Abstract
Recently, we reported the isolation of the Kv3.4 current in dorsal root ganglion (DRG) neurons and described dysregulation of this current in a spinal cord injury (SCI) model of chronic pain. These studies strongly suggest that rat Kv3.4 channels are major regulators of excitability in DRG neurons from pups and adult females, where they help determine action potential (AP) repolarization and spiking properties. Here, we characterized the Kv3.4 current in rat DRG neurons from adult males and show that it transfers 40–70% of the total repolarizing charge during the AP across all ages and sexes. Following SCI, we also found remodeling of the repolarizing currents during the AP. In the light of these studies, homomeric Kv3.4 channels expressed in DRG nociceptors are emerging novel targets that may help develop new approaches to treat neuropathic pain.
Keywords: action potential, Kv3.4, pain, potassium channel, spinal cord injury
Introduction
Kv3.4 channels have been historically difficult to isolate in neurons.1–6 In dorsal root ganglion (DRG) nociceptors, we showed that Kv3.4 currents can be easily isolated and that Kv3.4 channels are dysregulated following spinal cord injury (SCI).7,8 Previous to this work, Kv3.4 was detected in DRG neurons via immunohistochemistry, western blot and RT-PCR,9-11 but there was no compelling isolation of the corresponding K+ current. In the whole-cell configuration, a subtraction protocol revealed a Kv3.4-like current in small-diameter nociceptors.5 To isolate the Kv3.4 current more conclusively, we used the cell-attached configuration on DRG neurons from rat pups and a 1-s conditioning pulse to −30 mV. This strategy was sufficient to consistently observe high voltage-activated outward currents with a characteristic A-type profile (>80% of patches).7 Furthermore, we used pharmacological tools, single-cell qPCR and siRNA to establish that this current was mediated by Kv3.4 channels.7 The siRNA experiments also demonstrated action potential (AP) broadening suggesting that the Kv3.4 current regulates AP repolarization and duration. Subsequently, we also determined the expression of Kv3.4 channels in adult female rats using electrophysiology, single-cell qPCR and immunohistochemistry.8
The Kv3.4 N-terminal inactivation domain (NTID) underlies fast inactivation of the corresponding currents.12-14 Early reports showed modulation of fast Kv3.4 channel inactivation by oxidation, phosphorylation and PIP2 in heterologous expression systems.15-18 Demonstrating that inactivation modulation also occurs in neurons, we showed that PKC activation dramatically slows inactivation of the native Kv3.4 channel expressed in DRG nociceptors.7 Under physiological conditions, however, Kv3.4 channel inactivation is not modulated by PIP2.19,20 Revealing the neurophysiological impact of inactivation modulation by PKC, we also found that NTID phosphorylation by PKC shortened the AP duration in nociceptors, and Kv3.4 channel knockdown by siRNA eliminated this change.7
Implicating the Kv3.4 channel in pain pathology, several studies have found reduced expression of this K+ channel in various models of persistent neuropathic pain.8,10,11 Chien et al. supported this link further by demonstrating that intrathecal injection of anti-sense Kv3.4 oligonucleotides induces mechanical hypersensitivity in rats.11 Following SCI, we showed that Kv3.4 currents in DRG nociceptors are downregulated and additionally exhibit phenotype switching from fast inactivating A-type to delayed rectifier-type.8 Kv3.4 downregulation resulted from a decrease in surface expression as demonstrated by immunostaining followed by confocal microscopy. DRG nociceptors from SCI rats also displayed hyperexcitability associated with shortening the interspike interval (ISI), which was reversed in real-time by injection of computer-generated Kv3.4 currents via dynamic clamping.8 Overall, our studies suggest that the Kv3.4 conductance may play a dual role in DRG neurons7,8: (1) regulating action potential repolarization in a manner that depends on phosphorylation of the Kv3.4 NTID; and (2) helping regulate the ISI. Impairing the latter role as a result of downregulating Kv3.4 channels might contribute to the hyperexcitability that underlies persistent pain following SCI.
Here, we extended our studies to DRG neurons from adult male rats to investigate gender differences and continue to assess the functions of Kv3.4 channels by applying AP clamp protocols and dynamic clamping. Additionally, we investigated how Kv3.4 channel dysregulation in SCI might remodel AP homeostasis.
Results
Ubiquitous expression of Kv3.4 channels in rat nociceptors
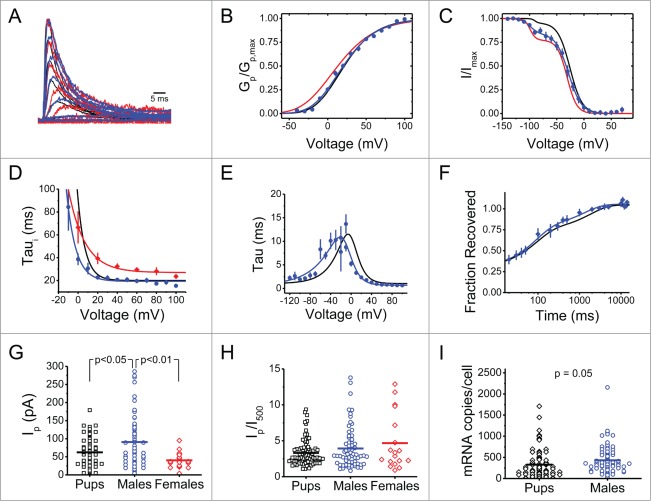
Kv3.4 channels are highly expressed in small-diameter DRG neurons from rat pups and adult female rats. If Kv3.4 channels are key players in an essential defense mechanism such as pain signaling, and are generally implicated in chronic pain, they must be similarly expressed in adult male rats. Accordingly, small-diameter DRG neurons from adult male rats express a robust high voltage-activated A-type K+ current with biophysical properties that closely match those from pups and adult females (Fig. 1 and Table 1). Interestingly, however, the peak Kv3.4 current from male rat DRG neurons is almost 2-fold larger than the equivalent current from pups and adult females (Fig. 1G). Supporting this finding further, adult male DRG neurons also express increased levels of the Kv3.4 mRNA transcript at the single-cell level (Fig. 1I). Nevertheless, the ratio of peak (IP) over sustained current at 500 ms (I500) did not change (Fig. 1H), suggesting a general increase in outward currents in the adult male DRG neuron. Another potentially interesting gender difference is apparent when comparing the time constants of inactivation, which are modestly longer in DRG neurons from female rats (Fig. 1D). Altogether, the Kv3.4 channel is robustly expressed in DRG nociceptors throughout development and its biophysical properties are generally similar between males and females.
