Abstract
ABSTRACT. Advances in organ regeneration have been facilitated by gentle decellularization protocols that maintain distinct tissue compartments, and thereby allow seeding of blood vessels with endothelial lineages separate from populations of the parenchyma with tissue-specific cells. We hypothesized that a reconstituted vasculature could serve as a novel platform for perfusing cells derived from a different organ: thus discordance of origin between the vascular and functional cells, leading to a hybrid repurposed organ. The need for a highly vascular bed is highlighted by tissue engineering approaches that involve transplantation of just cells, as attempted for insulin production to treat human diabetes. Those pancreatic islet cells present unique challenges since large numbers are needed to allow the cell-to-cell signaling required for viability and proper function; however, increasing their number is limited by inadequate perfusion and hypoxia. As proof of principle of the repurposed organ methodology we harnessed the vasculature of a kidney scaffold while seeding the collecting system with insulin-producing cells. Pig kidneys were decellularized by sequential detergent, enzymatic and rinsing steps. Maintenance of distinct vascular and collecting system compartments was demonstrated by both fluorescent 10 micron polystyrene microspheres and cell distributions in tissue sections. Sterilized acellular scaffolds underwent seeding separately via the artery (fibroblasts or endothelioma cells) and retrograde (murine βTC-tet cells) up the ureter. After three-day bioreactor incubation, histology confirmed separation of cells in the vasculature from those in the collecting system. βTC-tet clusters survived in tubules, glomerular Bowman's space, demonstrated insulin immunolabeling, and thereby supported the feasibility of kidney-to-pancreas repurposing.
Keywords: decellularization, diabetes, organogenesis, stem cells, tissue engineering
Introduction
Biomaterial tissue scaffolds are fundamental components of many regenerative medicine approaches, with decellularized whole-organs being a promising recent focus in the field. A major strategy has been to overcome the shortage of transplantable organs for human disease by processing tissues from animals and then reconstituting them with patient-derived cells.1 These biological scaffolds are produced by the sequential perfusion of detergents and enzymes to remove all the cellular components, thereby retaining much extracellular matrix (ECM) and basement membranes.2,3 The resilient components (e.g., collagens, laminin, fibronectin) are typically well-preserved by these decellularization protocols, less so for certain proteoglycan and glycosaminoglycans (GAGs).4,5 Advantageously, ECM proteins are largely conserved from person to person and across species, and therefore decellularized scaffold materials do not typically elicit an immune rejection response.6,7 In fact, there is mounting evidence that these types of scaffolds exert beneficial immunomodulatory effects.8–13 Much research heretofore has focused on the preservation of complex architectures which would permit the correct stereotypic placement of appropriate parenchymal cell types and allow recapitulation of organ-specific function (such as for liver, kidney or heart for transplantation).14–19 We now propose to go beyond concordance between a scaffold and its native reconstitution: instead to use decellularized whole organ scaffolds as a vascular platform to perfuse cells originally from a different tissue. We would thus harness these scaffolds’ extensive vasculature to grow hybrid organs. This approach addresses a heretofore unmet need for transplanting cells or organoids which otherwise fail due to insufficient blood perfusion and oxygenation.
As proof of concept we chose to use a pancreatic insulin-producing cell line (βTC-tet) since one of the limitations to their success in humans has been lack of adequate vascularization. It has become clear that for both survival and endocrine function there needs to be enough cells in islets to allow cell-to-cell signaling through pathways such as homeobox-1 (Pdx1).20 The conundrum has been that increasing the cell number improves their viability but exacerbates the hypoxia problem from under-perfusion. To overcome that problem and demonstrate feasibility of the hybrid organ (as well as trans-species) approach, our goal was to grow insulin-producing cells in highly vascularized swine kidney scaffolds. We devised a methodology to decellularize pig kidneys that assured integrity of the distinct tissue compartments. This allowed us to seed the swine vasculature separately from the collecting system, into which we seeded and cultured murine βTC-tet cells that demonstrated insulin immunolabeling.
