cells.16 This phosphorylation was mediated by Src family tyrosine kinases (STKs), with Fyn appearing to be the dominant kinase. There are indirect mechanisms to modulate TRPC. EGF activates Ras/Raf/Mek/Erk pathway. Erk1/2 in turn activates TRPC4. 17 On the contrary, EGF activates cPLA2/COX-2/Prostaglandin/AC/cAMP/PKA pathway. PKA activation in turn has a negative feedback effect on EGF-induced stimulation of the MAPK cascade at the level of Raf-1 in the ERK limb of this superfamily. PKA activated by accumulation of the cAMP inhibits the Raf-1 activation. 17 We also showed that PKA mediate inhibition of TRPC.18 Similar study regarding leptin and glucose regulation of TRPC4 was recently published.19 In this study, leptin is shown to activate TRPC4 channel and Ca2+ influx through TRPC4 activates AMPK that triggers translocation of ATP-dependent K+ channel (KATP) to the plasma membrane to hyperpolarize the resting membrane potential in pancreatic β-cell. Leptin activates TRPC4 in POMC,20,21,22 and kisseptin-secreting hypothalamic neuron.23 Through POMC, leptin increases energy expenditure without any effect on food intake and decreases insulin secretion. However, the exact mechanism how leptin activate TRPC4 at the molecular level, via tyrosine phosphorylation of TRPC4 like EGF or JAK2-PI3K-PIP3-PLCgamma1 pathway, needs more experiments. It seems that each growth factor activates each TRPC channel. PDGF activates TRPC6,24 BDNF TRPC3,25,26 and VEGF TRPC5.27
The physiological role of these channels was recently established, demonstrating that TRPC4 and TRPC6 are the molecular candidates for non-selective cation channels activated by the muscarinic receptor stimulation (mIcat) in visceral smooth muscle cells. mIcat mediates the physiological action of acetylcholine in evoking smooth muscle contraction.28 Studies in knock-out showed that TRPC4 plays and important role in vascular physiology. TRPC4−/− mice have markedly reduced store- and receptor-induced endothelial Ca2+ entry and impaired endothelium-dependent vasorelaxation. Knockout of TRPC4 strongly reduced acetylcholine-activated non-selective cation currents in visceral smooth muscle cells that are involved in the regulation of gastric motility. Airway tracheal and bronchial smooth muscles express TRPC4 and TRPC5 that are recognized as possible candidates in the pathogenesis of asthma and chronic obstructive pulmonary disease (COPD).5
Recently, single-nucleotide polymorphisms (SNPs) in the TRPC4 gene are associated with human diseases. For example, the TRPC4I957V mutation of SNP in the TRPC4 gene is associated with a reduced risk of myocardial infarction. This mutation leads to an increase in receptor-operated cation currents and Ca2+ entry. This mechanism underlying protection is an improved endothelial function, but this needs more investigation.29 In other study, photoparoxysmal responses (PPR) characterized by abnormal visual sensitivity of the brain to photic stimulation which is generally related to idiopathic generalized epilepsies (IGE) are related to various SNPs in the TRPC4 gene.30 This relation was not significant after corresponding corrections, this trend toward relation of TRPC4 variants and PPR/IGE is of especial interest and deserves further investigation since it could be shown that TRPC4 is involved in numerous functions related with epilepsy.7
Small G Protein Rasd1
Rasd1 was first discovered as a dexamethasone inducible monomeric Ras protein in AtT-20 mouse corticotroph cells in the year 199831 and is expressed at high concentrations in brain and at lower concentrations in heart, liver, kidney, skeletal muscle, pancreas, and placenta.32-34 Expression of Rasd1 is upregulated by steroid hormones—glucocorticoid, dexamethasone, and β-estradiol. In pancreatic β-cell, dexamethasone decreased insulin secretion while mRNA level of Rasd1 was shown to be increased.35 The upregulation of Rasd1 expression by glucocorticoids was due to glucocorticoid response element (GRE) in the 3'- flanking region of the human Rasd1 gene.36 Rasd1 has all of the conserved domains of the Ras superfamily required for guanine nucleotide binding, hydrolysis, and effector interaction. The full-length cDNA of Rasd1 predicts a 280-aminoacid protein with a calculated molecular mass of 31,700Da.31
Rasd1 selectively activates the Gαi/Gαo-protein signaling pathway and appears to act as a guanine nucleotide exchange factor for Gαi, somewhat mimicking GPCR.32,37 Also, it has been reported that Rasd1 may have a dual role in modulating the activation of AC2 (adenylyl cyclase 2) signaling by concurrently blocking PKC (protein kinase C) and Gβγ activity—2 proteins that function as activators of AC2. Rasd1 acts to negatively regulate PKCδ through an isoprenylation-dependent mechanism.38 As Rasd1 can also regulate Gβγ signaling,39-41 it may be that Rasd1 interferes with multiple inputs to AC2 that function in an additive or synergistic manner for maximal AC2 activity.
Although their physiological functions remain to be fully elucidated, Rasd1 has been implicated in the photic and nonphotic responsiveness of the circadian clock,42 in tonic inhibitor of atrial natriuretic factor (ANF) secretion and a modulator of hormone secretion in volume overload condition of heart by inhibiting protein regulation of ANF release.43 Also Rasd1 has a function to regulate neurotransmitter mediated behaviors44 and cancers.45
The functional relationship between TRPC4 and small GTPase Rasd1
In previous paper, we reported that Gαi proteins are potent activators of mTRPC4β channels.1,13 Also, Rasd1 is functionally classified as a guanine nucleotide exchange factor (GEF) for Gαi proteins46 and facilitates guanosine diphosphate (GDP) to GTP exchange of Gαi proteins and triggers it to be functionally active until intrinsic GTPase activity of Gαi protein turns off the signal by hydrolyzing GTP to GDP. Therefore, since the function of Rasd1 as a GEF of Gαi may resemble the activators of Gαi proteins, we expressed Rasd1 and mTRPC4β in HEK293 cells and detected the effect of Rasd1 on the activity of mTRPC4β. Rasd1S33V, Rasd1G81A and Rasd1A178V (a constitutively active form of Rasd1) induced large mTRPC4β current. In contrast, expressing dominant-negative form of Rasd1, Rasd1G31V, did not activate mTRPC4β current. In addition, to exclude the possibility that Rasd1 activates other TRP channels such as mTRPC4α, hTRPC5, or mTRPC6 since amino acid sequence homology shows more than 40% similarity among those channels,47 we tested whether Rasd1 activates these channels. However, Rasd1 could not activate mTRPC4α and mTRPC6 channels and reduced hTRPC5 currents. Also, we tested various Ras proteins other than Rasd1 to check the specificity of mTRPC4β to Rasd1. Among various Ras proteins, Rasd1 was the only Ras protein to increase mTRPC4β. Therefore, mTRPC4β is the unique channel activated by Rasd1 and Rasd1 is a unique TRP channel activator among various Ras proteins in Ras family.
