Abstract
Purpose
Intramuscular innervation of mammalian horizontal rectus extraocular muscles (EOMs) is compartmental. We sought evidence of similar compartmental innervation of the superior oblique (SO) muscle.
Methods
Three fresh bovine orbits and one human orbit were dissected to trace continuity of SO muscle and tendon fibers to the scleral insertions. Whole orbits were also obtained from four humans (two adults, a 17-month-old child, and a 33-week stillborn fetus), two rhesus monkeys, one rabbit, and one cow. Orbits were formalin fixed, embedded whole in paraffin, serially sectioned in the coronal plane at 10-μm thickness, and stained with Masson trichrome. Extraocular muscle fibers and branches of the trochlear nerve (CN4) were traced in serial sections and reconstructed in three dimensions.
Results
In the human, the lateral SO belly is in continuity with tendon fibers inserting more posteriorly on the sclera for infraducting mechanical advantage, while the medial belly is continuous with anteriorly inserting fibers having mechanical advantage for incycloduction. Fibers in the monkey superior SO insert more posteriorly on the sclera to favor infraduction, while the inferior portion inserts more anteriorly to favor incycloduction. In all species, CN4 bifurcates prior to penetrating the SO belly. Each branch innervates a nonoverlapping compartment of EOM fibers, consisting of medial and lateral compartments in humans and monkeys, and superior and inferior compartments in cows and rabbits.
Conclusions
The SO muscle of humans and other mammals is compartmentally innervated in a manner that could permit separate CN4 branches to selectively influence vertical versus torsional action.
Keywords: superior oblique muscle, trochlear nerve, anatomy
The mammalian superior oblique (SO) muscle consists of two potentially independent compartments with mechanical advantage for torsional versus vertical actions, innervated by separate trochlear nerve divisions. This provides anatomic basis for magnetic resonance imaging observations of differential compartmental SO function.
The partitioning hypothesis of English et al.1 proposed muscles as arrays of unique subunits capable of independent action due to innervation by different motor neuron pools. There is evidence of neuromuscular compartments in the feline rectus femoris, medial and lateral gastrocnemius, and soleus muscles.2 In humans, the peroneus longus,3 trapezius,4,5 and tibialis anterior6 muscles all have subdivisions innervated by separate motor neuron pools.
The extraocular muscles (EOMs) are composed of long, generally parallel fibers in continuity with similarly arranged tendon fibers, in both of which there is a high degree of independence of passive7 and actively generated force.8 In view of these properties, it is unsurprising that the compartmentalization paradigm has been extended to the rectus EOMs, where motor nerves innervating the horizontal rectus EOMs bifurcate into superior and inferior divisions innervating corresponding nonoverlapping compartments of muscle fibers.9,10 Since rectus EOM tendons are up to 12 mm broad at their scleral insertions, forces applied at different transverse points along the insertions would differ, and so have different vertical and potentially torsional effects.11 The inferior rectus (IR) EOM also exhibits organization corresponding to distinct lateral and medial compartments.10
Magnetic resonance imaging (MRI) has been employed in humans to demonstrate differential compartmental function of EOMs during physiological activation. For example, during vertical eye rotation, the superior compartment of the human medial rectus (MR) muscle exhibits contractile changes, with an absence of contractility in the inferior MR compartment, as well as either the inferior or superior lateral rectus (LR) compartment (Demer JL, Clark RA. IOVS 2013;54:ARVO E-Abstract 1302).
The human MR superior compartment is significantly more contractile than the inferior compartment during conjugate adduction, but only one-third as contractile during convergence.12 During ocular counterrolling, MRI indicated significant contractility in the inferior but not superior LR compartment, and borderline contractile changes in the superior but not inferior MR compartment.13 During fusional vergence induced by a vertical prism, normal humans exhibit MRI evidence of differential compartmental activity in the LR and IR EOMs.14 Since a substantial proportion of abducens palsy cases exhibit greater atrophy of the superior than inferior LR and also exhibit ipsilateral hypotropia, it has been suggested that selective superior compartment LR weakness may arise from selective pathology of the superior division of the abducens nerve.15 From these studies it has been clearly shown that multiple human EOMs have differential compartmental activity under physiological conditions.
It has long been recognized that the superior oblique (SO) muscle has both torsional and vertical actions.16 The SO tendon is broad at its scleral insertion, with an average length of 11 mm, varying from 7.5 to 12.7 mm.17–19 In central gaze, the anterior SO fibers of humans insert close to the equator, so that tension in them would mainly produce incycloduction. Conversely, the posterior SO fibers insert posterior to the equator, so their tension in central gaze would mainly cause infraduction. The Harada-Ito operation consists of lateral and anterior transposition of the anterior fibers of the SO tendon, exploiting the differential geometry of the SO tendon insertion to correct excyclotropia without vertical effect.20 The obvious potential for selective control of the torsional versus vertical actions of the normal SO makes it an intriguing candidate to investigate for possible compartmental innervation. The present study was conceived to investigate the anatomical plausibility in several species of selective compartmental control of the SO by examining its intramuscular innervation by the trochlear nerve (CN4), and the selectivity of its fiber insertions.
Methods
Gross Dissection
Three fresh bovine heads 20 to 30 months of age were obtained from a local slaughterhouse (Manning Beef LLC, Pico Rivera, CA, USA). The orbits were dissected to expose the SO muscle in its natural anatomic relationship. The SO was traced from the point of origin deep within the orbit to the scleral insertion and excised en bloc with the trochlea. The trochlea was then removed and SO unrolled in a Petri dish.