Materials and Methods
Scaffold preparation
All animal procedures were conducted under guidelines approved by the University of Florida IACUC as a tissue protocol or from a company as intact kidneys harvested from heparinized pigs (Midwest Research Swine, Inc., Gibbon, MN) at approximately 3–5 weeks of age and 5–12 kg weight, perfused with heparin followed by arterial rinsing with saline. The kidneys were isolated and then underwent decellularization using a 4-day protocol similar to that we previously described.2,3,21 The artery and ureter of each kidney were cannulated and connected to a peristaltic pumping system that incorporated pressure-relief protection. The remaining blood in the organs was rinsed out with 2L of heparinized isotonic saline infused via the artery, washed with a nonionic detergent (20L of 1.0% Triton X-100), phosphate-buffered saline (PBS) rinse (2L), then an ionic detergent (20L of 0.25% or 0.75% sodium dodecyl sulfate). A PBS rinse (2L) followed by a second nonionic detergent1 flush was also performed to assist in the clearance of remnant ionic detergent1 before the final PBS (40L), DNase (1L of 0.0025%) and 1% antibiotics/antimycotics (in PBS, 10L) rinses all at 1L/hr. Kidneys were sterilized with 1% MINNCARE® (4L, 4.5% peracetic acid and 22.0% hydrogen peroxide, Mar Cor, Plymouth, MN) or by irradiation with 12 to 16 kGy over 24 to 30 hours (Florida Accelerator Services and Technology, Gainesville, FL). Sterility of the decellularized organ was demonstrated by flowing DMEM supplemented with 10% FBS between 24 and 48 hours before cellular injection(s).
Imaging of kidney compartments
Pump flow rates and pressure profiles were determined to optimize decellularization without disrupting the integrity of the organ compartments. Collagen IV staining was used to show the preservation of the basement membranes. Protocols were developed to image and confirm the separation of seeding antegrade into the artery and retrograde via the ureter. This was accomplished first with polymer particles and then with cells. For the former, we used fluorescently-colored 10 micron polystyrene microspheres and ascertained their distribution by fluorescence imaging of the organ when intact and bisected using an IVIS Spectrum (Perkin Elmer, Waltham, MA) and microscopy. With a peristaltic pump, 2 ml of red 580/605 FluoSpheres® (3.6 × 106 spheres/ml, Life Technologies, Grand Island, NY) in 10 ml of saline were perfused through the artery and images were taken. A second 2 ml of red FluoSpheres® in 10 ml of saline was introduced 2 d later. Then 2 d later, 4 ml of yellow 505/515 FluoSpheres® (3.6 × 106 spheres/ml) in 150 ml of saline were introduced through the ureter and then reimaged with IVIS.
Cell seeding and immunohistochemistry
To establish conditions for cell seeding, we repopulated with murine βTC-tet cells (1.2 × 107cells) retrograde through the ureter with a peristaltic pump assisted with vacuum.14 Once pump flow and pressure parameters were optimized, immortalized GFP+ murine lung fibroblasts (1.4 × 107cells) were used to establish the pattern of distribution through the renal artery; the intact organ was then incubated in a bioreactor for at least 3 d. Cells were grown in in DMEM 4.5 g/L glucose with 10% FBS and 1% penicillin-streptomycin. Media was perfused at 15 ml/min with a peristaltic pump. The bioreactor was situated in a 5% CO2 incubator at 37°C and a separate pump was used to assure gas exchange. Proper oxygenation was verified with a sensor placed in the media (NeoFox, Dunedin, FL). Then the tissue was fixed (4% PFA), paraffin embedded, cut at 4 microns, stained with H&E and for anti-GFP (1:1000, Abcam, Cambridge, MA), and insulin (1:100, Ventana, Tucson, AZ) with Ventana CC1 retrieval and collagen IV (1:25 Dako, Carpinteria, CA) with Ventana protease II immunohistochemical labeling with hematoxylin counter staining (Ventana). Once optimal seeding and pumping conditions were determined using fibroblasts, we confirmed cell distribution and growth using mouse hemangioendothelioma endothelial (EOMA) cells (ATCC, Manassas, VA). Approximately 4.7 × 106 cells were vacuum seeded, which was repeated after static culture for 2 hr and then media flow commenced. On day 4 the scaffold was re-vacuum seeded with 4.9 × 107 EOMA cells. Media was changed every 2 d. On day 7 the media was removed and PBS was flushed through, followed by 10% neutral buffered formalin for 18 hr as preparation for histologic studies. Anti-CD31 (1:50, Santa Cruz Biotechnology, Dallas, TX) labeling was used for imaging the endothelial cells, and the collagen IV antibodies were utilized to define the basement membrane microstructure.