Figure 1.
Age- and gender-independent expression of Kv3.4 channels in small-diameter rat DRG neurons. (A) Normalized Kv3.4 currents from adult males (blue), pups (black) and females (red). Currents elicited from a 1 s conditioning pulse to −30 mV followed by step depolarizations from −80 to +100 mV. (B, C) Normalized peak conductance-voltage relation (Gp/Gp,max) and steady-state inactivation curve (I/Imax). The solid lines represent the best-fit Boltzmann functions. The steady-state inactivation curve from males had a non-inactivating component comprising 17% of the total amplitude, which was manually subtracted. Note that the curve has 2 distinct components. The best-fit parameters of the hyperpolarized component are V1/2 = −99 mV, k = 8 mV, and amplitude = 15%. (D–E) Time constants of activation (Taua), deactivation (Taud), and inactivation (Taui) with best fit exponential functions as follows: Taua,d = (0.13eV/13.0 + 0.04e−V/39.3)−1 + 0.66; Taui = 22.3eV/9.4 + 19.3; and Taui = 40.2eV/16.8 + 27.0 for male Taua,d, male Taui, and female Taui, respectively. (F) Recovery from inactivation at −100 mV. The solid lines are best-fit double exponential functions. For males, the fast time constant associated with low voltage-activated A-type currents is 87 ± 15 ms and represents 48% of the total amplitude, and the slow time constant associated with Kv3.4 channels represents 27% of the total current. (G) Scatter plot of Ip. Mean Ip is 91 ± 6 (N = 68) for adult males. (H) Scatter plot of Ip/I500. Mean Ip/I500 values are 3.3 ± 0.2 for pups (N = 96), 3.9 ± 0.4 for adult males (N = 66) and 4.7 ± 0.9 for adult females (N = 18). (I) Scatter plot of Kv3.4 mRNA copies/neuron in DRG neurons that express Kv3.4 mRNA. Kv3.4 copies/neuron are 328 ± 36 in pups (N = 82) and 431 ± 36 in adult males (N = 81). For all panels, data from pups and adult females were previously published in Ritter et al 2012 and 2015, respectively. 7,8 Some error bars are occluded by the symbols. Mean best-fit parameters are summarized in Table 1. P values are from Bonferroni corrected t tests if there are multiple comparisons and Student's t test for single comparisons.
Table 1.
Biophysical Properties of Kv3.4 channels in nociceptors. All parameters were calculated as previously described. 7,8 For z, the subscripts indicate the parameter from which the z was calculated: Gp – peak chord conductance relation; ssi–steady state inactivation curve; Taua – time constant of activation; Taud – time constant of deactivation; Taui – time constant of inactivation. Taur is the time constant of recovery from inactivation at −100 mV.
| Pupsa |
Adult Femalesb |
Adult Males |
|||||
|---|---|---|---|---|---|---|---|
| Activation | N | N | N | ||||
| V1/2 | 21.6 ± 2.0 mV | 79 | 16.8 ± 4.8 mV | 18 | 20.5 ± 2.6 mV | 39 | |
| Vs | −20.9 ± 1.5 mV | 79 | −25.5 ± 3.3 mV | 18 | −24.5 ± 1.9 mV | 39 | |
| zGp | 1.00 ± 0.04 e0 | 79 | 1.01 ± 0.09 e0 | 18 | 0.95 ± 0.04 e0 | 39 | |
| zTaua | 1.97 ± 0.45 e0 | 69 | 1.97 ± 0.64 e0 | 32 | |||
| zTaud | 1.00 ± 0.26 e0 | 12 | 0.65 ± 0.16 e0 | 7 | |||
| Inactivation | |||||||
| V1/2 | −25.7 ± 0.1 mV | 39 | −30.1 ± 1.6 mV | 18 | −28.1 ± 0.9 mV | 21 | |
| zssi | 2.30 ± 0.12 e0 | 39 | 3.40 ± 0.58 e0 | 18 | 2.16 ± 0.12 e0 | 21 | |
| Taur | 1.8 ± 0.4 s | 15 | 1.3 ± 0.4 s | 13 | |||
| zTaui | 3.31 ± 0.31 e0 | 76 | 1.51 ± 0.07 e0 | 17 | 2.70 ± 0.31 e0 | 34 | |
Data from Ritter et al.7
Data from Ritter et al.8
The Kv3.4 current drives action potential repolarization in DRG nociceptors
To elucidate how Kv3.4 channels help shape AP repolarization, we applied AP-clamping using a stereotypical nociceptor AP waveform as a voltage protocol to elicit outward K+ currents in the cell-attached configuration (Fig. 2A). Delivering the AP waveform from a holding voltage of −100 mV evoked the total outward current. Then, to isolate the high voltage-activated component of the current, a 1-s conditioning pulse to −30 mV preceded the AP waveform (to inactivate low voltage-activated A-type K+ currents; Figure 2A, top black trace). The high voltage component of the total outward current, corresponding to Kv3.4 channels, represents 67 ± 19% (N = 8) in rat pups (Fig. 2A&B). Establishing that the Kv3.4 channel contributes to this current, Kv3.4 siRNA inhibited ˜50% of the high voltage component (Fig. 2B). In adult male rats, the high voltage component amounts to 37 ± 11% (N = 12; Fig. 2C&D). To effectively drive AP repolarization, the high voltage-activated Kv3.4 current quickly reaches its maximum amplitude after the peak of the AP (Fig. 2A&C). It is also notable that the current passes its reversal potential and relaxes toward the zero current level as it deactivates during the afterhyperpolarization. Altogether, we can conclude that the Kv3.4 current in small-diameter DRG neurons shapes AP repolarization by contributing 40-70% of the total repolarizing charge transfer.
