Abstract
Progressive luminal acidification of intracellular compartments is important for their functions. Proton transport into the organelle's lumen is mediated by vacuolar ATPases (V-ATPases) large multi-subunit proton pumps organized into 2 domains, V0 and V1, working together as a rotary machine. The interaction of each subunit with specific partners plays a crucial role in controlling V-ATPase activity. Recently, we have shown that RILP, a Rab7 effector regulating late endocytic traffic and biogenesis of multivesicular bodies (MVBs), is a specific interactor of the V-ATPase subunit V1G1, a fundamental component of the peripheral stalk for correct V-ATPase assembly. RILP controls V1G1 stability and localization affecting V-ATPase assembly and function at the level of endosomes and lysosomes. The discovery of this new regulatory mechanism for V-ATPase opens new scenario to the comprehension of organelle's pH regulation and reveals a key role of RILP in controlling different aspects of endosome to lysosome transport.
Keywords: V-ATPase, RILP, V1G1, Rab7, atp6v1g1, Rab proteins, Membrane traffic, Endocytosis, Ubiquitin
Abbreviations
- V-ATPase
vacuolar ATPase
- MVBs
multivesicular bodies
- V1G1
subunit G1 of the V-ATPase
- EEs
early endosomes
- LEs
late endosomes
- RILP
Rab-Interacting Lysosomal Protein
- RAVE
Regulator of the ATPase of Vacuolar and Endosomal membranes
- EGF
Epidermal Growth Factor
The luminal pH of endocytic organelles and lysosomes is acidic; acidification increases progressively from endocytic vesicles and early endosomes (EEs) to late endosomes (LEs) and lysosomes. Each organelle maintains a specific internal pH, which is fundamental for its function.1,2 Whereas the pH of EEs is in the 6.8–6.1 range, the pH of LEs is around 5.5 and it reaches values of 4.5–4.7 in lysosomes, these organelles being significantly acidic (Fig. 1). The acidic pH of endocytic organelles not only provides the environment for the activation of the degradative enzymes, but it is also essential for: 1) membrane trafficking, 2) for sorting endosomes to promote the uncoupling of internalized ligand–receptor complexes following receptor-mediated endocytosis, or 3) for the inactivation of internalized pathogens.3,4 In addition acidification in EEs of mammalian cells is required for the formation of endosomal carrier vesicles or MVBs.4-7 Importantly, dysregulation of organelle's acidification is associated to a variety of human diseases, such as osteoporosis, osteopetrosis, male infertility, renal acidosis, and cancer.3 Several channels, transporters, and exchangers control pH of endo-lysosomes. Acidification involves a fine balance between H+ influx and efflux pathways, as well as counter-ion (anion and cation) conductance.1,8,9 The action of the proton pumps, known as the vacuolar (V)-ATPases, using free energy of ATP hydrolysis, promotes the transport of the H+ into organelles lumen generating a voltage difference across the membrane.1-3,9,10 Members of the ClC channel family, through the exchange of 2Cl−/H+, modulate luminal acidification providing anions that neutralize the gradual increase of positive charge in the lumen that inhibits V-ATPase activity.1,8,9,11,12 In addition cation-conductive pathways (for example K+ channels) might also dissipate the voltage generated by the V-ATPase.1,8,9,11The functional interaction between the G1 subunit of the V-ATPase proton pump and RILP (Rab-Interacting Lysosomal Protein), a factor involved in endocytosis, has recently been investigated.13 In this review we will discuss the molecular mechanism involved in this interaction and we will highlight the implications of this interaction in controlling V-ATPase activity and thus endosomes and lysosomes acidification.
Figure 1.