Results
Decellularization and preservation of compartments
The overall structure of the kidney remained complete (Fig. 1A, B). Architecture was optimally preserved during decellularization using fluid pressure and flow parameters of up to approximately 80 mm Hg and 1L/hr, (Fig. 1F–H) compared with native structures (Fig. 1C–E). Collagen IV staining illustrates the basement membrane remained intact as individual tubules and glomeruli are clearly seen (Fig. 1F-H arrowheads pointing at glomerular tufts).
Figure 1.
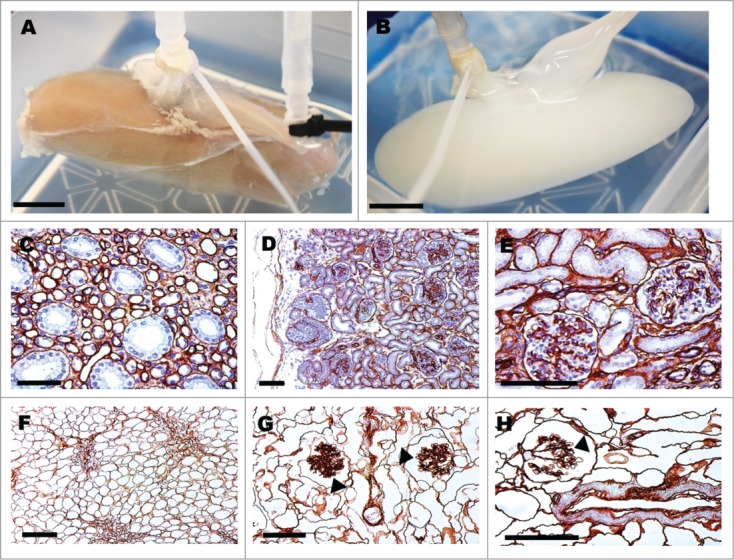
Pig kidneys pre- and post-decellularization and collagen-IV staining of glomerular structures. Gross image of pig kidney pre- (A) and post-decellularization (B), scale bar 1 cm. Collagen-IV immunohistochemical staining of basement membranes (C–H). Pig kidney histology before decellularization (C–E) and after (F–H); arrow heads point to acellular glomerular vascular tufts (G,H). Scale bar (C–H) 100 μm.
FluoSpheres seeding
Seeding with either the FluoSpheres® or cells was possible with positive pressure limited to 80 mm Hg, but was improved by applying 60–80 mm Hg vacuum. Figure 2A, B demonstrates antegrade distribution of the polystyrene particles (in red) from the arterial cannula and retrograde (in green) from the ureter to the medulla and corticomedullary junction, with some particles noted in the mid-cortex. Histologic sections (Fig. 2C, D) confirmed landing of FluoSpheres® from the tip of the ureter back via the collecting system as far as Bowman's space of glomeruli in the outer cortex.
Figure 2.
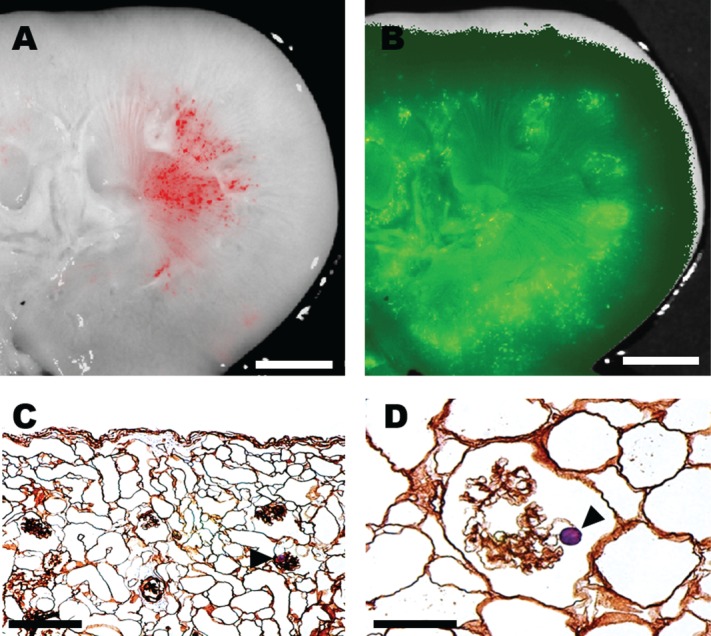
Fluorescent 10 μm polystyrene microspheres (FluoSpheres®) with antegrade perfusion in renal artery (red), (A) visualizing afferent arteriole and glomerulus and retrograde perfusion in ureter (green), (B). Scale bar (A, B) 1 cm. Ten μm polystyrene FluoSpheres® (arrowhead) injected retrograde in the ureter and reaching Bowman's space. Scale bar (C, D) 50 μm.