One fresh postmortem human head was obtained in conformity with legal requirements by designated tissue donation to research. After removal of the brain, the left orbit was unroofed without disturbance to the EOMs or associated connective tissues. The superior rectus (SR) muscle was disinserted from the globe and reflected to expose the SO. Distinctively colored sutures were placed to mark the medial and lateral margins of the SO belly, as well as the anterior and posterior edges of the SO tendon insertion near the sclera. The entire SO was then excised en bloc from the origin to the scleral insertion, including the trochlea, and placed in a Petri dish. After photography, the trochlea was carefully opened, and the SO tendon unrolled and laid flat in continuity with the EOM fibers.
Histology
Formalin-fixed orbits were acquired by tissue sharing from other laboratories after animals were killed upon completion of unrelated experimental protocols. Orbits were thus obtained from two rhesus macaque (M. Mulatta) monkeys, a rabbit, and a cow. Human orbits were obtained in conformity with legal requirements from male cadavers aged 65 years (H3), aged 44 years (H5), a 17-month-old (H7), and a 33-week-old stillborn fetus (H9). Samples from H3, H5, and H9 were obtained intact at autopsy and fixed by immersion in 10% neutral buffered formalin. Preparation of the H7 included freezing by a tissue bank to −78°C within 24 hours of death, thawing in 10% neutral buffered formalin, removing orbits en bloc with periorbita, decalcification of orbits in 0.003 M EDTA and 1.35 N HCl solution, dehydration in alcohols, processing in xylene, and finally embedding in paraffin. Orbits were coronally sectioned at 10-μm thickness, mounted on 50- × 75-mm glass slides, and stained with Masson trichrome (MT).
Micrographs were taken using a digital camera (Nikon D1X) mounted on a light microscope (Eclipse E800; Nikon, Tokyo, Japan). Objective magnification ×10 was used for orbits H5, H7, H9, M19, and M20. Objective magnification ×4 was used for H3 and the rabbit, and ×2 for the cow. Resolution was 16.9 megapixels per image. Using Adobe Photoshop CS5 Photomerge tool, multiple overlapping fields from each section were joined to produce a single high-resolution image incorporating the area of interest in each section. Imaging programs Adobe Photoshop CS5 (Adobe Systems, San Jose, CA, USA) and ImageJ64 (W. Rasband, National Institutes of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/, 1997–2009 [in the public domain]) were used for further processing.
The CN4 was readily identified and traced in each micrograph with distinctive colored overlay layers in posterior sections before its bifurcation, continuing anteriorly through successive sections where identifiably dividing cross sections could be unequivocally recognized until visual recognition became impossible. Overlay layers were created corresponding to each histological image in which nerve and muscle fibers were represented as solid colored cross sections. These nerve and muscle overlay images were merged into an image stack for reconstruction in three dimensions.
Overlap Calculations
Eight coronal sections of the anterior muscle belly, 60 μm apart for postnatal specimens and 40 μm apart for the prenatal specimen, were sampled for neural arborization. For each section, a straight line best dividing the two compartmental nerve arborizations was drawn relative to the long axis of the muscle. The mean boundary between compartments was calculated by averaging over all sections the orientation of the best fit lines. Any nerve branch crossing the boundary line was considered an overlap.
Results
Bovine Gross Anatomy
Gross dissection of the bovine orbit revealed in vivo relationships of the SO muscle and tendon (Fig. 1). The cartilaginous trochlea, through which the SO tendon traverses, is located superonasally. Anterior to the trochlear reflection, SO muscle fibers transition to SO tendon fibers that broadly insert on the sclera. One end of the insertion is anterior to the equator, while the other more posterior insertion end is at the equator. The SO was removed en bloc and trochlea excised to verify the relationship of SO muscle to tendon fibers. Forceps traction on fibers in the superior SO belly resulted in tension on the posterior insertion, suggesting a mechanical advantage for incycloduction. Forceps traction on the inferior SO belly fibers resulted in tension on the anterior insertion fibers, which would cause supraduction.
Figure 1.
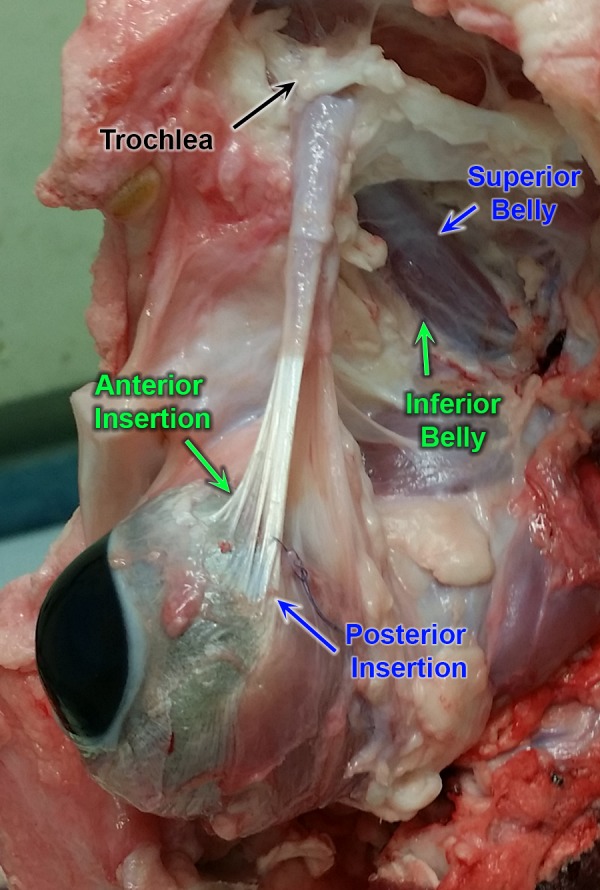
Gross dissection of bovine orbit showing scleral insertion of the superior oblique (SO) muscle and tendon. The orbital floor has been excised and the globe displaced inferiorly, and the superior rectus muscle excised to reveal continuity of the superior SO belly with tendon fibers inserting at the posterior scleral insertion close to the equator. The inferior SO belly is seen in continuity with tendon fibers inserting anteriorly on the sclera.