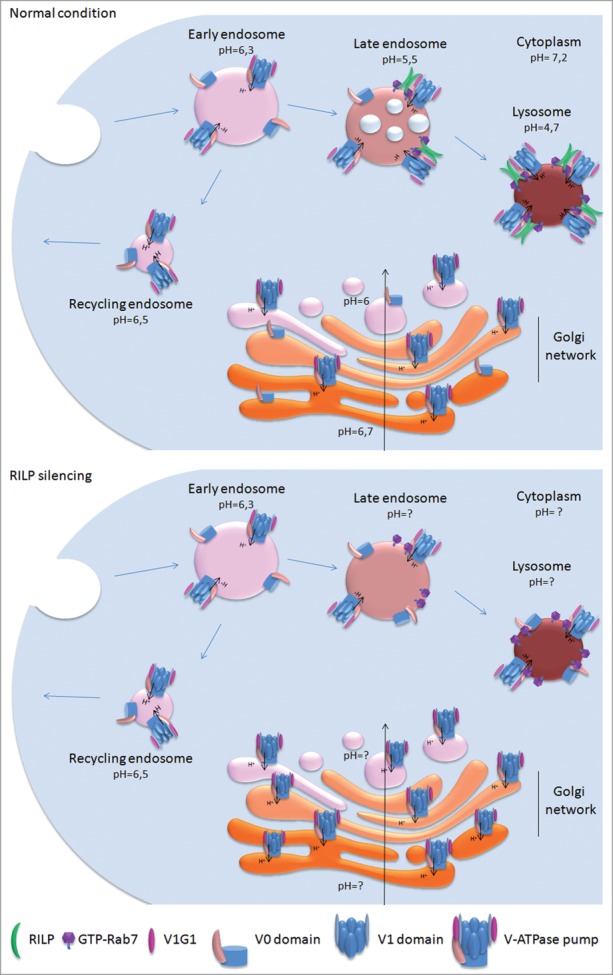
A model for the role of RILP in the recruitment of the V1 domain onto late endosome and lysosomes. The pH along the endocytic pathway gradually decreases from endosomes to lysosomes. The recruitment of RILP on endosomes and lysosomes, mediated by GTP-bound Rab7, promotes the subsequent recruitment of the V1 domain inducing reassembly of the V-ATPase, through the interaction of RILP with the V1G1 subunit. Depletion of RILP prevents the recruitment of V1G1 on late endosomal and lysosomal membranes promoting mislocalization of the V1G1 subunit (and possibly of the V1 domain) on Golgi membranes and affecting organelle's pH.
The V-ATPase proton pump
V-ATPases are large multisubunit complexes organized into 2 domains composed of 14 different subunits that are organized into an ATP-hydrolytic domain (V1) and a proton-translocation domain (V0) that work together as a rotary machine.3,10,14 The V1 domain is peripheral and is composed of 8 different subunits (A, B, C, D, E, F, G, and H) that hydrolyze ATP. The membrane V0 domain, composed of 6 subunits (a, c, c’, c,” d, and e), promotes the translocation of protons from the cytoplasm to the lumen or the extracellular space.3,10,14 Mammals present a rich diversity of V-ATPase subunit isoforms, expressed in a tissue-specific manner; for example, the G1 subunit isoform is expressed ubiquitously, whereas the G2 isoform localizes in neuronal synaptic vesicles.15 In most cases, the role of these various isoforms is not known, but a unique combination of subunit isoforms are found in specific cellular membranes.4 The activity of V-ATPases in vivo is regulated by several mechanisms including reversible dissociation of the V1 and V0 domains.3,4 The E and G subunit of the V1 domain are organized in 3 EG heterodimers that form 3 peripheral stalks.16-20 The stalks provide transient elastic energy during the rotary catalytic cycle and the physical link between the V1 and V0 domain of the pump. The G subunit can, indeed, interact with the a subunit of the V0 domain.17,21,22 In yeast, dissociation and assembly are independently controlled processes.4 The reassembly requires a protein complex called RAVE (regulator of the ATPase of vacuolar and endosomal membranes), composed of rav1, rav2, and of skp1, a component of ubiquitin ligase complexes.4 RAVE interacts with dissociated V1 domains by binding with subunits E and/or G.23
Functional interaction between RILP and V1G1
Recently, we have identified a new functional interaction between the G1 subunit of the V-ATPase V1 domain and the N-terminal domain of RILP.13 RILP is a downstream effector of the small GTPase Rab7, a Rab protein that controls transport to endocytic degradative compartments.24-28 Rab7, in its active GTP-bound form, recruits RILP on membrane.26-28 RILP is required for biogenesis of MVBs and, together with Rab7, for transfer of cargo from EEs, LEs, and MVBs to lysosomes.26,28-30 Our data indicate that RILP, when bound to Rab7, recruits V1G1 on endosomes and lysosomes as we demonstrated that RILP is able to bind simultaneously to V1G1 at its N-terminal half and to Rab7 at its C-terminal half.13 The recruitment of V1G1 is important for V-ATPase assembly and function.13,31,32 Interestingly, overexpression of RILP increases the colocalization of V1G1 with late endosomal and lysosomal markers whereas depletion of RILP delocalizes V1G1 from LEs and lysosomes to Golgi membranes (Fig. 1).13 As RILP-recruited V1G1 is also complexed with other V-ATPase subunits, binding of V1G1 to RILP seems to contribute to the correct assembly of the entire pump on lysosomal and endosomal membranes.13 Indeed, RILP depletion causes not only V1G1 