The sequence homology analysis demonstrate that Rasd1 is most closely related to members of the Ras superfamily of SMWG proteins, with 55% amino acid homology to Rap2B, 36% homology to Rit1, and 50% homology to the prototypical Ras protein, H-Ras.48,49. Recently, several nucleotide sequences predicting proteins with high degrees of homology to human Rasd1 (Genebank No. AF172846) have been reported, including mouse Rasd1 (Genebank No. AF009246), and Rat Rasd1(Genebank No.BC099136), which share 98% homology with human Rasd1. Human Rasd1 is located at chromosome 2q42. The most closely associated homologs to Rasd1 are human Rasd2 (Genebank No. BC013419), and mouse Rasd2 (Genebank No. BC026377), which share 62% homology with Rasd1 (Fig. 1). Ras superfamily proteins such as Rasd1, Rasd2, Rit1, H-Ras and Rap2B have highly conserved GTP binding pocket (Σ1–Σ4) domains and an effector loop which participates in protein-protein interactions with other signaling molecules and is necessary for full biological activity.48,50,51,52. The presumed structure of the Rasd1 contains several characteristic Ras superfamily motifs including the phosphate/magnesium binding regions GXXXXGK(S/T) (P-loop) ( Σ1), DXXG (Σ2), and the guanine base binding loops NKXD (Σ3) and EXSAK (Σ4).51,52,54 The motif regions G-1 to G-3 which are characteristic of GTPases are present in Rasd1.51,52. The C terminus possesses a typical CAAX motif,51,52,54 an important biochemical feature of a Ras superfamily. CAAX motif undergoes enzymatic posttranslational farnesylayion or prenylation, which related to its subcellular localization by promoting the translocation of the Rasd1 to the plasma membrane.51,54. We tested whether Rasd2 and Rit1 can activate TRPC4 without any activator like rasd1 (Fig. 2). Since Rasd1 proteins were tagged with cyan fluorescent protein (pECFP) proteins at the C-terminus, pECFP was transfected as a negative control for Rasd1. As shown in Fig. 2B, pECFP alone did not affect mTRPC4β current (1.39 ± 0.24 pA/pF, n = 20). Rasd2S40V, a constitutively active form of Rasd2, induced large mTRPC4β current (Fig. 2; 11.1 ± 2.23 pA/pF; n = 10). A mutation of serine at position 40 to valine (S40V) made Rasd2 unable to hydrolyse GTP to GDP, hence holding it constitutively active. This constitutively active Rasd2S40V induced strong morphological change.55 Rit1Q79L, a constitutively active form of Rit1, induced large mTRPC4β current (Fig. 2; 9.86 ± 4.82 pA/pF; n = 7). A mutation of glutamine at position 79 to leucine (Q79L) made Rit1 unable to hydrolyse GTP to GDP, hence holding it constitutively active. This constitutively active Rit1Q79L induced strong morphological change.55.
Figure 1.
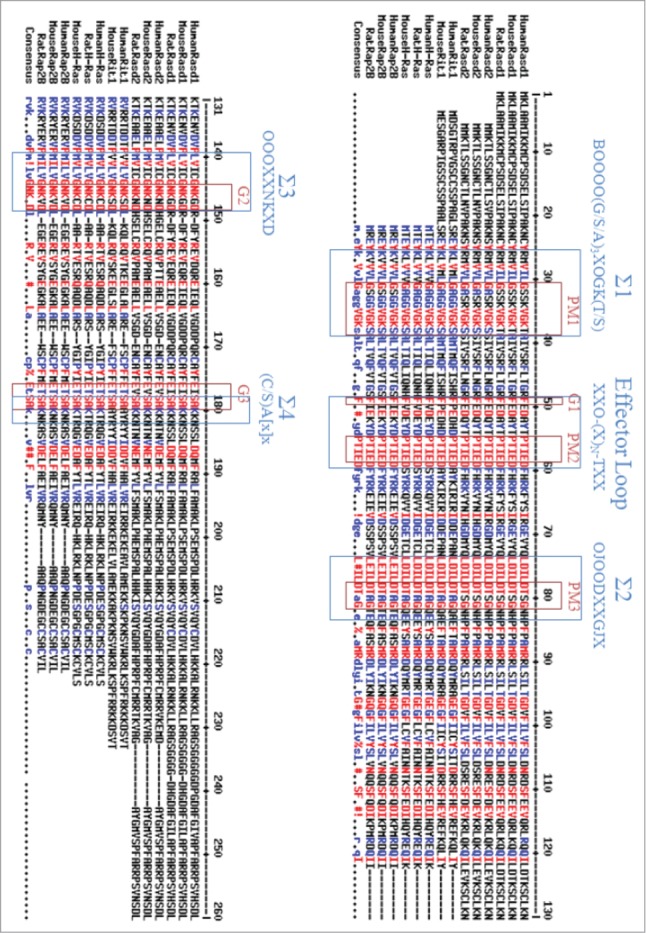
Sequence alignment of several forms of Rasd1, Rasd2, and Rit1 proteins. The following sequences were aligned by using multiple sequence alignment by Florence Corpet: human Rasd1 (Genebank No. AF172846), mouse Rasd1 (Genebank No. AF009246), rat Rasd1 (Genebank No.BC099136), human Rasd2 (Genebank No. BC013419), mouse Rasd2 (Genebank No. BC026377), rat Rasd2 (Genebank No.AF134409), human Rit1 (Genebank No.U71203), mouse Rit1 (Genebank No.U712505), human H-Ras (Genebank No. AF493916), mouse H-Ras (Genebank No. AY373386), rat H-Ras (Genebank No.M13011), human Rap2B (Genebank No. AF493915), mouse Rap2B (Genebank No. BC032168), and rat Rap2B (Genebank No.AF386786). Regions defining the GTP-binding and hydrolysis domain (Σ1-Σ4) are boxed and annotated with their identifying consensus sequences. Consensus abbreviations: B, basic residue; J, polar residue; O, hydrophobic residue; X, any residue. The three conserved GDP/GTP binding motifs (G1-G3), and phosphate-magnesium binding motifs (PM1-PM3) are boxed. The red colors below the sequence indicate amino acid identity, whereas the blue color indicates similarity.
Figure 2.
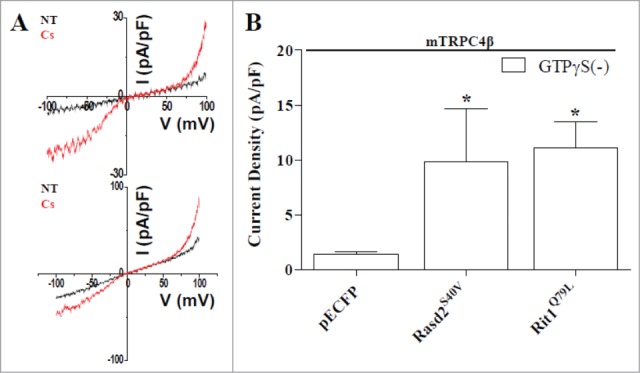
Rasd2 and Rit1 activates TRPC4 channel. (A) All panels indicate I-V relationship of currents measured from HEK293 cells expressing mouse TRPC4β-ECFP channel and constitutively active Rasd2S40V or Rit1Q79L proteins. We studied TRPC4 channel activity with Cs+-rich extracellular solution (Cs) since TRPC4 has greater permeability to Cs+ than Na+. Rasd2S40V and Rit1Q79L proteins activated TRPC4 channel without GTPγS. (B) Summarized current density measured above. Rasd2S40V or Rit1Q79L proteins induced TRPC4 current increase. The comparison between pECFP and Rasd2S40V or Rit1Q79L was carried out with Student's t-test. Statistical significance was denoted by an asterisk (*) at P< 0.05.