Human Gross Anatomy
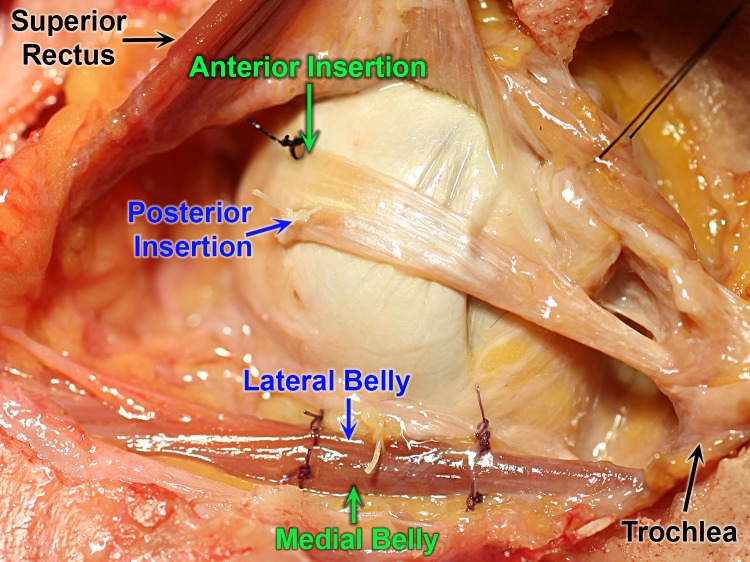
Anatomic relationships of the human SO were investigated by careful dissection of a fresh human cadaver orbit exposed by removal of the orbital roof, as seen in Figure 2. Colored marking sutures were placed to maintain unequivocal orientation of the medial and lateral SO belly, as well as the anterior and posterior poles of the scleral insertion (Fig. 2). These relationships were maintained following careful en bloc excision of the SO belly and tendon in continuity with the trochlea, which could after this exposure be freely slid anteriorly and posteriorly along the tendon. The trochlea cartilage was sharply incised and folded away to allow for unrolling of the SO tendon to a broad, flat configuration evocative of a rectus EOM tendon (Fig. 3). After this unrolling, it was evident that the medial SO belly was continuous with the tendon fibers that had inserted on the anterior portion of the sclera, and the lateral belly was continuous with the tendon fibers that had inserted posteriorly on the sclera. Forceps traction on muscle fibers of the lateral belly created tension visible in the posterior tendon fibers. Conversely, forceps traction on the medial belly created tension visible in the anterior tendon fibers.
Figure 2.
Superior view of left human orbit after reflection of superior rectus muscle, exposing the superior oblique (SO) muscle and tendon. Black and white sutures mark the SO belly and tendon margins at the scleral insertion.
Figure 3.

Isolated human SO muscle and tendon from dissection illustrated in Figure 2, following excision of the trochlea, allowing tendon to unroll flat. Fibers of the medial belly are continuous with the anterior pole of the tendon insertion, while fibers of the lateral muscle belly are continuous with the posterior scleral insertion.
Microscopic Anatomy of Rabbit
Uniquely in the present study, the SO in the rabbit originates from two heads (Fig. 4), between which the common CN4 trunk passes from posterior to anterior before dividing into two nonoverlapping distributions. More anteriorly the two SO heads unite into a single belly, albeit with a thin fissure demarcating them anteriorly. The superior compartment of the rabbit SO is much smaller than the inferior. The smaller CN4 branch innervates the superior SO head whose fibers insert relatively posteriorly on the sclera. The larger CN4 branch innervates the SO compartment whose fibers insert anteriorly on the sclera. The two CN4 branches course anteriorly parallel to the EOM fibers, remaining segregated in their respective superior and inferior compartments, as seen in the three-dimensional reconstruction in Figure 5. Unlike other species, the rabbit SO has no visible tendon; muscle fibers transverse the trochlea and insert directly on the sclera.
Figure 4.
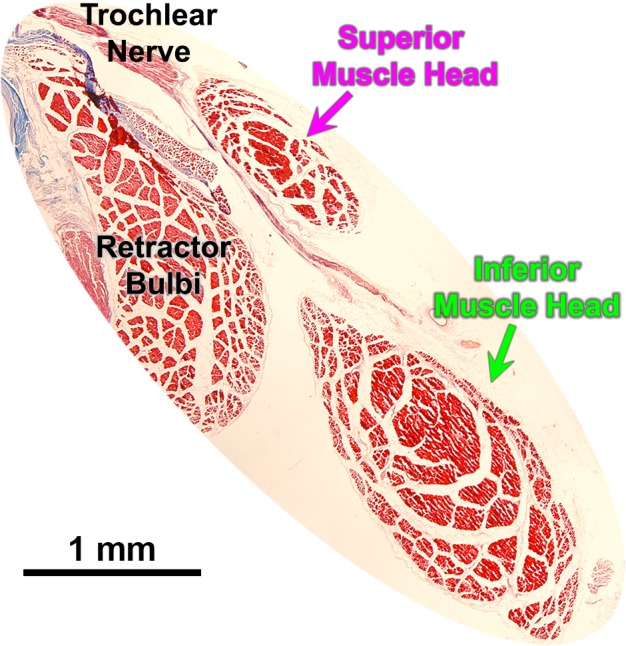
Histological section illustrating dual-headed origin of the rabbit superior oblique (SO) muscle. The main trunk of the trochlear nerve innervates the larger, inferior muscle head.