Figure 2.
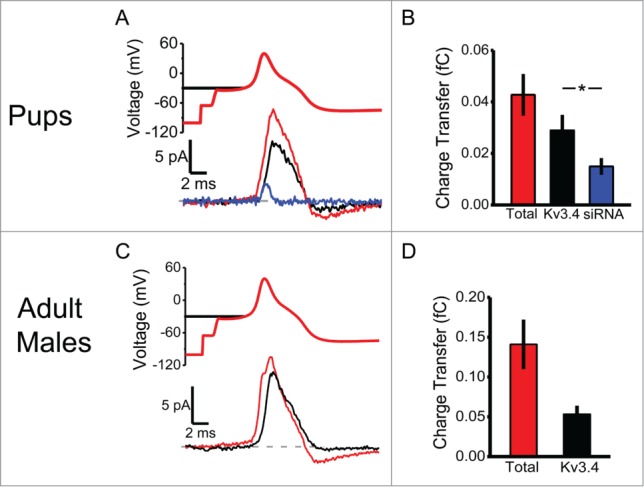
The Kv3.4 current transfers a significant fraction of the repolarizing charge during the AP in DRG neurons. (A) AP-clamp stimulus waveform (above) and the evoked currents (below). From the same neuron, either the AP waveform was preceded by a 1-s conditioning to −100 mV (total K+ current, red) or a 1-s conditioning pulse to −30 mV (Kv3.4 current, black). Representative recording from a separate neuron transfected with Kv3.4 siRNA and elicited from a 1-s conditioning pulse to −30 mV (blue). The data are from 7-day old pups. Dashed line represents 0 pA. (B) Total charge transfer during the AP (in fC) is 0.043 ± 0.008 at −100 mV and 0.029 ± 0.006 at −30 mV in control neurons and 0.015 ± 0.003 at −30 mV in Kv3.4 siRNA transfected neurons (N = 8 for all groups). (C, D) Same as in A&B but from adult male DRG neurons. Total charge transfer during the AP (in fC) is 0.141 ± 0.031 from −100 mV and 0.053 ± 0.011 from −30 mV (N = 12). Asterisks indicate P < 0.05 from a Student's t test.
Synthetic Kv3.4 currents modulate AP repolarization under dynamic-clamp conditions
The data presented so far strongly implicate Kv3.4 channels in the repolarization of the AP in DRG neurons. Thus, specific manipulations of this current should namely affect AP repolarization in a predictable manner. To test this idea, we conducted a dynamic-clamp experiment by injecting a computer-generated Kv3.4 current into a small-diameter DRG neuron. To generate the synthetic Kv3.4 current, we created a Markov model that both quantitatively and globally recapitulates the kinetic and voltage-dependent properties of the native Kv3.4 channel in DRG neurons (Fig. 3A-E).14 As expected, real-time injection of the best-fit synthetic Kv3.4 currents (“overexpression”) mainly shortens AP duration (Fig. 3F, blue trace). Conversely, real-time subtraction of the native Kv3.4 current by injection of a negative Kv3.4 synthetic current broadens the AP (Fig. 3F, red trace). The latter is consistent with the biological counterpart of this experiment, which shows a similar result following specific siRNA knockdown of the native Kv3.4 channels.7 The AP changes observed under dynamic-clamp conditions resulted primarily from speeding up or slowing down the initial repolarization phase of the AP (Fig. 3G). This is in agreement with AP-clamp experiments showing that Kv3.4 currents are primarily active during the repolarization phase of the action potential (Fig. 2). Overall, the results conclusively establish Kv3.4 channels as active key determinants of AP repolarization in small-diameter DRG neurons.
Figure 3.
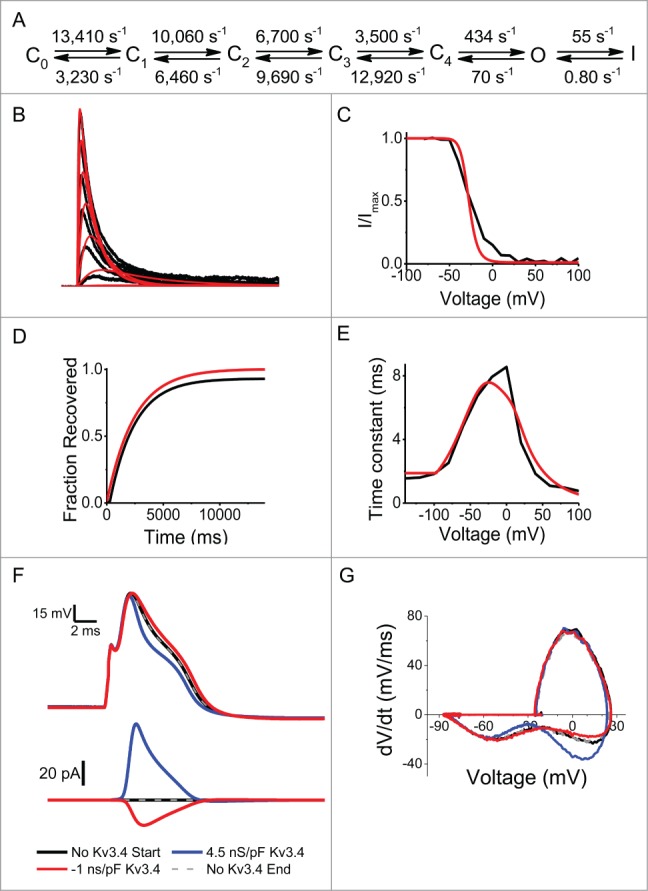
The Kv3.4 current regulates AP repolarization in DRG neurons under dynamic-clamping conditions. (A) Markov gating model of the fast-inactivating Kv3.4 channel with rate constants at V = 0 mV. For voltage dependence of the rate constants see Fineberg et al. 14 (B–E) Best global fit (red traces) to a family of Kv3.4 currents (B), mean steady state inactivation (C), mean recovery from inactivation at −100 mV (D) and mean Taua and Taud (E). For the steady-state inactivation curve, the sustained current and the low voltage-activated K+ current were manually subtracted. (F) Representative APs (top) and the corresponding Kv3.4 currents (below) during dynamic clamping of a DRG neuron. The APs were evoked by a 0.5-ms current injection stimulus. The injected Kv3.4 currents had a fast inactivating A-type phenotype. Dynamic clamping injections of the synthetic Kv3.4 current occurred in the following order: no Kv3.4 (start, black), 4.5 ns/pF (blue), −1 ns/pF (red), no Kv3.4 (end, gray dashed). The same time scale applies to voltage and current. (G) Phase plane plots corresponding to the APs in panel F. Note effects on the rate of AP repolarization.