Next, to characterize the activation mechanism of Gαi proteins between Rasd1 and mTRPC4β channels, we used pertussis toxin (PTX). PTX has been widely used as a reagent to characterize the involvement of heterotrimeric G proteins in signaling. This toxin catalyzes the ADP ribosylation of α subunits of Gαi family, and this modification prevents the occurrence of the receptor-G protein interaction.28 Because the population of functional Gαi is strongly reduced by PTX, we examined whether PTX antagonizes the action of Rasd1. Rasd1-activated mTRPC4β currents from Rasd1S33V-expressing cells at −60 mV were significantly reduced when PTX was pretreated. However, when GTPγS was added to internal solution as a positive control for the activation, PTX could not reduce GTPγS activated mTRPC4β currents from Rasd1S33V-expressing cells. From these results, we reasoned that Rasd1 requires certain population of functional Gαi proteins to activate mTRPC4β channels. Muscarinic acetylcholine receptor type 2 (M2R), for example, is strong mTRPC4β channel activator, and functional Gαi proteins are quintessential for its action onto mTRPC4β channels. In a sense, if Rasd1 and M2R both exist, the competitive action for functional Gαi protein would be expected. Therefore, we expressed both Rasd1 and M2R in HEK293 cells and tested how the mTRPC4β channel current results. Without Rasd1, M2R strongly activated mTRPC4β channels in response to extracellular carbachol (100 μM). When Rasd1 was co-expressed, however, M2R-activated mTRPC4β current was significantly reduced which clearly demonstrates competitive action between Rasd1 and M2R. Therefore, activation signaling of Rasd1 to mTRPC4β involves Gαi protein, and certain population of functional Gαi protein is essential for activation of mTRPC4β by Rasd1.
There are several types of Gαi proteins in HEK293 cells in which the level of expression decreases following order Gαi2 > Gαi3 > Gαi1.56 To verify the subtype of Gαi proteins, first, we tested the effect of the subtype of wild-type (WT) Gαi proteins on mTRPC4β currents. WT Gαi2 or Gαi3 protein itself increased the mTRPC4β currents in HEK293 cells without activator GTPγS. However, Gαi1 did not increase mTRPC4β currents without Rasd1S33V. mTRPC4β currents were increased by activating endogenous Gαi proteins in HEK cells via expressed Rasd1S33V. Therefore, we co-expressed mTRPC4β channel, Rasd1S33V, and WT Gαi protein subtypes to test further effect of expressed WT Gαi protein subtypes based on the endogenous Gαi protein subtypes in HEK cells on mTRPC4β currents by Rasd1S33V. mTRPC4β currents by Rasd1S33V with Gαi1 protein, Gαi2 protein or Gαi3 protein showed no difference compared with that by Rasd1S33V protein alone, or expression of WT Gαi2 or Gαi3proteins alone. Because Rasd1 fully activated endogenous Gαi proteins in HEK cells, mTRPCβ currents may not be further increased by co-expressing Gαi proteins with Rasd1S33V. However, Rasd1S33V-activated mTRPC4β currents were increased dramatically by co-expressing Gαi1 protein with Rasd1S33Vcompared with that by expression of WT Gαi1 protein alone. These result suggested that either Rasd1 activates Gαi1 protein more potently or Rasd1 is expressed at the low level in HEK cells. Next, we tested the effect of dominant negative Gαi proteins on Rasd1S33V-activated mTRPC4β currents. Dominant negative Gαi1G202T and Gαi3G202T significantly reduced Rasd1-activated mTRPC4β currents, but Gαi2G203T did not affect. Therefore, Gαi1 and Gαi3 proteins are crucial for activation of mTRPC4β by Rasd1 with more potent effect on Gαi2 protein.
Since Rasd1 is GEF of Gαi proteins, it removes GDP from α subunit and changes GDP to GTP. This exchange dislocates α subunit from betagamma subunits (Gβγ). Although classical pathway of heterotrimeric G protein describes α subunit mostly, the release of Gβγ subunit is equally important. Therefore, we tested the involvement of Gβγ subunits in Rasd1 activation of mTRPC4β. When Gβ1 and Gγ2 proteins or Gβ1 and Gγ7 proteins were co-expressed with mTRPC4β channels, neither of them was able to activate mTRPC4β channels. Also, co-expression of β-adrenergic receptor kinase (βARK) that scavenges free Gβγ proteins did not reduce Rasd1-activated mTRPC4β currents. Furthermore, GβW99A (holds G proteins as a heterotrimer55 making G proteins to be insensitive to activating signaling) and GβI80A (keeps Gβγ dimers in free form55) mutant did not induce significant difference in Rasd1 activation of mTRPC4β. From these results, Gβγ signaling can be excluded from possible signaling candidates of Rasd1. In previous study, we experimented about the relation between various small G protein (Ras, Rho, Rab proteins) and TRPC4 ion channel. As results, we found that Rasd1 activated the largest TRPC4 ion channels. In addition, both Rasd2 and Rit also activated TRPC4 with a lesser degree. It is known that Rasd2 can bind Gβγ and Gαi and Rit can also bind Gαo.57 Since TRPC4 can be activated by Gαi and Gαo, TRPC4 could activated by not only Rasd1 and Rasd2 acting Gαi but also Rit acting on Gαo. Taken together, Rasd1 selectively activated TRPC4 channels, and it was the only Ras protein among Ras protein family that can activate TRPC4 channels. For this to occur, it was found that certain population of functional Gαi1 and Gαi3 proteins are essential.
Together, the results indicate that activation of Gαi subunits by Rasd1 is the primary mechanism for activating TRPC4. This raised the question of whether activation of the channels requires direct interaction of Rasd1 with the Gαi subunits. We tested whether Rasd1 and Gαi1 interacts directly (Fig. 3A). To characterize the association between Rasd1 with Gαi1 in vivo, HEK cells were transfected with Rasd1-CFP and Gαi1, and their association was analyzed by co-immunoprecipitation. Immunoprecipitation of Gαi pulled down Rasd1-CFP (Fig. 3A). We also used FRET method (Fig. 3B). Key prerequisites for our experiments of interaction of Rasd1 and G proteins were strategic attachment of CFP or YFP to G protein. To study G protein subunit interactions in cell, CFP or YFP were inserted into the αBC-loop within the α-helical domain of the Gαi, a domain that has been used previously to insert various sequences into Gα-subunits.58 Gαi2(WT)-YFP was well targeted to the plasma membrane. Rasd1-CFP showed the greatest FRET efficiency with Gαi2(WT)-YFP (Fig. 3B). There was no significant FRET of Rasd1 with TRPC4, TRPC5, or Gβγ. Whether Gαi1(WT)-YFP or Gαi3(WT)-YFP has more FRET efficiency than Gαi2(WT)-YFP or not needs further studies.