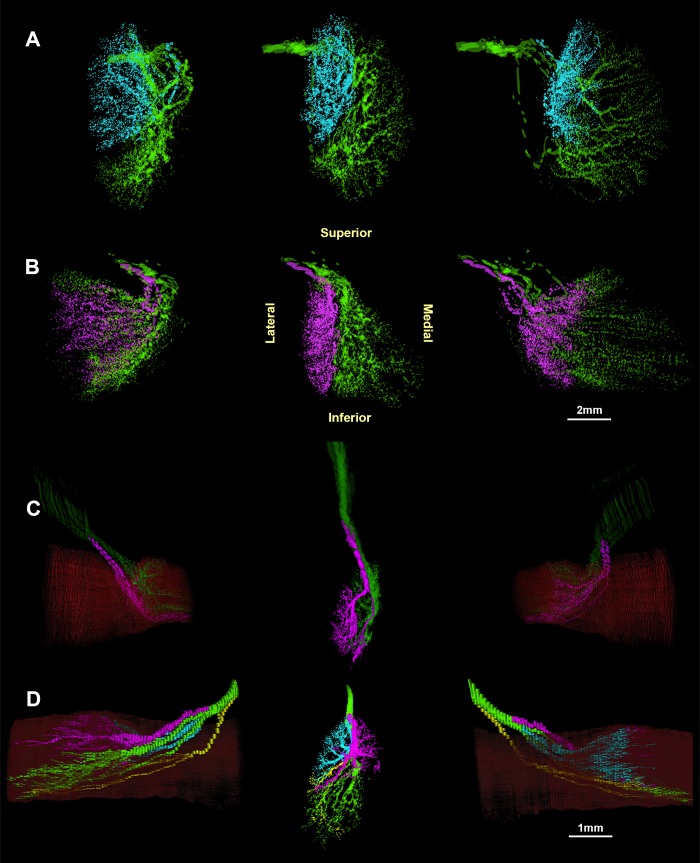
Figure 5.
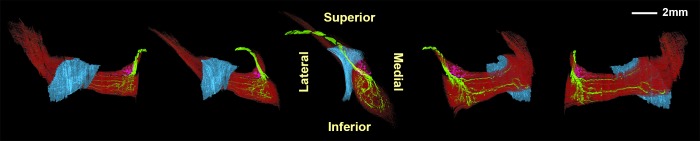
Three-dimensional reconstruction showing two divisions of the rabbit trochlear nerve (CN4) within the superior oblique (SO) muscle: a small superior division (magenta) and a large inferior division (green). The trochlea is shown in blue, with muscle fibers in red. The SO muscle arises from two heads at its origin, flanking CN4. The center image is a posterior view, while side images show various perspectives rotated about a vertical axis.
Bovine Microscopic Anatomy
Three-dimensional reconstruction of the superior and inferior divisions of the bovine CN4 illustrates that its two divisions arborize in nonoverlapping compartments whose dividing border aligns with the minor axis of the elliptical muscle cross-section (Fig. 6). A single CN4 trunk in the posterior bovine orbit approaches the superolateral surface of the SO belly and bifurcates just external to it. The inferior division in turn bifurcates, sending branches along the medial and lateral surfaces of the SO that course anteriorly to arborize within the inferior half of the EOM. The superior division continues further anteriorly in the orbit along the superior surface of the SO belly, ultimately entering its superior surface to arborize. Some intramuscular nerves also course posteriorly from the entry point.
Figure 6.
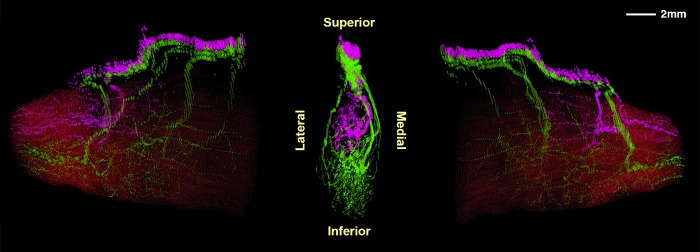
Bovine superior oblique muscle and trochlear nerve (CN4), reconstructed in three dimensions. Center image is a posterior view of CN4 only, with muscle fibers rendered transparent. Flanking images show perspectives rotated 80° to the left and right with muscle fibers overlaid in red. CN4 branches penetrate the muscle and project both anteriorly and posteriorly. CN4 bifurcates into superior (magenta) and inferior (green) branches arborizing within nonoverlapping intramuscular territories.
Microscopic Anatomy of Monkey
In monkey orbits M19 and M20, CN4 bifurcated on the superolateral surface of the SO belly and projected anteriorly into two zones of minimally overlapping arborizations innervating generally parallel bundles of muscle fibers (Fig. 7). In each monkey orbit, the superior CN4 division entered and arborized in the superior belly, while the inferior division innervated the inferior portion, respecting a demarcation oriented medial to lateral.
Figure 7.
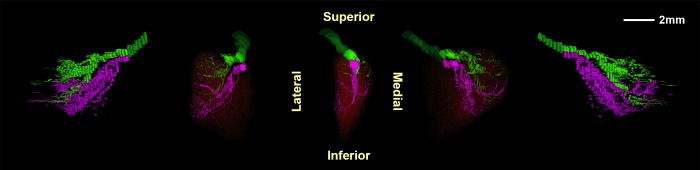
Three-dimensional reconstructions of the superior (green) and inferior (magenta) branches of the intramuscular trochlear nerve in monkey specimen M19. Center image is a posterior view of the nerve with muscle fibers superimposed in red. Flanking images show the nerve and muscle in rotated perspectives 40° and 80° to the left and right.