Cell seeding and distribution
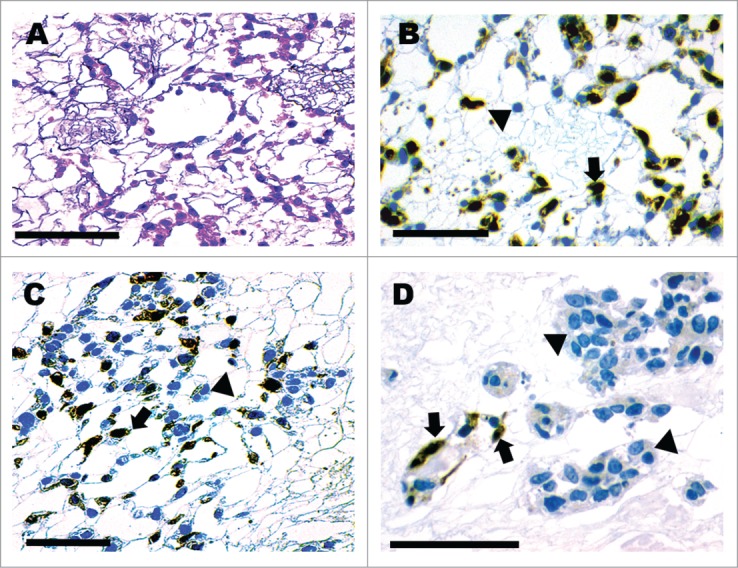
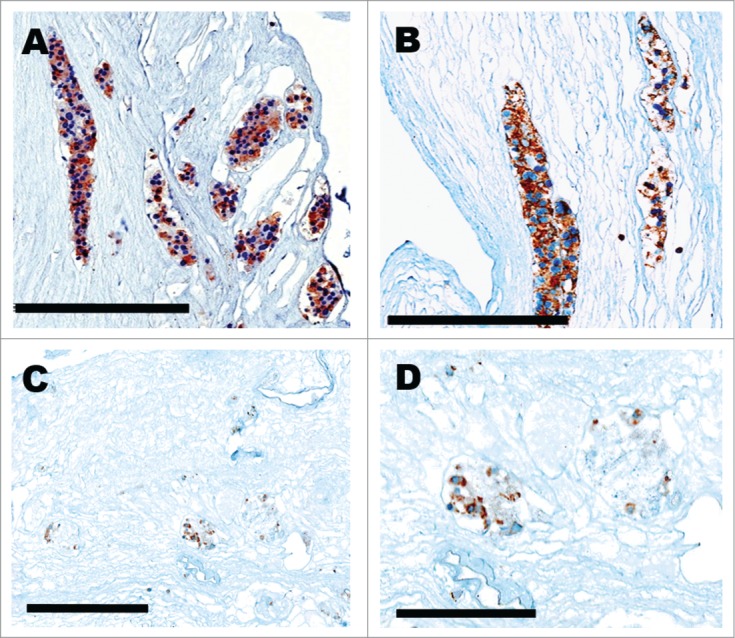
Figure 3A–C shows seeding and survival of arterially perfused GFP+ murine lung fibroblast cells extending to the peritubular capillary networks. Their distribution was distinct from the retrograde-delivered βTC-tet cells that were seeded and survived within the collecting system tubules and Bowman's spaces (Figs. 3D and 4). Figure 3D shows the presence of fibroblast cells in the vasculature (GFP+) and the βTC-tet (GFP-) cells in adjacent tubules. βTC-tet cells within the tubules and Bowman's spaces were also positive for insulin production after a 3-day perfusion culture as evidence by immunohistochemical staining (Fig. 4). After these results were obtained by iterative refinements in the pumping and seeding protocols, the findings were confirmed using the endothelioma cells. As demonstrated in Figure 5, the morphology and anti-CD31 labeling showed that the cells remained within the vascular compartment's vessels, lined the luminal surfaces (Fig. 5B–D), and successfully populated glomerular tufts (Fig. 5A)
Figure 3.