Figure 3.
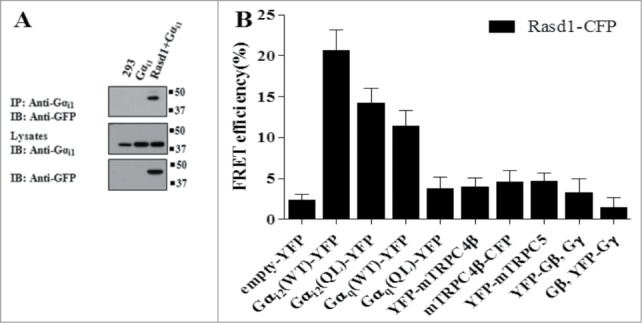
Gαi and TRPC4β interact with Rasd1. Rasd1-CFP and Gαi1 was co-transfected in HEK293 cells. Immunoprecipitation was performed with Gαi1followed by immunoblotting with GFP antibody. Total proteins of Rasd1-CFP and Gαi1 were detected using GFP and Gαi1 antibody. (B) FRET efficiency was measured in HEK293 cells Rasd1-CFP, Gαi2 WT-YFP, Gαi2 QL-YFP, Gq WT-YFP, Gq QL-YFP, YFP-mTRPC4β, mTRPC4β-CFP, YFP-mTRPC5, YFP-GβGγ, and GβYFP-Gγ. Among these proteins, Rasd1 bound Gαi2 WT, Gαi2 QL, and Gq WT. QL: Constitutively active form Q205L to impair GTPase activity of Gαi2.
The roles of glucocorticoids and leptin via TRPC4 in insulin secretion
Dexamethasone (a synthetic glucocorticoid) is a widely used immunosuppressant and insulin secretion regulator. In a previous research, Kemppainen et al.31 suggested that dexamethasone increased the expression of Rasd1 mRNA in murine AtT-20 corticotroph cells. Therefore, we experimented whether dexamethasone increases mTRPC4β current by activating Rasd1. mTRPC4β current was significantly increased with dexamethasone. With these results on hands, we tested whether Rasd1 activation by dexamethasone is sufficient to generate TRPC4-like current in INS-1 cells, since both Rasd1 and TRPC4 are detectable in pancreatic β-cells.
100 nM dexamethasone was sufficient to generate cationic current in INS-1 cells, and the I-V curve resembled that of TRPC4, suggesting that dexamethasone activates TRPC4 in INS-1 cells. Also, inhibition of Rasd1 with small interfering RNA (siRNA) blunted dexamethasone-induced TRPC4 current in INS-1 cells, indicating that dexamethasone activates TRPC4 via Rasd1 in INS-1 cells. Though Rasd1 is functionally classified as a guanine nucleotide exchange factor (GEF) for Gαi proteins, Lellis-Santos et al.35 suggested that dexamethasone induced a rapid and sustained increase in Rasd1 mRNA expression in MIN6 cells (3.5- and fold15- compared with control after 24 and 48 h of dexamethasone treatment). We also showed that dexamethasone increased protein expression level of Rasd1. Therefore, we think that dexamethasone triggers TRPC4-like cationic current in INS-1 cells at least via increasing protein expression level of Rasd1. Whether dexamethasone enhances the GEF activity of Rasd1 or not needs more experiments. The finding of TRPC4 current in pancreatic β-cell in response to dexamethasone was interesting since similar study regarding leptin and glucose regulation of TRPC4 was recently published.19 Leptin was first discovered in 1994 by Zang et al.59 and it is the most important and widely studied hormones in the control of energy balance.60 Leptin is a 16 kDa protein mostly secreted from adipose tissue which has a critical role regulating body weight and energy homeostasis.61,62 Leptin signals via the leptin receptors (LepRs), which exists as several isoforms. These receptors are expressed in the brain and in peripheral tissues.63 In the hypothalamus leptin acts as an anorexigenic hormone regulating the melanocortine/neuropeptide Y system to reduce food intake, increase energy expenditure, and decrease body weight.64 However, circulating leptin levels are increased in obese humans,65 suggesting that obesity may be either a result or a cause of leptin resistance.66-68 Leptin is an important regulator of the immune system. Leptin-deficient and leptin receptor-deficient mice exhibit thymic atrophy and are immunodeficient.69 Leptin is also considered a proinflammatory adipokine because of the actions it exerts in several cells of the immune system, including monocytes/macrophages, dendritic cells, neutrophils, eosinophils, basophils, natural killer cells, and lymphocytes.70-73 Qiu et al.74 suggested that a Janus 2 tyrosine kinase (Jak 2)-dependent pathway via stimulation of PI3 kinase and PLCgamma1 activated TRPC channels. Therefore, we think that leptin through a Jak2–PI3 kinase–PLCgamma pathway may activate TRPC4β channels in INS-1 cells. In this study, leptin is shown to activate TRPC4 channel and Ca2+ influx through TRPC4 activates AMPK that triggers translocation of ATP-dependent K+ channel (KATP) to the plasma membrane to hyperpolarize the resting membrane potential in pancreatic β-cell. Another study reported that dexamethasone increased the protein expression of Rasd1 in pancreatic β-cells and diminished insulin secretion.35 These studies indicated that Rasd1 and TRPC4 could be common molecular candidates in controlling insulin secretion in β-cells (Fig. 4).
Figure 4.