Microscopic Anatomy of Human
Orientation of the human SO is evident from the coronal cross section of the orbit. Magnified views of the SO along with labeled nerve branches are shown in Figure 8. The main CN4 trunk in postnatal human specimens also displayed topographical arrangement, traveling forward in the orbit along the orbital surface of the SO, and bifurcating immediately before entering the EOM belly (Fig. 9).
Figure 8.
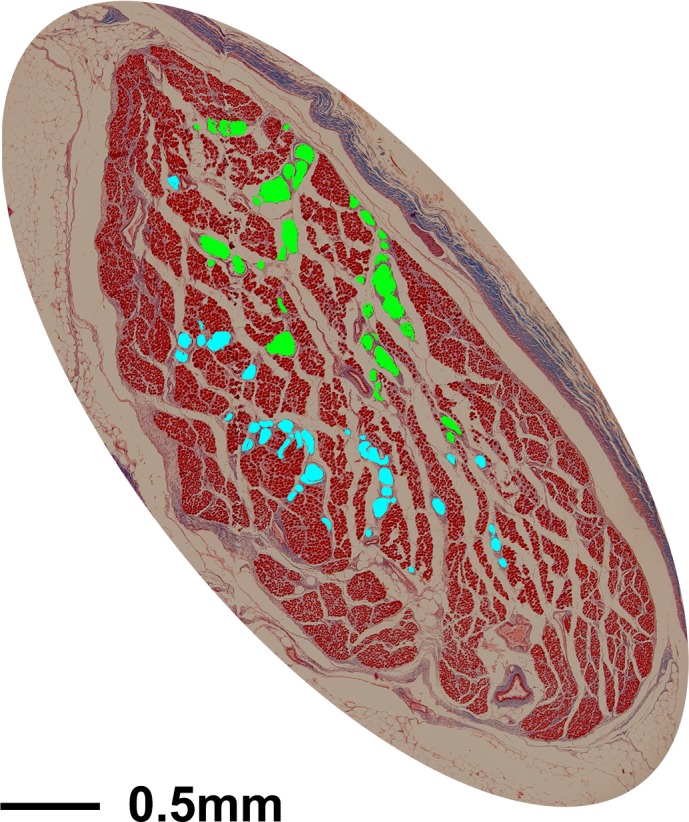
Cross section of human superior oblique muscle stained with Masson trichrome. In all sections, the two divisions of the trochlear nerve were serially traced using different-color masks corresponding to each branch, as shown here.
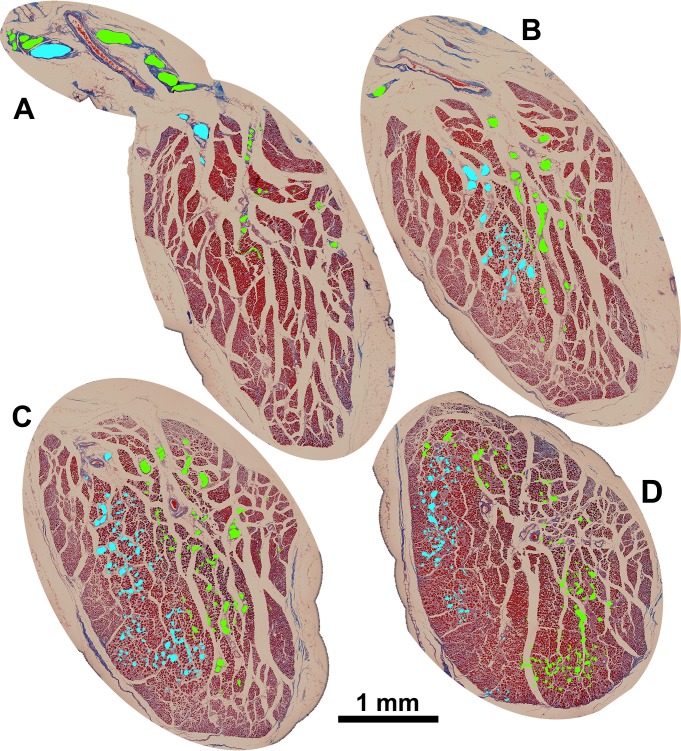
Figure 9.
Coronal cross section of human SO muscle 3.54 cm posterior to corneal surface, with branches of the trochlear nerve (CN4) highlighted by colored overlays (A). CN4 can be seen bifurcating external to the muscle, arborizing in distinct compartments in sections taken approximately 3.26 cm (B), 3.08 cm (C), and 2.86 cm (D) posterior to the corneal surface.
The human CN4 bifurcated into similar-sized, nonoverlapping medial and lateral divisions (Figs. 10A–C). There was minimal overlap between the medial and lateral innervation territories (Figs. 11A–C) as each division repeatedly arborized into smaller branches traveling almost parallel to the EOM fibers. Although orientation of the border separating the two innervation territories varied among specimens, it roughly paralleled the major axis of the elliptical SO cross section.
Figure 10.
Human intramuscular trochlear nerve rendered in multiple perspectives by rotation about a vertical axis. (A) 65-year-old male, (B) 44-year-old male, and (C) 17-month-old male all show two minimally overlapping medial and lateral divisions. (D) 33-week-old male fetus does not show two distinct branches. Flanking images in (C) and (D) show muscle fibers in red.