mislocalization, but also a different intracellular distribution of LysoTracker Red (a marker of acidic compartments), suggesting alterations in V-ATPase activity.13 In addition, RILP depletion strongly delays degradation of the EGF receptor and affects MVBs formation again suggesting V-ATPase impairment.29,30 Taken together, these data indicate that RILP, binding to V1G1, recruits the V-ATPase proton pump on late endosomal and lysosomal membranes promoting endosomal acidification and maturation. The correct amount of the V1G1 subunit is fundamental for V-ATPase activity.31,32 Alterations in V1G1 expression destabilize the E subunit modulating V-ATPase assembly and affect cathepsin D maturation similarly to what happens in cells treated with the vacuolar pump inhibitor BafilomycinA1.13,31,32 Moreover, V1G1 depletion causes accumulation of transferrin in acidic compartments suggesting alteration of trafficking at the endosomal level.13 RILP strictly controls also V1G1 abundance, possibly in order to avoid V-ATPase proton pump activation on other organelles. Indeed, when RILP is not recruited on endosomes and lysosomes, as in the case of Rab7 silencing or of RILP overexpression, it induces ubiquitination-dependent proteasomal degradation of V1G1.13 The proteasomal degradation of V1G1 could prevent mislocalization of V1G1 and thus altered activity of the proton pump on other compartments. Rab7T22N, a Rab7 mutant unable to recruit RILP to late endosomal/lysosomal membranes, causes a strong decrease in LysoTracker Red staining probably associated to an increase of pH of intracellular organelles.26 In cells expressing Rab7T22N mutant, RILP binds V1G1 but can't recruit it on late endosomal and lysosomal membranes, probably affecting V-ATPase assembly and function on these organelles. At the same time, RILP could promote V1G1 degradation possibly preventing an increase of acidification in other organelles as observed in RILP-depleted cells.13 The progressively acidification of endocytic organelles has a central role in endosomes maturation. Acidification, triggered by V-ATPase, in EEs of mammalian cells is required for the formation MVBs.4-7 Defects of vesicular acidification block transport from early to LEs.6,33 Different mechanisms control V-ATPase activity: 1) selection of V-ATPase isoforms and V-ATPase concentration on the membrane, 2) chemiosmotic mechanism modulated by the Cl−/H+ and Na+/H+ exchange mediated by channels and transporters, 3) changes in the efficiency of coupling proton transport, and 4) control of reversible dissociation and reassembly of the pump.3,4,17,34 Our work discovered a new molecular mechanism involved in the control of V-ATPase reassembly at the level of endosomes and lysosomes in mammalian cells. The discovery of the functional interaction between RILP and V1G1 opens a new scenario on the regulation of the V-ATPase. RILP and Rab7, regulating V1G1 and thus V-ATPase assembly and activity at the endosomal level, may trigger downstream pathways giving rise to MVBs formation and endosomal progression. Thus, modulation of Rab7 and RILP could help to regulate V-ATPase assembly and activity, with important implications for the development of therapies against genetic and acquired diseases caused by V-ATPase alterations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Work done in the author's laboratories was supported by AIRC (Associazione Italiana per la Ricerca sul Cancro, Investigator Grant N. 14709 to C.B.), Telethon-Italy (Grant GGP09145 to C.B.) and MIUR (Ministero dell’Istruzione, dell’Università e della Ricerca, Grant PRIN2010–2011 to C.B.). M.D.L. was supported by a triennal FIRC (Fondazione Italiana per la Ricerca sul Cancro) fellowship.