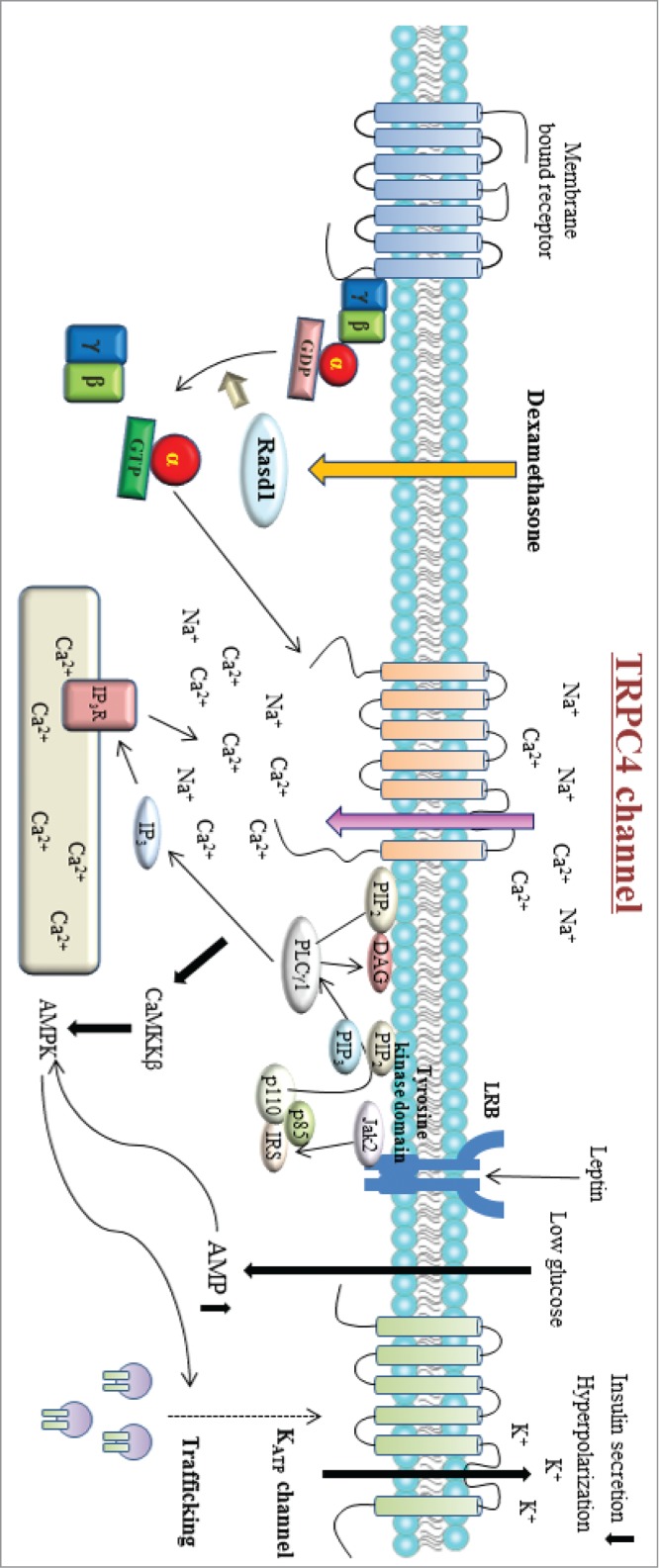
Schematic diagram for the signaling pathway involved in dexamethasone-induced TRPC4 channel activity. Rasd1 is classified as a guanine nucleotide exchange factor (GEF) for Gαi proteins. Dexamethasone enhanced Rasd1 that promotes GDP to GTP exchange in Gαi proteins and triggers it to be functionally active until the intrinsic GTPase activity of Gαi proteins turns off the signal by hydrolyzing GTP to GDP. Gαi-GTP protein activates TRPC4β channel. Leptin binds to its receptor (LRB) to activate Jak2, which phosphorylates IRS proteins and activates PI3 kinase. PI3 kinase activates PLCγ1 to increase TRPC4 channel activity.
Summary
We showed 1) that Rasd1 small G protein activates TRPC4 channel via GEF for Gαi proteins. Two) Related small G proteins Rasd2 and Rit1 also activated TRPC4 channels. Three) Rasd1 interacts with Gαi protein rather than TRPC4 or Gβγ. Four) In pancreatic β cell line, INS-1, Rasd1 induced TRPC4-like current.
In the previous studies, we reported that Gαi/o proteins are essential for the activation of TRPC4.1,2,13. Related to Gαi protein, Rasd1 and homologs also activated TRPC4 by inducing GTP exchange for Gαi proteins.75 Interestingly, PTX did not inhibit Rasd1-induced current in the presence of intracellular GTPγS.75 In the beginning, we thought that Gαi proteins are really essential for the activation of TRPC4. However, we could not obtain the clear evidence showing the mutant responding to GTPγS but not Gαi proteins. Any mutant that did not respond to Gαi2 protein also did not respond to GTPγS.1 On the contrary, α-spectrin domain (730–758 amino acids) deleting mutant showed the response to Gαi protein but not to GTPγS, suggesting that Gαi protein-dependent pathway might be different from more general activation pathways by GTPγS including Gαi protein pathways. We need TRPC4 mutants which respond to GTPγS but not to Gαi proteins for clear distinction between Gαi protein pathway and GTPγS dependent pathway or to find other activation pathways independent from Gαi protein pathways. It will also be important to test whether TRPC4 currents can be recorded simultaneously with intracellular calcium concentration to see the relationship of TRPC4 with calcium itself. Finally, as was done for GIRK, we should study further the binding site for intracellular calcium itself, the effect of polyamine and Mg2+ on TRPC4 in the inside-out patch mode, the roles of PtdIns(4,5)P2 and PKC in the desensitization and the role of G proteins in channel assembly and trafficking in addition to their known role in cellular signaling.
Clinically, the effect of glucocorticoid is important in care of hospitalized diabetes mellitus (DM) patients.76 It is known that long-term prescription of dexamethasone could lead to insulin resistance and decrease in insulin secretion.76 If the patient has been treated with dexamethasone as an anti-inflammatory drug or immunosuppressant, diabetes mellitus as his/her underlying disease could occur as a paradoxical effect of the drug. The relationship among Rasd1, TRPC4, and insulin secretion might suggest new therapeutic agent for this particular clinical situation. However, the exact mechanism how leptin activates TRPC4 at the molecular level, via tyrosine phosphorylation of TRPC4 like EGF or JAK2-PI3K-PIP3-PLCgamma1 pathway, needs more experiments.
It is well known that insulin secretion from pancreatic β-cells is increased by parasympathetic nervous system. When we tried to activate TRPC4 channel with carbachol, however, carbachol did not activate TRPC4 current in INS-1 cells (Fig. 5) although muscarinic M3 receptor is expressed in pancreatic β-cells.77 There must be some missing links between muscarinic stimulation and insulin secretion. Miguel et al.78 showed that acetylcholine (ACh) acts on different receptor subtypes producing both a stimulatory (M1, M3) and an inhibitory (M2, M4) action on insulin release. Gautam et al.78 also showed that mutant mice selectively lacking the M3 muscarinic acetylcholine receptor subtype in pancreatic β-cells display impaired glucose tolerance and greatly reduced insulin release. In contrast, transgenic mice selectively overexpressing M3 receptors in pancreatic β-cells show a profound increase in glucose tolerance and insulin release. Moreover, these mutant mice are resistant to diet-induced glucose intolerance and hyperglycemia. Gautam et al.79 suggested that β-cell M3 muscarinic receptors play a key role in maintaining proper insulin release and glucose homeostasis. On the other hand, Miguel et al.80 showed an important functional cooperation between the cholinergic neurotransmitter ACh and the incretin hormone GLP-1 on insulin secretion mediated through the M3 muscarinic receptor subtype. However, the insulinotropic action of ACh was associated with a paradoxical inhibitory effect on GLP-1 stimulated cAMP production, achieved through a novel PTX- and pirenzepine-sensitive M1 muscarinic receptor activated pathway. These results suggest that intracellular Ca2+ and cAMP are important for the insulinotropic action of ACh. Considering the role of TRPC4 in M2 and M3 muscarinic receptor signaling, there is still the possibility for TRPC4 to be involved in the insulinotropic action of ACh in native pancreatic β-cells.