Three-day incubation of scaffold showing antegrade seeding of vasculature and retrograde seeding of collecting system. H&E (A). GFP+ cells line peritubular capillary vessels (B–D) while a GFP− βTC-tet cell population (D) occupy tubular structures. GFP+ cells are marked by arrows and the GFP− βTC-tet cells with arrowheads (B–D). Scale bar 100 μm.
Figure 4.
3-day incubation of scaffold after retrograde seeding of pancreatic β cells. Cells showing positive insulin immunoreactivity localized to the collecting tubules (A, B) and glomeruli (C, D). Scale bar 200 μm (A–C) 100 μm (D).
Figure 5.
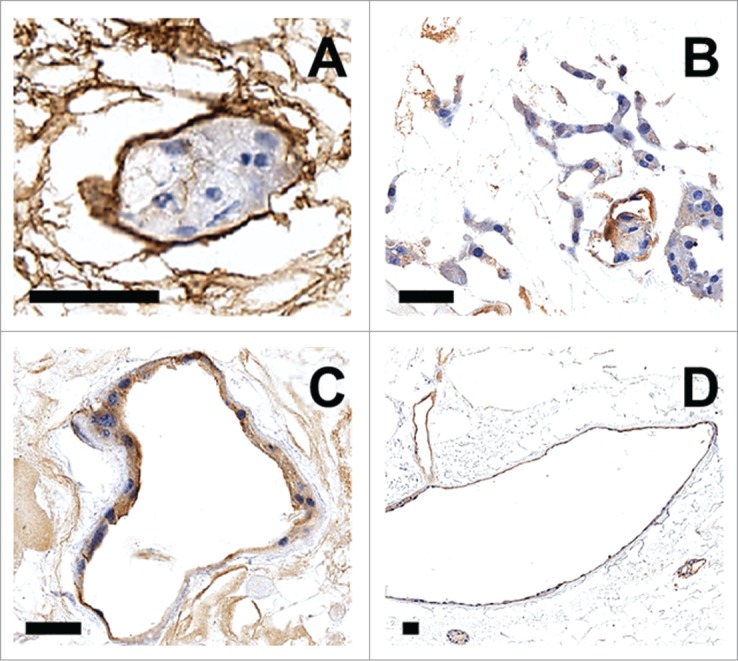
(A) Collagen IV immunohistochemical staining of glomerular tuft containing EOMA cells. (B–D) CD31 immunohistochemical staining of EOMA cells lining blood vessels. Scale bar = 50 μM.
Discussion
Although there has been varied success in restoring organ-specific functionality and structure to repopulated biological scaffolds, we believe a common theme in many reports has been progress in recapitulating the vasculature. Some investigators have used cells already in the endothelial lineage (e.g., human umbilical vascular endothelial cells).22 In an alternative approach we have previously shown23 that precursor (embryonic stem) cells show evidence for differentiation within vessels: expressing endothelial lectin and VEGFR2 (Flk1). Furthermore the cells produce embryologically correct (fetal) collagen IV α 1 and 2 isoforms, laminin and label appropriately for mouse basement membrane antigens within what was originally a rat scaffold. We believe that robust vasculatures such as that within kidneys can be harnessed to perfuse cells from other organs. Here we show for the first time successful repurposing of kidney scaffolds for growth of insulin-producing β cells.
An integral and necessary aspect of this technology is the maintenance of separate vascular and nonvascular (e.g. collecting system) compartments. This allows for distinct seeding and incubation conditions as visualized with FluoSpheres® and cells. We achieved β cell population of the collecting compartment and endothelial cell-line seeding of the blood vessels (including glomeruli).