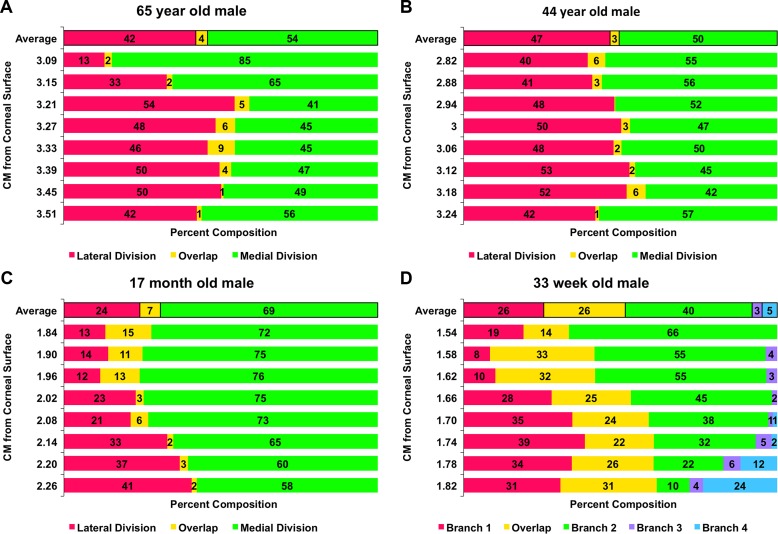
Figure 11.
Percent of SO cross section innervated by CN4 divisions, indicated in green or magenta, at intervals along its length. Postnatal specimens having two major divisions exhibit minimal overlap (yellow), averaging 4% for the 65-year-old male (A), 3% for the 33-year-old male (B), and 7% for the 17-month-old male (C). The prenatal specimen (D) had significantly more overlap of 26% among four marked branches.
The foregoing postnatal compartmental organization of the intramuscular SO was not observed in the 33-week stillborn old male fetus (Fig. 10D). In this fetus, the CN4 trunk divided in the posterior orbit into four branches that entered the superior orbital surface of the SO belly. These branches then arborized anteriorly within the EOM with further daughter branches generally traveling parallel to the EOM fibers, but with considerable overlap among the arborizations (Fig. 11D).
Discussion
We here report the novel finding that motor innervation of the mature mammalian SO muscle is partitioned into minimally overlapping compartments, each innervated by a separate branch of CN4 derived from a bifurcation of the main trunk external to the EOM belly. This compartmental innervation pattern is consistent across four species, both lateral and frontal eyed: rabbit, cow, monkey, and human. This configuration therefore seems likely to be a general feature of the mammalian SO.
Compartmental SO innervation appears generally specialized to favor specific oculorotary functions that depend on the geometry of the SO's broad insertion. In lateral-eyed animals such as cow and rabbit, tension on the posterior pole of the tendon insertion at the globe equator would incycloduct in central gaze, while the opposite pole anterior to the equator would supraduct. A similar geometry probably prevails in the other lateral-eyed animal in this study, the rabbit. However, Fink19 noted that bovine oblique EOMs have a more posterior insertion closer to the globe equator. In frontal-eyed primates such as monkey and human, the functions of the SO in central gaze have been classically understood to be incycloduction, infraduction, and abduction.16 Eye position within the orbit influences SO action: Incycloduction has historically been considered the main action in abduction, while infraduction is considered the main action in adduction.
The present anatomical findings have a fundamental implication for neural control of SO function. In adults of all four species studied here, CN4 bifurcates into divisions that selectively innervate two corresponding compartments of SO muscle fibers, and these are in continuity with topographically arranged tendon fibers whose insertion sites would have different mechanical advantages for vertical duction versus cycloduction. In the postnatal SO, the two CN4 bifurcations are minimally overlapping, so that corresponding daughter branches remain in their respective compartments throughout the EOM length. Fibers of each compartment are generally parallel, as are the transitions to separate tendon fibers that insert differentially on the sclera. This implies that innervation in the two CN4 divisions would implement different proportions of vertical duction and cycloduction, allowing a potential additional degree of differential neural control over these actions. This finding in SO extends the known neuromuscular compartmentalization of the triceps brachii muscle,21 the trapezius muscle,5 and cricothyroid muscle,22 as well as compartmentalization of mammalian horizontal rectus EOMs,9,10 and the partially overlapping compartmentalization of the IR muscle.23
The rabbit exhibits the most dramatic example of independent SO compartmentalization. In rabbit the main CN4 trunk enters between two unequal-sized SO heads at its origin in the deep orbit. As CN4 bifurcates, a small branch innervates a small superior EOM compartment, while a more substantial branch arborizes in a larger inferior belly. The compartments maintain relative proportions throughout EOM length. Unlike what is seen in other species, the rabbit SO has no tendon; EOM fibers transverse the trochlea to insert on the sclera. Thus it was possible in rabbit to trace EOM fibers themselves directly to their scleral insertion points, and clearly recognize an intercompartmental gap throughout the entire length of the SO. While anatomically prominent, SO compartmentalization in rabbit is likely to be functionally similar to that in primates.
In humans, the boundary between the two SO compartments roughly parallels the major axis of the EOM's elliptical coronal section, separating the SO into predominately medial and lateral zones. Conversely, in monkey, rabbit, and cow specimens, the short axis roughly approximates the dividing line between superior and inferior compartments. Regardless of species, EOM fibers in each compartment parallel the EOM's long dimension. In rabbit, cow, and monkey, superior SO compartment EOM fibers terminate on the posterior scleral insertion, while the inferior compartment EOM fibers terminate on anterior sclera. Since the relationship between the SO insertion and the globe equator varies by species, the relative torsional and vertical actions would also vary correspondingly. In humans the arrangement is slightly rotated so that the lateral compartment terminates retroequatorially on the posterior scleral insertion and the medial compartment terminates more anteriorly at a relatively equatorial position.