Figure 5.

Carbachol did not activate TRPC4-like currents in INS-1 cells. One hundred micromolars of carbachol induced TRPC4-like current in INS-1 cells measured by perforated patch clamp configuration. Under carbachol stimulation, TRPC4-like current did not activated by muscarinic receptors. Black arrow heads (Cs) and red arrow heads (Cs + CCh) indicate the time points where corresponding I-V curves were obtained.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIP) (#2014R1A5A2009936, 2010–0019472 and 2012R1A2A1A01003073). J.W., J. M., and K. H. were supported by the graduate program of BK Plus Program from the Korea government (MSIP).
References
- 1.Jeon JP, Lee KP, Park EJ, Sung TS, Kim BJ, Jeon JH, So I. The specific activator of TRPC4 by Gi protein subtype. Biochem Biophys Res Commun 2008; 377:538-543; PMID:18854172; http://dx.doi.org/ 10.1016/j.bbrc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Jeon JP, Roh SE, Wie J, Kim J, Kim H, Lee KP, Yang D, Jeon JH, Cho NH, Kim IG, et al.. Activation of TRPC4β by Gαi subunit increases Ca2+ selectivity and controls neurite morphogenesis in cultured hippocampal neuron. Cell Calcium 2013; 54(4):307-319; PMID:24011658; http://dx.doi.org/ 10.1016/j.ceca.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Jeon JP, Hong C, Kim J, Myeong J, Jeon JH, So I. An essential role of PtdIns(4,5)P2 for maintain the activity of the transient receptor potential canonical (TRPC)4β. Pflugers Arch Eur J Physiol 2013; 465:1011-21; PMID: 23417604; http://dx.doi.org/ 10.1007/s00424-013-1236-x. [DOI] [PubMed] [Google Scholar]
- 4.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, et al.. Genes expressed in human tumor endothelium. Science 2000; 289:1197-1202; PMID:10947988; http://dx.doi.org/ 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 5.Nilius B, Owasinik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 2007; 87:165-217; PMID:17237345; http://dx.doi.org/ 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 6.Ong SA, Tan JJ, Tew WL, Chen KS. (2011) Rasd1 modulates the coactivator function of NonO in the cyclic AMP pathway. PLoS One 2011; 6(9):e24401; PMID: 21915321; http://dx.doi.org/ 10.1371/journal.pone.0024401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilius B, Flockerzi V. Mammalian Transient Receptor Potential (TRP) Cation Channels. 2014; 222 Springer. [PubMed] [Google Scholar]
- 8.Miehe S, Bieberstein A, Arnould I, Ihdene O, Rutten H, Strubing C. The Phospholipid-binding Protein SESTD1 Is a Novel Regulator of the Transient Receptor Potential Channels TRPC4 and TRPC5. J Biol Chem 2010; 285:12426-12434; PMID:20164195; http://dx.doi.org/ 10.1074/jbc.M109.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu MX. Association of Mammalian Trp4 and Phospholipase C Isozymes with a PDZ Domain-containing Protein, NHERF. J Biol Chem 2000; 275:37559-37564; PMID:10980202; http://dx.doi.org/ 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- 10.Mery L, Strauss B, Dufour JF, Krause KH, Hoth M. The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci 2002; 155:3497-3508; PMID:12154080. [DOI] [PubMed] [Google Scholar]
- 11.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic Evidence Supporting Caveolae Microdomain Regulation of Calcium Entry in Endothelial Cells. J Biol Chem 2007; 282:16631-16643; PMID:17416589; http://dx.doi.org/ 10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 12.Fowler Ma, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic Expression of TRPC4 and TRPC5 Channels in the Rodent Brain. Plos One 2007; 2(6):e573; PMID:17593972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon JP, Hong C, Park EJ, Jeon JH, Cho NH, Kim IG, Choe H, Muallem S, Kim HJ, So I. Selective Gαi subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J Biol Chem 2012; 287:17029-17039; PMID:22457348; http://dx.doi.org/ 10.1074/jbc.M111.326553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, Wu M, Xu J, Long S, Yang P, et al.. Identification of ML204, a Novel potent Antagonist That Selectively Modulates Native TRPC4/C5 channels. J Biol Chem 2011; 286:33436-46; PMID:21795696; http://dx.doi.org/ 10.1074/jbc.M111.274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graziani A, Poteser M, Heupel WM, Schleifer H, Krenn M, Drenckhahn D, Romanin C, Baumgartner W, Groschner K. Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: evidence for a regulatory TRPC4-β-catenin interaction. J Biol Chem 2010; 285:4213-23; PMID:19996314; http://dx.doi.org/ 10.1074/jbc.M109.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odell AF, Scott JL, Van Helden DF. Epidermal growth factor induces tyrosine phosphorylation, membrane insertion, and activation of transient receptor poten tial channel 4. J Biol Chem 2005; 280:37974-87; PMID:16144838; http://dx.doi.org/ 10.1074/jbc.M503646200. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Sun X, Wang Z, Ning G, Zhang F, Kong J, Lu L, Reinach P.S. EGF stimulates Growth by Enhancing Capacitative Calcium Entry in Corneal Epithelial Cell. J Membrane Biol 2003; 194:47-58; PMID:14502442; http://dx.doi.org/ 10.1007/s00232-003-2025-9. [DOI] [PubMed] [Google Scholar]
- 18.Sung TS, Jeon JP, Kim BJ, Hong C, Kim SY, Kim J, Jeon JH, Kim HJ, Suh CK, Kin SJ, et al.. Molecular determinants of PKA-dependent inhibition of TRPC5 channel. Am J Physiol Cell physiol 2011; 301:823-832; PMID:21734191; http://dx.doi.org/ 10.1152/ajpcell.00351.2010. [DOI] [PubMed] [Google Scholar]
- 19.Park SH, Ryu SY, Yu WJ, Han YE, Ji YS, Oh K, Sohn JW, Lim A, Jeon JP, Lee H, et al.. Leptin promotes K(ATP) channel trafficking by AMPK signaling in pancreatic β-cells. Proc Natl Acad Sci 2013; 110:12673-78; PMID:23858470; http://dx.doi.org/ 10.1073/pnas.1216351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn JW, Williams KW. Functional heterogeneity of arcuate nucleus pro-opiomelanocortin neurons: implications for diverging melanocortin pathways. Mol Neurobiol. 2012; 45(2):225-33; PMID:22328135; http://dx.doi.org/ 10.1007/s12035-012-8240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013; 152(3):612-9; PMID:23374353; http://dx.doi.org/ 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn JW, Elmquist JK, Williams KW. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013; 36(9):504-12; PMID:23790727; http://dx.doi.org/ 10.1016/j.tins.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology. 