SCI remodels the outward currents that shape the AP in DRG nociceptors
Following SCI, whereas Kv3.4 channel surface expression is reduced and its inactivation rate is slowed, the AP shape and duration undergo no significant change.8 To investigate the basis of the unexpected absence of consistent AP changes, we conducted AP-clamp experiments in adult female rats from 3 groups: (1) naïve (no surgical procedure); (2) 2-weeks post laminectomy; and (3) 2-weeks post SCI groups. We hypothesized that the high-voltage outward charge transfer evoked by the AP waveform should correlate with the peak amplitude of corresponding A-type Kv3.4 currents evoked by a step depolarization. Following SCI, however, modulated currents (slow inactivating or non-inactivating) may deviate from this relationship. Accordingly, we found correlation between peak A-type current and charge transfer in neurons from naïve animals (Fig. 4, black symbols, Pearson Correlation coefficient 0.79, P = 0.002, N = 12). Similarly, recordings from the laminectomy group yielded a correlation that agrees with that from the naïve group (Fig. 4, blue symbols). Data from the SCI group split into 3 categories (Fig. 4A, red traces). A small fraction followed the control correlation (SCI1), while many recordings clustered as small high voltage-activating currents that transfer little charge during the AP (SCI2). A third category of recordings deviated from the control correlation (SCI3), mainly showing that peak currents were associated with larger charge transfer during the AP as compared to controls (Fig. 4B). Inspection of the distinct currents in the SCI group revealed heterogeneity resulting from slow inactivation, greater sustained current and activation at more negative voltages. Particularly, currents that exhibited no inactivation (SCI3) were consistently outside the control correlation and displayed leftward shifted chord conductance-voltage relations (Fig. 4C&D). This change could have contributed to a larger overall charge transfer during the AP.
Figure 4.
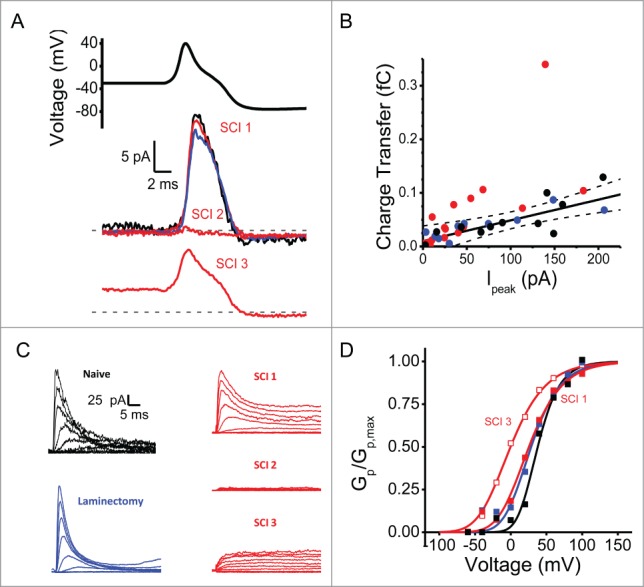
Heterogeneity of voltage-gated K+ currents following SCI. (A) AP-clamp stimulus and representative currents from naïve (black), laminectomy (blue), and SCI (red) groups. (B) Relationship between Ipeak at +100 mV and charge transfer during AP-clamping. Solid black line is the best-fit linear regression (y = 0.010+(3.9*10−5)×; R2 = 0.58). The dashed lines mark the 95% confidence limits. (C) Outward current families from the same patches used for AP-clamping in panel A. Currents were elicited as in Figure 1A. These currents are replotted from Ritter et al. 8 Note the heterogeneous distribution of current phenotypes in the SCI group (SCI1, SCI2 and SCI3). (D) Normalized peak chord conductance-voltage relations from recordings in C (except SCI2, which exhibited negligible outward currents). On average the SCI currents deviating from the correlation had a V1/2 = −22 ± 4 mV (N = 5).
Discussion
The results from voltage-, AP- and dynamic-clamping experiments help establish the Kv3.4 current as a major K+ current that provides substantial repolarizing charge transfer during the downstroke of the AP in small-diameter DRG neurons from pups and adult rats. Following SCI, we also showed that K+ current heterogeneity partly resulting from modulation of Kv3.4 channel inactivation kinetics and expression might help shape of the AP under pathological conditions.