References
- 1. Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 2010; 11:50-61; PMID:19997129; http://dx.doi.org/ 10.1038/nrm2820 [DOI] [PubMed] [Google Scholar]
- 2. Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol 2012; 74:69-86; PMID:22335796; http://dx.doi.org/ 10.1146/annurev-physiol-012110-142317 [DOI] [PubMed] [Google Scholar]
- 3. Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 2007; 8:917-29; PMID:17912264; http://dx.doi.org/ 10.1038/nrm2272 [DOI] [PubMed] [Google Scholar]
- 4. Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 2008; 20:415-26; PMID:18511251; http://dx.doi.org/ 10.1016/j.ceb.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal β COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol 1996; 133:29-41; PMID:8601610; http://dx.doi.org/ 10.1083/jcb.133.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clague MJ, Urbé S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem 1994; 269:21-4; PMID:8276796 [PubMed] [Google Scholar]
- 7. Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319:1244-7; PMID:18309083; http://dx.doi.org/ 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- 8. Demaurex N. pH Homeostasis of cellular organelles. News Physiol Sci 2002; 17:1-5; PMID:11821527 [DOI] [PubMed] [Google Scholar]
- 9. Huotari J, Helenius A. Endosome maturation. EMBO J 2011; 30:3481-500; PMID:21878991; http://dx.doi.org/ 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cipriano DJ, Wang Y, Bond S, Hinton A, Jefferies KC, Qi J, Forgac M. Structure and regulation of the vacuolar ATPases. Biochim Biophys Acta 2008; 1777:599-604; PMID:18423392; http://dx.doi.org/ 10.1016/j.bbabio.2008.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scott CC, Gruenberg J. Ion flux and the function of endosomes and lysosomes: pH is just the start: the flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays 2011; 33:103-10; PMID:21140470; http://dx.doi.org/18307107 10.1002/bies.201000108 [DOI] [PubMed] [Google Scholar]
- 12. Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 2008; 43:3-36; PMID:18307107; http://dx.doi.org/ 10.1080/10409230701829110 [DOI] [PubMed] [Google Scholar]
- 13. De Luca M, Cogli L, Progida C, Nisi V, Pascolutti R, Sigismund S, Di Fiore PP, Bucci C. The Rab-interacting lysosomal protein (RILP) regulates vacuolar ATPase acting on the V1G1 subunit. J Cell Sci 2014; In press; http://dx.doi.org/ 10.1242/jcs.142604. [DOI] [PubMed] [Google Scholar]
- 14. Toei M, Saum R, Forgac M. Regulation and isoform function of the V-ATPases. Biochemistry 2010; 49:4715-23; PMID:20450191; http://dx.doi.org/ 10.1021/bi100397s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murata Y, Sun-Wada GH, Yoshimizu T, Yamamoto A, Wada Y, Futai M. Differential localization of the vacuolar H+ pump with G subunit isoforms (G1 and G2) in mouse neurons. J Biol Chem 2002; 277:36296-303; PMID:12133826; http://dx.doi.org/ 10.1074/jbc.M200586200 [DOI] [PubMed] [Google Scholar]
- 16. Oot RA, Huang LS, Berry EA, Wilkens S. Crystal structure of the yeast vacuolar ATPase heterotrimeric EGC(head) peripheral stalk complex. Structure 2012; 20:1881-92; PMID:23000382; http://dx.doi.org/ 10.1016/j.str.2012.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marshansky V, Rubinstein JL, Grüber G. Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta 2014; 1837:857-79; PMID:24508215; http://dx.doi.org/ 10.1016/j.bbabio.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 18. Diepholz M, Venzke D, Prinz S, Batisse C, Flörchinger B, Rössle M, Svergun DI, Böttcher B, Féthière J. A different conformation for EGC stator subcomplex in solution and in the assembled yeast V-ATPase: possible implications for regulatory disassembly. Structure 2008; 16:1789-98; PMID:19081055; http://dx.doi.org/ 10.1016/j.str.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 19. Muench SP, Huss M, Song CF, Phillips C, Wieczorek H, Trinick J, Harrison MA. Cryo-electron microscopy of the vacuolar ATPase motor reveals its mechanical and regulatory complexity. J Mol Biol 2009; 386:989-99; PMID:19244615; http://dx.doi.org/ 10.1016/j.jmb.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 20. Kitagawa N, Mazon H, Heck AJ, Wilkens S. Stoichiometry of the peripheral stalk subunits E and G of yeast V1-ATPase determined by mass spectrometry. J Biol Chem 2008; 283:3329-37; PMID:18055462; http://dx.doi.org/ 10.1074/jbc.M707924200 [DOI] [PubMed] [Google Scholar]