We believe that it is advantageous to utilize biological scaffolds to regenerate vasculature, in that they inherently preserve chemical signals and permit mechanical shear stress signaling from the peristaltic pumping of the incubation media. In our previous work,23 the evidence for precursor cells to mature into an endothelial lineage was obtained without added growth or differentiation factors; only the matrix and mechanical forces were present. Growth factors could be added to the scaffold or the media so as to further enhance vascularization, as has been successful when sustained release of VEGF was incorporated into synthetic collagen hydrogel or other proangiogenic polymer models.24,25 The geometry of the peritubular capillaries is also favorable due to its proximity to the tubular structures wherein trans-organ cells can be placed. This is relevant to recent evidence for the existence of cross-talk between cells in kidney capillary and tubule compartments.26 There are even reports of vascular beds providing signals to hormone-producing pancreatic cells in a synthetic polymeric model that incorporated endothelial cells and fibroblasts.27 With adequate vascularization one might take advantage of other advances in polymer-based substrates for islet cell growth.28,29
Repurposing of organ scaffolds is particularly promising for pancreatic islet cells. We believe that the kidney's highly vascular structure will permit viability and functionality of the large numbers of cells that are needed by islets for the required cell-to-cell signaling through such pathways as homeobox-1.30 Unfortunately, one reason that pancreatic islet cell transplants have failed in man31,32 (e.g., when engrafted to the liver) is that the large clusters of cells cannot be maintained without appropriate oxygenation.28 Lowering the cell number so as to allow adequate nutrient perfusion not only reduces vital signaling but is disadvantageous in that it limits the amount of insulin produced.33 A theoretical therapeutic strategy would thus be to repurpose an intact decellularized swine kidney: repopulate it with cells derived from the human patient (e.g. vascular and islet cells derived from induced pluripotent stem cells); grow the organ in a bioreactor with appropriate media and growth factors; and then transplant the hybrid graft. Utilization of pig organs for xenotransplantation, rather than smaller ones from rodents, has been studied by other investigators and will necessitate scaling up of the amount of cells and bioreactor time in order to achieve full-tissue regeneration. Homogeneous repopulation of the vessels, for example, would be necessary to allow for stable reanastomosis and to minimize thrombotic tendencies. There is also motivation for using a repurposed scaffold organ, such as kidney, rather than a decellularized pancreas. Although there has been recent notable reported success for the latter approach,34,35 other investigators have previously highlighted the difficulties working with that organ: architectural complexity due to its exocrine function (only up to 2% of cells are thought to be insulin-secreting) and there is a concern that porcine pancreatic extracellular matrix may be disrupted by decellularization protocols.35 While the kidney scaffold's vasculature is more robust and thus superior to nearly all other organs, a potential limitation to the repurposed-tissue strategy is that the collecting system extracellular milieu might not be optimal for pancreatic cells36,37 without further chemical modification or use of appropriate growth factors.25 Lack of native pancreatic matrix may be more important when using relatively undifferentiated precursors,35 as indeed our findings do support the survival of insulin-producing cells.
Lastly, we suggest that biological scaffolds have additional merits over totally synthetic architectures in that the matrix has been reported to have immunomodulatory properties.38–40 Fishman et al.39 have studied effects on cell-mediated immunity and report decreased cytokine production, transition to an M2 macrophage phenotype and down regulation of T-cell sensitization. This is of importance since, apart from issues of oxygenation, human islet cell transplants have also failed due to inflammation and rejection of cadaveric donor tissue. Encapsulation of islet cells41 with synthetic or semi-synthetic substances as an immune barrier is actively being studied by many investigators,42 but to date immunoisolation techniques have not met with long-term success.24,28,43,44 It is not clear whether those difficulties can be overcome by recent advances in the synthesis (e.g., for alginates) and multi-layering of the coating materials.33,42 Although not taking advantage of vascular structures, it is possible that rejection can be attenuated by transplanting micro-organelles of islet cells grown in decellularized biological scaffolds. For example, this might be accomplished by dissection of islets surrounded by acellular kidney matrix and subsequent implantation into an organ such as the liver.
In conclusion, we have demonstrated proof of concept for repurposing a biological scaffold, kidney-to-pancreas, so as to provide both extensive vascularization of large numbers of cells and the benefits from matrix- and cell-to-cell signaling.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. Bryon Petersen and his laboratory at the University of Florida (UF) for significant logistical support of this work, the UF-MBI CTAC for collection of the IVIS data as well as Kevin Walker and Katie Kingham of the North Florida/South Georgia Veterans Health System (NF/SGVHS), the UF Molecular Pathology Core and the Sanford Burnham Prebys Medical Discovery Institute Histology Core Facility at Lake Nona in Orlando, FL for histological work and imaging.
Funding
Financial support in part by the Gatorade Research Foundation.