The Kv3.4 channel underlies a dominant voltage-gated K+ current in nociceptors
Gold et al. first showed a Kv3.4-like current in small-diameter, capsaicin sensitive DRG neurons.5 Others also found Kv3.4 immunoreactivity and mRNA expression in these neurons.9-11,21 We isolated the rat A-type Kv3.4 current and determined its molecular identity.7 Kv1.4, Kv4.1 and Kv4.3 channels also underlie A-type K+ currents in rat DRG.10,11,22-25 However, in contrast to Kv3.4 currents, these currents are low voltage-activated and are generally eliminated in our recordings by a conditioning pulse to −30 mV.5,22 Evidence of their presence is apparent in the steady-state inactivation curve, which typically exhibits 2 components: a small hyperpolarized component corresponding to the low voltage-activated A-type currents, and a dominant depolarized component (>80%) corresponding to the Kv3.4 current (Fig. 1C). Additionally, immunohistochemistry suggests that Kv1.4 and Kv4.× channels are expressed in subsets of nociceptors, whereas Kv3.4 channels are almost always expressed in small-diameter neurons.8,11,22,23,25
Gold et al. additionally recorded high voltage-activated delayed rectifying outward currents in almost every DRG neuron.5 These currents resemble those mediated by Kv3.1 and Kv3.2 channels. However, we did not find Kv3.1 and Kv3.2 mRNA transcripts.7 Accordingly, the Ip/I500 ratio is consistently >2 in cell-attached macropatches (Fig. 1H), showing that the high voltage-activated current is generally inactivating. In cultured mouse DRG neurons, others reported Kv3.1, Kv3.2 and Kv3.3 channel immunostaining and currents, but did not investigate Kv3.4 channels.26 We isolated an A-type Kv3.4-like current in acutely dissociated mouse DRG neurons, suggesting that Kv3.4 channel expression is conserved in rodents and possibly other mammals (Ritter and Covarrubias, unpublished). To explain why we consistently observe the A-type phenotype of the Kv3.4 channel, we propose that the recording configuration may influence the channel's inactivation properties. Others generally used the whole-cell configuration,5,25,26 whereas we have always recorded from cell-attached patches. This is potentially significant because, in addition to phosphorylation, oxidation also eliminates Kv3.4 N-type inactivation.16,17 If the neuron is dialyzed with an oxidizing or PKC activating intracellular solution in the whole-cell configuration, Kv3.4 channels might switch from A-type to delayed rectifier-type. Further experimentation is, however, necessary to support this hypothesis.
A homomeric Kv3.4 channel is the most plausible molecular correlate of the high voltage-activated A-type K+ current in cell-attached patches from small-diameter DRG neurons because it exhibits kinetic, voltage-dependent and modulatory properties that are closely matched by those of heterologously expressed Kv3.4 channels.7,8,12-14,17,18 Moreover, Kv3.4 siRNA nearly ablates the high voltage-activated K+ current in small-diameter DRG neurons.7 Although fast-inactivating Kv3.4 subunits and slow-inactivating Kv3.× subunits could form heteromultimers with intermediate properties,4,27-29 we did not observe the expected intermediate slowing of macroscopic inactivation in DRG nociceptors under normal conditions (Fig. 1A&D). These findings can have significant implications in explorations of the Kv3.4 channel as a specific therapeutic target in DRG neurons.
The overall age and gender-independent properties of the Kv3.4 current are also potentially relevant (Fig. 1). We have previously proposed that the Kv3.4 channel is a significant player in pain signaling (below), which might underlie an analgesic homeostatic mechanism in mammals.7,8 Because pain signaling is crucial for survival, all molecules involved in nociception are expected to be mature early in life and both sexes must have a similar complement of signaling mechanisms. We do not know whether slower Kv3.4 inactivation and apparently smaller outward currents in female rats (Fig. 1D&G) reflect differences in pain thresholds, but it is a tantalizing possibility, which requires further investigation.
Physiological roles of the Kv3.4 channel in nociceptors
Given the high voltage-activation property of the Kv3.4 channel and the dramatic phosphorylation-dependent modul-ation of its inactivation property, the respective current is well suited to regulate AP duration and repolarization rate (Fig. 3).7 The AP- and dynamic-clamping experiments reported here provide compelling evidence for this role by showing that the Kv3.4 current transfers a substantial fraction of the repolarizing charge (Figs. 2 and 4). The Kv3.4 channel is expressed in all compartments of small-diameter nociceptors and, therefore, regulation of AP repolarization by this channel might generally impact the excitability of these neurons.8,11,30 At the primary synapse in the dorsal horn, changes in AP duration and repolarization rate could affect vesicular glutamatergic transmission and subsequent pain signaling. Phosphorylation-dependent inhibition of Kv3.4 inactivation and the resulting shortening of the AP at the dorsal horn synapse could be the basis of homeostatic analgesia during normal pain signaling (Fig. 5).7 Interestingly, 2-weeks post SCI, we detected no change in the AP duration even though Kv3.4 channel expression was reduced.8 Here we show that relatively small non-inactivating currents with a negatively shifted voltage dependence of activation contribute a greater charge transfer during the AP (Fig. 4). Remodeled Kv3.4 channels and/or other to-be-identified K+ channels that are part of a compensatory mechanism might mediate the currents in the SCI group. We have also previously reported dynamic-clamping results suggesting that the Kv3.4 channel, independently of its inactivation phenotype, can inhibit repetitive spiking in putative nociceptors and, consequently, pain signaling8; however, the basis of this regulation is not fully understood.
Figure 5.