- 21. Norgett EE, Borthwick KJ, Al-Lamki RS, Su Y, Smith AN, Karet FE. V1 and V0 domains of the human H+-ATPase are linked by an interaction between the G and a subunits. J Biol Chem 2007; 282:14421-7; PMID:17360703; http://dx.doi.org/ 10.1074/jbc.M701226200 [DOI] [PubMed] [Google Scholar]
- 22. Song CF, Papachristos K, Rawson S, Huss M, Wieczorek H, Paci E, Trinick J, Harrison MA, Muench SP. Flexibility within the rotor and stators of the vacuolar H+-ATPase. PLoS One 2013; 8:e82207; PMID:24312643; http://dx.doi.org/ 10.1371/journal.pone.0082207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smardon AM, Tarsio M, Kane PM. The RAVE complex is essential for stable assembly of the yeast V-ATPase. J Biol Chem 2002; 277:13831-9; PMID:11844802; http://dx.doi.org/ 10.1074/jbc.M200682200 [DOI] [PubMed] [Google Scholar]
- 24. Bucci C, Chiariello M. Signal transduction gRABs attention. Cell Signal 2006; 18:1-8; PMID:16084065; http://dx.doi.org/ 10.1016/j.cellsig.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 25. Bucci C, Frunzio R, Chiariotti L, Brown AL, Rechler MM, Bruni CB. A new member of the ras gene superfamily identified in a rat liver cell line. Nucleic Acids Res 1988; 16:9979-93; PMID:3057452; http://dx.doi.org/ 10.1093/nar/16.21.9979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell 2000; 11:467-80; PMID:10679007; http://dx.doi.org/ 10.1091/mbc.11.2.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol 2001; 11:1680-5; PMID:11696325; http://dx.doi.org/ 10.1016/S0960-9822(01)00531-0 [DOI] [PubMed] [Google Scholar]
- 28. Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J 2001; 20:683-93; PMID:11179213; http://dx.doi.org/ 10.1093/emboj/20.4.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Progida C, Malerød L, Stuffers S, Brech A, Bucci C, Stenmark H. RILP is required for the proper morphology and function of late endosomes. J Cell Sci 2007; 120:3729-37; PMID:17959629; http://dx.doi.org/ 10.1242/jcs.017301 [DOI] [PubMed] [Google Scholar]
- 30. Progida C, Spinosa MR, De Luca A, Bucci C. RILP interacts with the VPS22 component of the ESCRT-II complex. Biochem Biophys Res Commun 2006; 347:1074-9; PMID:16857164; http://dx.doi.org/ 10.1016/j.bbrc.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 31. Tomashek JJ, Graham LA, Hutchins MU, Stevens TH, Klionsky DJ. V1-situated stalk subunits of the yeast vacuolar proton-translocating ATPase. J Biol Chem 1997; 272:26787-93; PMID:9334266; http://dx.doi.org/ 10.1074/jbc.272.42.26787 [DOI] [PubMed] [Google Scholar]
- 32. Ohira M, Smardon AM, Charsky CM, Liu J, Tarsio M, Kane PM. The E and G subunits of the yeast V-ATPase interact tightly and are both present at more than one copy per V1 complex. J Biol Chem 2006; 281:22752-60; PMID:16774922; http://dx.doi.org/ 10.1074/jbc.M601441200 [DOI] [PubMed] [Google Scholar]
- 33. Baravalle G, Schober D, Huber M, Bayer N, Murphy RF, Fuchs R. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res 2005; 320:99-113; PMID:15714281; http://dx.doi.org/ 10.1007/s00441-004-1060-x [DOI] [PubMed] [Google Scholar]
- 34. Lafourcade C, Sobo K, Kieffer-Jaquinod S, Garin J, van der Goot FG. Regulation of the V-ATPase along the endocytic pathway occurs through reversible subunit association and membrane localization. PLoS One 2008; 3:e2758; PMID:18648502; http://dx.doi.org/ 10.1371/journal.pone.0002758 [DOI] [PMC free article] [PubMed] [Google Scholar]