2011; 152(4):1503-14; PMID:21285322; http://dx.dio.org/ 10.1210/en.2010-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003; 284(2):C316-30; PMID:12529250; http://dx.doi.org/ 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- 25.Mizoguchi Y, Kato TA, Seki Y, Ohgidani M, Sagata N, Horikawa H, Yamauchi Y, Sato-Kasai M, Hayakawa K, Inoue R, et.al. Brain-derived neurotrophic factor (BDNF) induces sustained intracellular Ca2+ elevation through the up-regulation of surface transient receptor potential 3 (TRPC3) channels in rodent microglia. J Biol Chem. 2014; 289(26):18549-55; PMID:24811179; http://dx.doi.org/ 10.1074/jbc.M114.555334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vohra PK, Thompson MA, Sathish V, Kiel A, Jerde C, Pabelick CM, Singh BB, Prakash YS. TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle. Biochim Biophys Acta. 2013; 1833(12):2953-60; PMID:23899746; http://dx.doi.org/ 10.1016/j.bbamcr.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Pan Q, Meng H, Jiang Y, Mao A, Wang T, Hua D, Yao X, Jin J, Ma X. Enhancement of vascular endothelial growth factor release in long-term drug-treated breast cancer via transient receptor potential channel 5-Ca2+-hypoxia-inducible factor 1α pathway. Pharmacol Res. 2015; 93C:36-42; http://dx.doi.org/ 10.1016/j.phrs.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Tsai SC, Adamik R, Kanaho Y, Hewlett EL, Moss J. Effects of guanyl nucleotides and rhodopsin on ADP-ribosylation of the inhibitory GTP-binding component of adenylate cyclase by pertussis toxin. J Biol Chem 1984; 259:15320-15323; PMID:6439719. [PubMed] [Google Scholar]
- 29.Jung C, Gene GG, Tomas M, Plata C, Selent J, Pastor M, et al.. A gain-of-function SNP in TRPC4 cation channel protects against myocardial infarction. Cardiovasc Res 2011; 91:465-471; PMID:21427121; http://dx.doi.org/ 10.1093/cvr/cvr083. [DOI] [PubMed] [Google Scholar]
- 30.von Spiczak S, Muhle H, Helbiq I, de Kovel CG, Hamper J, Gaus V, et al.. Association study of TRPC4 as a candidate gene for generalized epilepsy with photosensitivity. Neuromolecular Med 2010; 12(3):292-9; PMID:20574736; http://dx.doi.org/ 10.1007/s12017-010-8122-x. [DOI] [PubMed] [Google Scholar]
- 31.Kemppainen RJ, Behrend EN. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem 1998; 273:3129-31; PMID:9452419; http://dx.doi.org/ 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- 32.Agretti P, de Marco G, Pinchera A, Vitti P, Bernal J, Tonacchera M. Ras homolog enriched in striatum inhibits the functional activity of wild type thyrotropin, follicle-stimulating hormone, luteinizing hormone receptors and activating thyrotropin receptor mutations by altering their expression in COS-7 cells. J Endocrinol Invest 2007; 30:279-284; PMID:17556863; http://dx.doi.org/ 10.1007/BF03346294. [DOI] [PubMed] [Google Scholar]
- 33.Bernal J, Crespo P. Analysis of Rhes activation state and effector function. Methods Enzymol 2005; 407:535-542; PMID:16757351. [DOI] [PubMed] [Google Scholar]
- 34.Tu Y, Wu C. Cloning, expression and characterization of a novel human Ras-related protein that is regulated by glucocorticoid hormone. Biochim Biophys Acta 1999; 1489:452-456; PMID:10673050; http://dx.doi.org/ 10.1016/S0167-4781(99)00197-9. [DOI] [PubMed] [Google Scholar]
- 35.Lellis-Santos C, Sakamoto LH, Bromati CR, Nogueira TC, Leite AR, Yamanaka TS, Kinote A, Anhê GF, Bordin S. The regulation of Rasd1 expression by glucocorticoids and prolactin controls peripartum maternal insulin secretion. Endocrinology 2012; 153:3668-78; PMID:22700767; http://dx.doi.org/ 10.1210/en.2012-1135. [DOI] [PubMed] [Google Scholar]
- 36.Kemppainen RJ, Cox E, Behrend EN, Brogan MD, Ammons JM. Identification of a glucocorticoid response element in the 3'-flanking region of the human Dexras1 gene. Biochim Biophys Acta 2003; 1627:85-89; PMID:12818426; http://dx.doi.org/ 10.1016/S0167-4781(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 37.Vargiu P, Morte B, Manzano J, Perez J, de Abajo R, Gregor Sutcliffe J, Bernal J. Thyroid hormone regulation of rhes, a novel Ras homolog gene expressed in the striatum. Brain Res Mol Brain Res 2001; 94:1-8; PMID:11597759. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen CH, Watts VJ. Dexamethasone-induced Ras protein 1 negatively regulates protein kinase C δ: implications for adenylyl cyclase 2 signaling. Mol Pharmacol 2006; 69:1763-71; PMID:16489124; http://dx.doi.org/ 10.1124/mol.105.019133. [DOI] [PubMed] [Google Scholar]
- 39.Graham TE, Prossnitz ER, Dorin RI. Dexras1/AGS-1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinases. J Biol Chem 2002; 277:10876-10882; PMID:11751935; http://dx.doi.org/ 10.1074/jbc.M110397200. [DOI] [PubMed] [Google Scholar]
- 40.Takesono A, Nowak MW, Cismowski M, Duzic E, Lanier SM. Activator of G-protein signaling 1 blocks GIRK channel activation by a G-protein-coupled receptor: apparent disruption of receptor signaling complexes. J Biol Chem 2002; 277:13827-13830; PMID:11842095; http://dx.doi.org/ 10.1074/jbc.M201064200. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen CH, Watts VJ. Dexras1 blocks receptor mediated heterologous sensitization of adenylyl cyclase 1. Biochem Biophys Res Commun 2005; 332:913-920; PMID:15913563; http://dx.doi.org/ 10.1016/j.bbrc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 42.Cheng HY, Obrietan K. Dexras1: shaping the responsiveness of the circadian clock. Semin Cell Dev Biol 2006; 17:345-351; PMID:16765612; http://dx.doi.org/ 10.1016/j.semcdb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 43.McGrath MF, Ogawa T, de Bold AJ. Ras dexamethasone-induced protein 1 is a modulator of hormone secretion in the volume overloaded heart. Am J Physiol Heart Circ Physiol 2012; 302:H1826-37; PMID:22408026; http://dx.doi.org/ 10.1152/ajpheart.01085.2011. [DOI] [PubMed] [Google Scholar]
- 44.Harrison LM, He Y. Rhes and AGS1/Dexras1 affect signaling by dopamine D1 receptors through adenylyl cyclase. J Neurosci Res 2011; 89:874-882; PMID:21374700; http://dx.doi.org/ 10.1002/jnr.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaidyanathan G, Cismowski MJ, Wang G, Vincent TS, Brown KD, Lanier SM. The Ras-related proteinAGS1/RASD1 suppresses cell growth. Oncogene 2004; 23:5858-5863; PMID:15184869; http://dx.doi.org/ 10.1038/sj.onc.1207774. [DOI] [PubMed] [Google Scholar]
- 46.Sato M, Blumer JB, Simon V, Lanier SM. Accessory proteins for G proteins: partners in signaling. Annu Rev Pharmacol Toxicol 2006; 46:157-1587; PMID:16402902; http://dx.doi.org/ 10.1016/j.yrtph.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Lee YM, Kim BJ, Kim HJ, Yang DK, Zhu MH, Lee KP, So I, Kim KW. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol Gastrointest Liver Physiol 2003; 284:G604-616; PMID:12631560; http://dx.doi.org/ 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- 48.Graham TE, Key TA, Kilpatrick K, Dorin RI. Dexras1/AGS-1, a steroid hormone-induced guanosine triphosphate-binding protein, inhibits 3',5'-cyclic adenosine monophosphate-stimulated secretion in AtT-20 corticotroph cells. 2001; 142(6):2631-40; PMID:11356714. [DOI] [PubMed] [Google Scholar]