In the prenatal human, CN4 divides prior to entry into the SO belly into roughly four major branches whose subsequent arborizations overlap considerably. We speculate that the embryonic SO innervation may be diffuse rather than compartmentally selective, but later developmental stages may be subject to pruning and refinement to obtain the observed selective adult pattern of two nonoverlapping innervation territories. However, confirmation of this supposition would require the study of multiple specimens at various stages of development, an enterprise probably best accomplished in a nonprimate mammal.
In all of the species examined, differential action of the two SO compartments appears mechanically plausible. The CN4 bifurcation is external to the SO, which minimizes possible mechanical hindrance to independent contraction of the compartments due to anchorage by a common, rigid nerve trunk. Moreover, there is minimal overlap, ranging from an average of 3% to 7%, between the two intramuscular nerve arborizations that could produce transverse force coupling between the two compartments. Helveston et al.18 supposed on the basis of ultrastructure that each SO tendon fiber can act independently through its entire length due to the scant lateral connections among adjacent fibers. Force transmission after marginal z-tenotomy of the SO tendon confirms this minimal transverse force coupling among adjacent tendon fibers.7
We concur with Helveston et al.18 that SO tendon fibers are continuous from the myotendinous junction to the scleral insertion with minimal lateral connections. However, we did not find evidence for the telescoping or slide-by tendon movement postulated by Helveston et al.19 Unlike the dense coaxial structure in the Helveston et al.18 artistic depiction of the tendon, gross anatomical dissection in human demonstrated that the SO tendon transiting the trochlea is simply rolled up in the coronal plane; a cross section would appear as a spiral, perhaps for Helveston et al. difficult to distinguish from a coaxial arrangement of complete circles. The tendon begins rolling at the myotendinous junction, rolls up compactly along its long axis while passing through the trochlea, then unrolls anterior to the trochlea as it fans out to the scleral insertion. When unrolled, the SO appears similar to other rectus EOMs: broad, flat, and with fibers continuing in parallel from myotendinous junction to the insertion. This insertion in humans is mechanically organized to produce differential advantage to the lateral and medial muscle compartments. The lateral SO belly is in continuity with the posterior insertion that has mechanical advantage for infraduction, while the medial belly is in continuity with the anterior insertion near the equator, and thus has mechanical advantage for incycloduction.
The anatomic plausibility of differential compartmental function in the human SO is supported functionally by MRI showing significant differential contractility during vertical fusional vergence.14 Using a bootstrap procedure that systematically varied the presumed orientation of the boundary between the medial and lateral SO compartments, Demer and Clark14 suggested that the functional boundary lies approximately 30° from the major axis of the elliptical cross section of the SO, although this angle was 15° in two orbits and 45° in six orbits. The present finding of alignment of the intercompartmental border with the major axis of the SO cross section seems reasonably consistent with the MRI data, since postmortem orbits are subject to differential shrinkage distortion of up to 30% that could alter angular orientation, and since orbits were embedded after removal of the calcified bony walls and trochlea. The boundary between SO compartments varied somewhat among the three postnatal human specimens studied microscopically here. During fusional vergence induced by vertical prism viewing by the contralateral eye, MRI demonstrated a 4% increase in posterior partial volume (PPV), a measure of contractility, in the lateral but not medial SO compartment.14 Conversely, the medial SO compartment of the prism viewing eye concurrently exhibited an 8% reduction in PPV that was not observed in the contralateral eye.14 Furthermore, nonuniform atrophy found in many cases of SO palsy is consistent with selective compartmental denervation atrophy. Neurogenic atrophy confined to one compartment would be anisotropic, resulting in an elongated residual cross section as frequently observed.24
Within the primate SO, there exist orbital layer (OL) fibers lying circumferentially around the periphery of the belly that insert on the collagenous SO sheath that transits the trochlea and ultimately inserts on the medial aspect of the SR pulley. These OL fibers are smaller than the central global layer (GL) fibers, which are in continuity with the tendon and insert on the sclera.25 We did not recognize separate nerve arborizations for the OL and GL. This finding is similar to the absence of obvious differential motor nerve branches specific to the OL and GL of the rectus EOMs, and implies that putative differential innervation to OL and GL may be at a fine scale.
Topographic organization of motoneurons innervating neuromuscular compartments has been recognized in feline lateral26 and medial gastrocnemius motor nuclei.27 We suspect that such an organization map might exist for the CN4 nucleus as well. Although there has been no study seeking evidence of independent motor neuron pools controlling the two SO compartments, we observed that independent fiber bundles are regularly arranged within the CN4 trunk. Such a segregated organization might conceivably continue anterograde to reflect compartmentalization within the CN4 nucleus. It may be possible to utilize retrograde tracers beginning from the initial branch point to trace each nerve fiber bundle back to its origin in the nucleus. Somatotopy running along the nerve bundle could allow further investigation using selective stimulation of CN4 similar to McNeal and Bowman's28 selective activation of medial gastrocnemius in dogs.
Acknowledgments
Supported by U.S. Public Health Service, National Eye Institute Grants EY08313 and EY0331, and an Unrestricted Grant from Research to Prevent Blindness. J. Demer holds the Leonard Apt Professorship of Pediatric Ophthalmology.