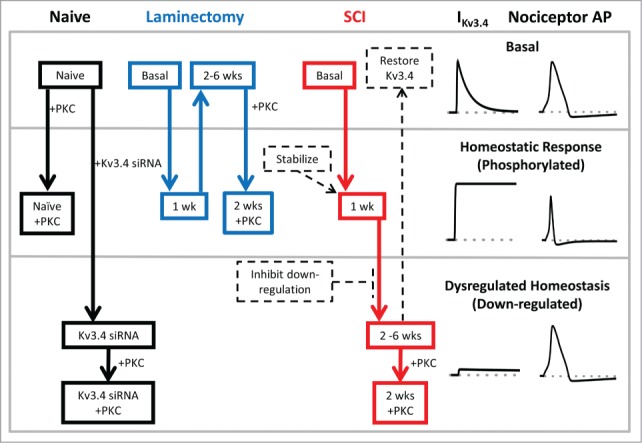
Schematic of Kv3.4 channel function, modulation and dysfunction induced by SCI. Under naïve and basal conditions, Kv3.4 channels mediate an A-type K+ current, which contributes significantly to the repolarization of the AP of DRG nociceptors (top section). In response to an acute insult (e.g., surgery or an early stage of the SCI), DRG neuron excitability increases and the associated release of inflammatory factors may trigger signaling cascades. The latter may then activate PKC, which phosphorylates the Kv3.4 NTID. This phosphorylation induces Kv3.4 phenotype switching from A-type (fast inactivating) to delayed rectifier-type (non-inactivating). Consequently, the nociceptor AP is shortened and pain signaling is inhibited (middle section; homeostatic analgesia). If the injury is severe and long lasting (SCI), Kv3.4 channels additionally undergo downregulation (probably as a result of additional phosphorylation at other sites). The loss of Kv3.4 channels effectively impairs homeostatic analgesia in the DRG and causes persistent peripheral hyperexcitability and pain sensitization (bottom section, dysregulated Kv3.4). This scheme reveals potential Kv3.4-based therapeutic strategies to mitigate SCI-induced persistent neuropathic pain by normalizing the excitability of nociceptors (dashed arrows and boxes). For instance, 1) Kv3.4 openers and Kv3.4 overexpression to restore Kv3.4 functional activity, 2) inhibition of signaling mechanisms that underlie functional downregulation and loss of surface expression, and 3) stabilization of the Kv3.4 non-inactivating phenotype.
Kv3.4 channel downregulation and peripheral pain sensitization
Several studies suggest that downregulation of Kv3.4 channel expression in DRG neurons is linked to allodynia and hyperalgesia resulting from direct trauma of sensory fibers or SCI.8,10,11 In the light of the physiological roles discussed above, the loss of Kv3.4 channels in nociceptors could contribute to enhanced firing rates and broadening of the AP, which would promote pain sensitization. Essentially, the loss of homeostatic analgesia that depends on the Kv3.4 channel and its modulation by PKC is conducive to the development of persistent peripheral hyperexcitability and persistent pain (Fig. 5). Under pathological conditions, however, a putative combination of injury-induced changes might help explain why the ISI is shortened while the somatic AP shape and duration remain relatively unaltered after SCI. Kv3.4 downregulation could contribute to an overall reduction in K+ conductance,31 which would be responsible for shortening of the interspike interval. At the same time, while Kv3.4 downregulation is expected to prolong the somatic AP, phenotype switching from A-type to delayed rectifier-type and changes in other ionic conductances (e.g., downregulation of voltage-gated Ca2+ channels and compensatory K+ channels) might tend to shorten it. To investigate these possibilities, it will be necessary to precisely determine how SCI alters the ensemble of ionic conductances that regulate spiking and shape the AP in different compartments of DRG neurons. In addition, while the molecular mechanism that governs the conversion of the Kv3.4 channel from A-type to delayed rectifier-type is known,18,32 more work is necessary to understand how SCI induces downregulation of the Kv3.4 channel in DRG nociceptors. These investigations are crucial steps toward exploiting the Kv3.4 channel as a promising therapeutic target for persistent neuropathic pain.
Methods
Animals
All animals were purchased from Taconic Farms and were treated as approved by the Thomas Jefferson University Institutional Animal Care and Use Committee. Seven-day old pups and adult male (200-250 grams) Sprague-Dawley rats were killed by decapitation under general anesthesia (2-3% isoflurane). Adult female Sprague-Dawley rats (200-250 grams) were killed by an overdose of anesthesia (ketamine at 285 mg/kg and xylazine at 30 mg/kg; administered intraperitoneally) followed by thoracotomy and perfusion of 400 ml 0.9% sterile saline. SCI and laminectomy surgeries were performed as previously described.8
Cell culture and molecular biology
DRG neurons were harvested and dissociated as described previously.7,8,14,22 Dissociated DRG neurons were plated on glass poly-ornithine coated coverslips and incubated at 37°C until use (<24 hours for controls and up to 48 hours for siRNA). Kv3.4 siRNA (Santa Cruz Biotechnology) was transfected using nucleofection as previously described.7 Single-cell RT-PCR was performed as described previously.7
Electrophysiology
Dissociated DRG neurons were used for electrophysiological experiments at room temperature (20–24°C) as previously described.7,8,14 A computer running Clampex 10.2 (Molecular Devices) was used to collect and store all data except for dynamic-clamp recordings, which were collected and stored using QuB v. 1.4.0.517 (www.qub.buffalo.edu). For dynamic clamp experiments the liquid junction potential of +15.5 mV was corrected online. Cell-attached macropatch recordings had a liquid junction potential of 0 mV and thus required no correction. In cell-attached macropatch experiments the bath and pipette solution contained (in mM): 130 choline-Cl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 50 sucrose, pH 7.4. For whole-cell dynamic-clamp recordings, the external (bath) solution contained (in mM): 130 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, pH 7.4. The internal (pipette) solution in current-clamp recordings contained (in mM): 130 K-Mes, 1 CaCl2, 1 EGTA, 10 HEPES, 2 Mg-ATP, 0.3 Tris-GTP, pH 7.3. AP clamp recordings were done as previously described33,34 except using an average AP waveform in cell-attached patches.