- 49.Colicelli J. Human Ras superfamily proteins and related GTPases. 2004; Sci STKE 2004(250):RE13; PMID:15367757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature 1990; 348(6297):125-132; PMID:2122258; http://dx.doi.org/ 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 51.Thapliyal A, Verma R, Kumar N. Small G Proteins Dexras1 and RHES and Their Role in Pathophysiological Processes. Int J Cell Biol 2014; PMID:24817889; http://dx.doi.org/doi: 10.1155/2014/308535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature 1991; 349(6305):177-127. [DOI] [PubMed] [Google Scholar]
- 53.Rojas AM, Fuentes G, Rausell A, Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J cell Biol 2012; 196(2):189-201; PMID:22270915; http://dx.doi.org/ 10.1083/jcb.201103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valencia A, Chardin P, Wittinghofer A, Sander C. The ras Protein Family Evolutionary Tree and Role of Conserved Amino Acids. Biochemistry 1991; 30(19):4637-4648; PMID:2029511. [DOI] [PubMed] [Google Scholar]
- 55.Heo WD, Tobias M. Switch-of-function mutants based on morphology classification of ras superfamily small GTPases. Cell 2003; 113:315-328; PMID:12732140; http://dx.doi.org/ 10.1016/S0092-8674(03)00315-5. [DOI] [PubMed] [Google Scholar]
- 56.Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as reveled by microarray analysis. BMC Genomics 2011; 12:1474-2164; PMID:21214938; http://dx.doi.org/ 10.1186/1471-2164-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vargiu P, De Abajo R, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P Bernal J. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene 2004;23:559-568; PMID:14724584; http://dx.doi.org/ 10.1038/sj.onc.1207161. [DOI] [PubMed] [Google Scholar]
- 58.Myeong J, Kwak M, Jeon JP, Hong C, Jeon JH, So I. Close Spatio-Association of Transient Receptor Potential Canonical (TRPC)4 channel with Gαi in TRPC4 activation process. Am J Physiol Cell Physiol. 2015. In press; PMID:25788576; http://dx.doi.org/ 10.1152/ajpcell.00374.2014. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425-432; PMID:7984236. [DOI] [PubMed] [Google Scholar]
- 60.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763-770; PMID:9796811; http://dx.doi.org/ 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 61.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995;269:540-543; PMID:7624776; http://dx.doi.org/ 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 62.Rosenbaum M, Leibel RL. Twenty years of leptin: role of leptin in energy homeostasis in humans, J Endocrinol 2014;223:T83-T96; PMID:25063755; http://dx.doi.org/ 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications, Ann Intern Med 2010;152:93-100; PMID:20083828; http://dx.doi.org/ 10.7326/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res 2004;59:267-285; PMID:14749506; http://dx.doi.org/ 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 65.Jéquier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci 2002;967:379-388; PMID:12079865. [DOI] [PubMed] [Google Scholar]
- 66.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292-5; PMID:8532024; http://dx.doi.org/ 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 67.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1995;1:1311-4; PMID:7489415; http://dx.doi.org/ 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 68.Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG, Xu AW. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes 2010;59:894-906; PMID:20068134; http://dx.doi.org/ 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest 1999;104:1051-59; PMID:10525043; http://dx.doi.org/ 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moraes-Vieira PMM, Bassi EJ, Araujo RC, Câmara NO, Leptin as a link between the immune system and kidney-related diseases: leading actor or just a coadjuvant?. Obes Rev 2012;13:733-743; PMID:22498577; http://dx.doi.org/ 10.1111/j.1467-789X.2012.00997.x. [DOI] [PubMed] [Google Scholar]
- 71.Ahima RS. Adipose tissue as an endocrine organ. Obesity 2006;14:242S-9S; PMID:17021375. [DOI] [PubMed] [Google Scholar]
- 72.Moraes-Vieira PM, Larocca RA, Bassi EJ, Peron JP, Andrade-Oliveira V, Wasinski F, Araujo R, Thornley T, Quintana FJ, Basso AS et al.. Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells,” Eur J Immunol 2014;44:794-806; PMID:24271843; http://dx.doi.org/ 10.1002/eji.201343592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772-783; PMID:16998510; http://dx.doi.org/ 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 74.Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560-1565; PMID:20107083; http://dx.doi.org/ 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wie J, Kim J, Ha K, Zhang YH, Jeon JH, So I. Dexamethasone activates transient receptor potential canonical 4 (TRPC4) channels via Rasd1 small GTPase pathway. Pflugers Arch. 2014 Dec 14. [Epub ahead of print]:2550:2319. [DOI] [PubMed] [Google Scholar]
- 76.Powers AC. Harrison's principles of internal medicine 17th edn. McGrawHill 2008; 338:2275-2304. [Google Scholar]
- 77.Gilon P, Henquin JC. Mechanisms and physiological significance of cholinergic control of pancreatic beta-cell function. Endocr Rev 2001; 22(5): 565-604; PMID:11588141. [DOI] [PubMed] [Google Scholar]
- 78.Miguel JC, Abdel-Wahab YH, Mathias PC, Paulo C.F. Mathias, Peter R. Flatt. Muscarinic receptor subtypes mediate stimulatory and paradoxical inhibitory effects on an insulin-secreting beta cell line. Biochim Biophys Acta, 2002, 1569(1-3): 45-50; PMID:11853956; http://dx.doi.org/ 10.1016/S0304-4165(01)00232-X. [DOI] [PubMed] [Google Scholar]
- 79.Gautam D, Han SJ, Hamdan FF, Jongrye Jeon, Bo Li, Jian Hua Li, Yinghong Cui, David Mears, Huiyan Lu, Chuxia Deng, et al.. A critical role for b cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab, 2006, 3(6): 449-61. [DOI] [PubMed] [Google Scholar]
- 80.Miguel JC, Abdel-Wahab YH, Green BD, Paulo CF. Mathias Peter R. Flatt. . Cooperative enhancement of insulinotropic action of GLP-1 by acetylcholine uncovers paradoxical inhibitory effect of β cell muscarinic receptor activation on adenylate cyclase activity Biochem Pharmacol, 2003, 65(2):283-92; PMID:12504804. [DOI] [PubMed] [Google Scholar]