Disclosure: A. Le, None; V. Poukens, None; H. Ying, None; D. Rootman, None; R.A. Goldberg, None; J.L. Demer, None
References
- 1. English AW,, Wolf SL,, Segal RL. Compartmentalization of muscles and their motor nuclei - the partitioning hypothesis. Phys Ther. 1993; 73: 857–867. [DOI] [PubMed] [Google Scholar]
- 2. Kim MW,, Kim JH,, Yang YJ,, Ko YJ. Anatomic localization of motor points in gastrocnemius and soleus muscles. Am J Phys Med Rehabil. 2005; 84: 680–683. [DOI] [PubMed] [Google Scholar]
- 3. Mendez GA,, Gatica VF,, Guzman EE,, Soto AE. Evaluation of the neuromuscular compartments in the peroneus longus muscle through electrical stimulation and accelerometry. Braz J Phys Ther. 2013; 17: 427–434. [DOI] [PubMed] [Google Scholar]
- 4. Larsen CM,, Juul-Kristensen B,, Olsen HB,, Holtermann A,, Sogaard K. Selective activation of intra-muscular compartments within the trapezius muscle in subjects with subacromial impingement syndrome. A case-control study. J Electromyogr Kinesiol. 2014; 24: 58–64. [DOI] [PubMed] [Google Scholar]
- 5. Holtermann A,, Roeleveld K,, Mork PJ,, et al. Selective activation of neuromuscular compartments within the human trapezius muscle. J Electromyogr Kinesiol. 2009; 19: 896–902. [DOI] [PubMed] [Google Scholar]
- 6. Bowden JL,, McNulty PA. Mapping the motor point in the human tibialis anterior muscle. Clin Neurophysiol. 2012; 123: 386–392. [DOI] [PubMed] [Google Scholar]
- 7. Shin A,, Yoo L,, Demer JL. Viscoelastic characterization of extraocular Z-myotomy. Invest Ophthalmol Vis Sci. 2015; 56: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shin A,, Yoo L,, Demer JL. Independent active contraction of extraocular muscle compartments. Invest Ophthamol Vis Sci. 2015; 56: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng M,, Poukens V,, da Silva Costa RM,, Yoo L,, Tychsen L,, Demer JL. Compartmentalized innervation of primate lateral rectus muscle. Invest Ophthalmol Vis Sci. 2010; 51: 4612–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costa RMD,, Kung J,, Poukens V,, Yoo L,, Tychsen L,, Demer JL. Intramuscular innervation of primate extraocular muscles: unique compartmentalization in horizontal recti. Invest Ophthalmol Vis Sci. 2011; 52: 2830–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demer JL. Compartmentalization of extraocular muscle function. Eye. 2015; 29: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demer JL,, Clark RA. Magnetic resonance imaging of differential compartmental function of horizontal rectus extraocular muscles during conjugate and converged ocular adduction. J Neurophysiol. 2014; 112: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark RA,, Demer JL. Differential lateral rectus compartmental contraction during ocular counter-rolling. Invest Ophthalmol Vis Sci. 2012; 53: 2887–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demer JL,, Clark RA. Magnetic resonance imaging demonstrates compartmental muscle mechanisms of human vertical fusional vergence. J Neurophysiol . In press. [DOI] [PMC free article] [PubMed]
- 15. Clark RA,, Demer JL. Lateral rectus superior compartment palsy. Am J Ophthalmol. 2014; 15: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demer JL. Extraocular muscles. : Jaeger EA,, Tasman PR, Clinical Ophthalmology. Philadelphia: Lippincott Williams and Wilkins; 2000: 1–23. [Google Scholar]
- 17. Fink WH. Surgical anatomy of the superior oblique muscle. Arch Ophthalmol. 1950; 43: 942–943. [PMC free article] [PubMed] [Google Scholar]
- 18. Helveston EM,, Merriam WW,, Ellis FD,, Shellhamer RH,, Gosling CG. The trochlea: a study of the anatomy and physiology. Ophthalmology. 1982; 89: 124–133. [DOI] [PubMed] [Google Scholar]
- 19. Fink WH. Surgery of the Vertical Muscles of the Eye. Springfield IL: Thomas; 1962: 41. [Google Scholar]
- 20. Metz HS,, Lerner H. The adjustable Harada-Ito procedure. Arch Ophthalmol. 1981; 99: 624–626. [DOI] [PubMed] [Google Scholar]
- 21. Lucas-Osma AM,, Collazos-Castro JE. Compartmentalization in the triceps brachii motoneuron nucleus and its relation to muscle architecture. J Comp Neurol. 2009; 516: 226–239. [DOI] [PubMed] [Google Scholar]
- 22. Mu LC,, Sanders I. The human cricothyroid muscle: three muscle bellies and their innervation patterns. J Voice. 2009; 23: 21–28. [DOI] [PubMed] [Google Scholar]
- 23. da Silva Costa RM,, Kung J,, Poukens V,, Demer JL. Nonclassical innervation patterns in mammalian extraocular muscles. Curr Eye Res. 2012; 37: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin SY,, Demer JL. Superior oblique shape in superior oblique palsy. Am J Ophthalmol. 2015; 159: 1169–1179, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kono R,, Poukens V,, Demer JL. Superior oblique muscle layers in monkeys and humans. Invest Ophthalmol Vis Sci. 2005; 46: 2790–2799. [DOI] [PubMed] [Google Scholar]
- 26. Weeks OI,, English AW. Compartmentalization of the cat lateral gastrocnemius motor nucleus. J Comp Neurol. 1985; 235: 255–267. [DOI] [PubMed] [Google Scholar]
- 27. Weeks OI,, English AW. Cat triceps surae motor nuclei are organized topologically. Exp Neurol. 1987; 96: 163–177. [DOI] [PubMed] [Google Scholar]
- 28. McNeal DR,, Bowman BR. Selective activation of muscles using peripheral nerve electrodes. Med Biol Eng Comput. 1985; 23: 249–253. [DOI] [PubMed] [Google Scholar]