Kinetic modeling
IChMASCOT was used as previously described35-37 to obtain the best global fit of a Markov kinetic model to DRG nociceptor Kv3.4 currents. The global fit involves the simultaneous fitting of multiple voltage and time dependent current properties. IChMASCOT is freely available for download at: http://www.jadesantiago.com/Electrophysiology/IChMASCOT/Download.aspx. For use in this work, Dr. Jose de Santiago-Castillo kindly provided the new β version of IChMASCOT (v.0.5.2). Simulated currents from the model were generated using IonChannelLab.38 Dynamic clamp experiments were performed as previously described using QuB dynamic clamp software.8,39,40
Data analysis
Data was analyzed as previously described.7,8 Charge transfer during the AP was determined by integrating the current evoked by the AP waveform. The integration was conducted between the time at AP threshold and the time at which the AP waveform returns to resting membrane potential following the AHP. This range was based on the AP waveform used as a voltage protocol from a holding voltage of −100 mV (Fig. 2A, red trace). The same range was used regardless of whether or not there was a conditioning pulse to −30 mV (Fig. 2A, black trace).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ms. Tanziyah Muqeem for critically reading the manuscript and Ms. Tamara Hala, Dr. Cojen Ho and Dr. Michael O'Leary for technical assistance. Also, we thank all members of the Covarrubias and Lepore Labs for their support and feedback.
Funding
This work was supported by NINDS NS079855-01A1 (MC), Farber Family Foundation (MC), Sigma Xi GIAR No. G20120315160396 (DMR), F31 NS090689-01 (BMZ), Craig Nielsen Foundation Grant 190140 (ACL), and Paralyzed Veterans of America Grant 160837 (ACL).
References
- 1.Riazanski V, Becker A, Chen J, Sochivko D, Lie A, Wiestler OD, Elger CE, Beck H. Functional and molecular analysis of transient voltage-dependent K+ currents in rat hippocampal granule cells. J Physiol 2001; 537:391–406; PMID:11731573; http://dx.doi.org/ 10.1111/j.1469-7793.2001.00391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martina M, Yao GL, Bean BP. Properties and functional role of voltage-dependent potassium channels in dendrites of rat cerebellar Purkinje neurons. J Neurosci 2003; 23:5698–707; PMID:12843273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekar LK, Loewen ME, Cao K, Sun X, Leis J, Wang R, Forsyth GW, Walz W. Complex expression and localization of inactivating Kv channels in cultured hippocampal astrocytes. J Neurophysiol 2005; 93:1699–709; PMID:15738276; http://dx.doi.org/ 10.1152/jn.00850.2004 [DOI] [PubMed] [Google Scholar]
- 4.Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci 2003; 6:258–66; PMID:12592408; http://dx.doi.org/ 10.1038/nn1019 [DOI] [PubMed] [Google Scholar]
- 5.Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol 1996; 75:2629–46; PMID:8793767 [DOI] [PubMed] [Google Scholar]
- 6.Yeung SYM, Thompson D, Wang Z, Fedida D, Robertson B. Modulation of Kv3 subfamily potassium currents by the sea anemone toxin BDS: significance for CNS and biophysical studies. J Neurosci 2005; 25:8735–45; PMID:16177043; http://dx.doi.org/ 10.1523/JNEUROSCI.2119-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritter DM, Ho C, O'Leary ME, Covarrubias M. Modulation of Kv3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion neurons. J Physiol 2012; 590:145–61; PMID:22063632; http://dx.doi.org/ 10.1113/jphysiol.2011.218560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritter DM, Zemel BM, Hala TJ, O'Leary ME, Lepore AC, Covarrubias M. Dysregulation of Kv3.4 channels in dorsal root Ganglia following spinal cord injury. J Neurosci 2015; 35:1260–73; PMID:25609640; http://dx.doi.org/ 10.1523/JNEUROSCI.1594-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X-H, Byun H-S, Chen S-R, Cai Y-Q, Pan H-L. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem 2010; 114:1460–75; PMID:20557422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan K-Z, Xu Q, Zhang X-M, Zhao Z-Q, Mei Y-A, Zhang Y-Q. Targeting A-type K+ channels in primary sensory neurons for bone cancer pain in a rat model. Pain 2012; 153:562–74; PMID:22188869; http://dx.doi.org/ 10.1016/j.pain.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 11.Chien L-Y, Cheng J-K, Chu D, Cheng C-F, Tsaur M-L. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 2007; 27:9855–65; PMID:17855600; http://dx.doi.org/ 10.1523/JNEUROSCI.0604-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudy B, Sen K, Vega-Saenz De Miera E, Lau D, Ried T, Ward DC. Cloning of a human cDNA expressing a high voltage-activating, TEA-sensitive, type-A K+ channel which maps to chromosome 1 band p21. J Neurosci Res 1991; 29:401–12; PMID:1920536; http://dx.doi.org/ 10.1002/jnr.490290316 [DOI] [PubMed] [Google Scholar]
- 13.Schröter KH, Ruppersberg JP, Wunder F, Rettig J, Stocker M, Pongs O. Cloning and functional expression of a TEA-sensitive A-type potassium channel from rat brain. FEBS Lett 1991; 278:211–6; PMID:1840526; http://dx.doi.org/ 10.1016/0014-5793(91)80119-N [DOI] [PubMed] [Google Scholar]
- 14.Fineberg JD, Ritter DM, Covarrubias M. Modeling-independent elucidation of inactivation pathways in recombinant and native A-type Kv channels. J Gen Physiol 2012; 140:513–27; PMID:23109714; http://dx.doi.org/ 10.1085/jgp.201210869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver D, Lien C-C, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science 2004; 304:265–70; PMID:15031437; http://dx.doi.org/ 10.1126/science.1094113 [DOI] [PubMed] [Google Scholar]
- 16.Ruppersberg JP, Stocker M, Pongs O, Heinemann SH, Frank R, Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature 1991; 352:711–4; PMID:1908562; http://dx.doi.org/ 10.1038/352711a0 [DOI] [PubMed] [Google Scholar]