References
- 1. Guyette JP, Gilpin SE, Charest JM, Tapias LF, Ren X, Ott HC. Perfusion decellularization of whole organs. Nat Protoc 2014; 9:1451-68; PMID:24874812; http://dx.doi.org/ 10.1038/nprot.2014.097 [DOI] [PubMed] [Google Scholar]
- 2. Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD. Embryonic Stem Cells Proliferate and Differentiate when Seeded into Kidney Scaffolds. J Am Soc Nephrol 2009; 20:2338-47; PMID:19729441; http://dx.doi.org/ 10.1681/ASN.2008111196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shupe T, Williams M, Brown A, Willenberg B, Petersen BE. Method for the decellularization of intact rat liver. Organogenesis 2010; 6:134-6; PMID:20885860; http://dx.doi.org/ 10.4161/org.6.2.11546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater 2009; 5:1-13; PMID:18938117; http://dx.doi.org/ 10.1016/j.actbio.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 5. Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng 1996; 2:209-17; PMID:19877943; http://dx.doi.org/ 10.1089/ten.1996.2.209 [DOI] [PubMed] [Google Scholar]
- 6. Badylak SE, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol 2008; 20:109-16; PMID:18083531; http://dx.doi.org/ 10.1016/j.smim.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown NH. Extracellular matrix in development: insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb Perspect Biol 2011; 3:a005082; PMID:21917993; http://dx.doi.org/ 10.1101/cshperspect.a005082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage Phenotype as a Determinant of Biologic Scaffold Remodeling. Tissue Eng Part A 2008; 14:1835-42; PMID:18950271; http://dx.doi.org/ 10.1089/ten.tea.2007.0264 [DOI] [PubMed] [Google Scholar]
- 9. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 2008; 20:86-100; PMID:18162407; http://dx.doi.org/ 10.1016/j.smim.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF. Macrophage Participation in the Degradation and Remodeling of Extracellular Matrix Scaffolds. Tissue Eng Part A 2009; 15:1687-94; PMID:19125644; http://dx.doi.org/ 10.1089/ten.tea.2008.0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sicari BM, Dziki JL, Siu BF, Medberry CJ, Dearth CL, Badylak SF. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials 2014; 35:8605-12; PMID:25043569; http://dx.doi.org/ 10.1016/j.biomaterials.2014.06.060 [DOI] [PubMed] [Google Scholar]
- 12. Wolf MT, Dearth CL, Ranallo CA, LoPresti ST, Carey LE, Daly KA, Brown BN, Badylak SF. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials 2014; 35:6838-49; PMID:24856104; http://dx.doi.org/ 10.1016/j.biomaterials.2014.04.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slivka PF, Dearth CL, Keane TJ, Meng FW, Medberry CJ, Riggio RT, Reing JE, Badylak SF. . Fractionation of an ECM hydrogel into structural and soluble components reveals distinctive roles in regulating macrophage behavior. Biomaterials Sci 2014; 2:1521-34; http://dx.doi.org/ 10.1039/C4BM00189C [DOI] [PubMed] [Google Scholar]
- 14. Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 2013; 19:646-51; PMID:23584091; http://dx.doi.org/ 10.1038/nm.3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robertson MJ, Dries-Devlin JL, Kren SM, Burchfield JS, Taylor DA. Optimizing Recellularization of Whole Decellularized Heart Extracellular Matrix. Plos One 2014; 9:e90406; PMID:24587354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, Edward K, La Francesca S, Sakamoto J, Vega S, et al. Production and Assessment of Decellularized Pig and Human Lung Scaffolds. Tissue Eng Part A 2013; 19:2045-62; PMID:23638920; http://dx.doi.org/ 10.1089/ten.tea.2012.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013; 34:6760-72; PMID:23787110; http://dx.doi.org/ 10.1016/j.biomaterials.2013.05.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 2010; 16:814-U120; PMID:20543851; http://dx.doi.org/ 10.1038/nm.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, Yoo JJ. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 2012; 33:7756-64; PMID:22841923; http://dx.doi.org/ 10.1016/j.biomaterials.2012.07.023 [DOI] [PubMed] [Google Scholar]