- 17.Covarrubias M, Wei A, Salkoff L, Vyas TB. Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate. Neuron 1994; 13:1403–12; PMID:7993631; http://dx.doi.org/ 10.1016/0896-6273(94)90425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck EJ, Sorensen RG, Slater SJ, Covarrubias M. Interactions between multiple phosphorylation sites in the inactivation particle of a K+ channel. Insights into the molecular mechanism of protein kinase C action. J Gen Physiol 1998; 112:71–84; PMID:9649584; http://dx.doi.org/ 10.1085/jgp.112.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruse M, Hammond GRV, Hille B. Regulation of voltage-gated potassium channels by PI(4,5)P2. J Gen Physiol 2012; 140:189–205; PMID:22851677; http://dx.doi.org/ 10.1085/jgp.201210806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruse M, Hille B. The phosphoinositide sensitivity of the K(v) channel family. Channels (Austin) 2013; 7:530–6; PMID:23907203; http://dx.doi.org/ 10.4161/chan.25816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li N, Lu Z, Yu L, Burnstock G, Deng X, Ma B. Inhibition of G protein-coupled P2Y2 receptor induced analgesia in a rat model of trigeminal neuropathic pain. Mol Pain 2014; 10:21; PMID:24642246; http://dx.doi.org/ 10.1186/1744-8069-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phuket TRN, Covarrubias M. Kv4 Channels Underlie the Subthreshold-Operating A-type K-current in Nociceptive Dorsal Root Ganglion Neurons. Front Mol Neurosci 2009; 2:3; PMID:19668710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 2001; 98:13373–8; PMID:11698689; http://dx.doi.org/ 10.1073/pnas.231376298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binzen U, Greffrath W, Hennessy S, Bausen M, Saaler-Reinhardt S, Treede R-D. Co-expression of the voltage-gated potassium channel Kv1.4 with transient receptor potential channels (TRPV1 and TRPV2) and the cannabinoid receptor CB1 in rat dorsal root ganglion neurons. Neurosci 2006; 142:527–39; http://dx.doi.org/ 10.1016/j.neuroscience.2006.06.020 [DOI] [PubMed] [Google Scholar]
- 25.Vydyanathan A, Wu Z-Z, Chen S-R, Pan H-L. A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J Neurophysiol 2005; 93:3401–9; PMID:15647393; http://dx.doi.org/ 10.1152/jn.01267.2004 [DOI] [PubMed] [Google Scholar]
- 26.Bocksteins E, Van de Vijver G, Van Bogaert P-P, Snyders DJ. Kv3 channels contribute to the delayed rectifier current (IK) in small cultured mouse dorsal root ganglion (DRG) neurons. Am J Physiol Cell Physiol 2012; 303(4):C406–15; PMID:22673617[AQ3] [DOI] [PubMed] [Google Scholar]
- 27.Covarrubias M, Wei AA, Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron 1991; 7:763–73; PMID:1742024; http://dx.doi.org/ 10.1016/0896-6273(91)90279-9 [DOI] [PubMed] [Google Scholar]
- 28.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 2001; 24:517–26; PMID:11506885; http://dx.doi.org/ 10.1016/S0166-2236(00)01892-0 [DOI] [PubMed] [Google Scholar]
- 29.Weiser M, Vega-Saenz De Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci 1994; 14:949–72; PMID:8120636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooke RE, Atkinson L, Batten TFC, Deuchars SA, Deuchars J. Association of potassium channel Kv3.4 subunits with pre- and post-synaptic structures in brainstem and spinal cord. Neurosci 2004; 126:1001–10; http://dx.doi.org/ 10.1016/j.neuroscience.2004.03.051 [DOI] [PubMed] [Google Scholar]
- 31.Tsantoulas C, McMahon SB. Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci 2014; 37:1–13; PMID:24461875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antz C, Bauer T, Kalbacher H, Frank R, Covarrubias M, Kalbitzer HR, Ruppersberg JP, Baukrowitz T, Fakler B. Control of K+ channel gating by protein phosphorylation: structural switches of the inactivation gate. Nat Struct Biol 1999; 6:146–50; PMID:10048926; http://dx.doi.org/ 10.1038/5833 [DOI] [PubMed] [Google Scholar]
- 33.Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci 2002; 22:10277–90; PMID:12451128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 2007; 8:451–65; PMID:17514198; http://dx.doi.org/ 10.1038/nrn2148 [DOI] [PubMed] [Google Scholar]
- 35.Kaulin YA, De Santiago-Castillo JA, Rocha CA, Covarrubias M. Mechanism of the modulation of Kv4:KChIP-1 channels by external K+. Biophys J 2008; 94:1241–51; PMID:17951301; http://dx.doi.org/ 10.1529/biophysj.107.117796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dougherty K, De Santiago-Castillo JA, Covarrubias M. Gating charge immobilization in Kv4.2 channels: the basis of closed-state inactivation. J Gen Physiol 2008; 131:257–73; PMID:18299396; http://dx.doi.org/ 10.1085/jgp.200709938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amarillo Y, De Santiago-Castillo JA, Dougherty K, Maffie J, Kwon E, Covarrubias M, Rudy B. Ternary Kv4.2 channels recapitulate voltage-dependent inactivation kinetics of A-type K+ channels in cerebellar granule neurons. J Physiol 2008; 586:2093–106; PMID:18276729; http://dx.doi.org/ 10.1113/jphysiol.2007.150540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santiago-Castillo JA, De Covarrubias M, Sánchez-Rodríguez JE, Perez-Cornejo P, Arreola J. Simulating complex ion channel kinetics with IonChannelLab. Channels (Austin) 2010; 4:422–8; PMID:20935453; http://dx.doi.org/ 10.4161/chan.4.5.13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milescu LS, Yamanishi T, Ptak K, Mogri MZ, Smith JC. Real-time kinetic modeling of voltage-gated ion channels using dynamic clamp. Biophys J 2008; 95:66–87; PMID:18375511; http://dx.doi.org/ 10.1529/biophysj.107.118190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milescu LS, Yamanishi T, Ptak K, Smith JC. Kinetic properties and functional dynamics of sodium channels during repetitive spiking in a slow pacemaker neuron. J Neurosci 2010; 30:12113–27; PMID:20826674; http://dx.doi.org/ 10.1523/JNEUROSCI.0445-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]