- 20. Li SW, Koya V, Li Y, Donelan W, Lin P, Reeves WH, Yang LJ. Pancreatic duodenal homeobox 1 protein is a novel β-cell-specific autoantigen for type I diabetes. Lab Invest 2010; 90:31-9; PMID:19901909; http://dx.doi.org/ 10.1038/labinvest.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ross EA, Abrahamson DR, St John PL, Clapp WL, Williams MJ, Terada N, Hamazaki T, Ellison GW, Batich CD. Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis 2012; 8:49-55; PMID:22692231; http://dx.doi.org/ 10.4161/org.20209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 2013; 19:646-51; PMID:23584091; http://dx.doi.org/ 10.1038/nm.3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ross EA, Abrahamson DR, St John P, Clapp WL, Williams MJ, Terada N, Hamazaki T, Ellison GW, Batich CD. Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis 2012; 8:49-55; PMID:22692231; http://dx.doi.org/ 10.4161/org.20209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coronel MM, Stabler CL. Engineering a local microenvironment for pancreatic islet replacement. Curr Opin Biotechnol 2013; 24:900-8; PMID:23769320; http://dx.doi.org/ 10.1016/j.copbio.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vernon RB, Preisinger A, Gooden MD, D'Amico LA, Yue BB, Bollyky PL, Kuhr CS, Hefty TR, Nepom GT, Gebe JA. Reversal of diabetes in mice with a bioengineered islet implant incorporating a type I collagen hydrogel and sustained release of vascular endothelial growth factor. Cell Transplant 2012; 21:2099-110; PMID:23231959; http://dx.doi.org/ 10.3727/096368912X636786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE. Tubulovascular Cross-Talk by Vascular Endothelial Growth Factor A Maintains Peritubular Microvasculature in Kidney. J Am Soc Nephrol 2014; 26(5):1027-38; PMID:25385849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaufman-Francis K, Koffler J, Weinberg N, Dor Y, Levenberg S. Engineered vascular beds provide key signals to pancreatic hormone-producing cells. PLoS One 2012; 7:e40741; PMID:22808248; http://dx.doi.org/ 10.1371/journal.pone.0040741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smink AM, Faas MM, de Vos P. Toward engineering a novel transplantation site for human pancreatic islets. Diabetes 2013; 62:1357-64; PMID:23613549; http://dx.doi.org/ 10.2337/db12-1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hussey AJ, Winardi M, Han XL, Thomas GP, Penington AJ, Morrison WA, Knight KR, Feeney SJ. Seeding of pancreatic islets into prevascularized tissue engineering chambers. Tissue Eng Part A 2009; 15:3823-33; PMID:19558221; http://dx.doi.org/ 10.1089/ten.tea.2008.0682 [DOI] [PubMed] [Google Scholar]
- 30. Li SW, Koya V, Li Y, Donelan W, Lin P, Reeves WH, Yang LJ. Pancreatic duodenal homeobox 1 protein is a novel β-cell-specific autoantigen for type I diabetes. Lab Invest 2010; 90:31-9; PMID:19901909; http://dx.doi.org/ 10.1038/labinvest.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343:230-8; PMID:10911004; http://dx.doi.org/ 10.1056/NEJM200007273430401 [DOI] [PubMed] [Google Scholar]
- 32. Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes 2005; 54:2060-9; PMID:15983207; http://dx.doi.org/ 10.2337/diabetes.54.7.2060 [DOI] [PubMed] [Google Scholar]
- 33. Calafiore R, Basta G. Clinical application of microencapsulated islets: actual prospectives on progress and challenges. Adv Drug Deliv Rev 2014; 67-68:84-92; PMID:24184490 [DOI] [PubMed] [Google Scholar]
- 34. Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013; 34:6760-72; PMID:23787110; http://dx.doi.org/ 10.1016/j.biomaterials.2013.05.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, Farney AC, Stratta RJ, Atala A, Opara EC, et al. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials 2013; 34:5488-95; PMID:23583038; http://dx.doi.org/ 10.1016/j.biomaterials.2013.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant 2009; 18:1-12; PMID:19476204; http://dx.doi.org/ 10.3727/096368909788237195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orlando G, Gianello P, Salvatori M, Stratta RJ, Soker S, Ricordi C, Domínguez-Bendala J. Cell Replacement Strategies Aimed at Reconstitution of the β-Cell Compartment in Type 1 Diabetes. Diabetes 2014; 63:1433-44; PMID:24757193; http://dx.doi.org/ 10.2337/db13-1742 [DOI] [PubMed] [Google Scholar]
- 38. Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, Raeder RH, Metzger DW. Xenogeneic extracellular matrix grafts elicit a Th2-restricted immune response. Transplantation 2001; 71:1631-40; PMID:11435976; http://dx.doi.org/ 10.1097/00007890-200106150-00024 [DOI] [PubMed] [Google Scholar]
- 39. Fishman JM, Lowdell MW, Urbani L, Ansari T, Burns AJ, Turmaine M, North J, Sibbons P, Seifalian AM, Wood KJ, et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc Natl Acad Sci U S A 2013; 110:14360-5; PMID:23940349; http://dx.doi.org/ 10.1073/pnas.1213228110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol 2004; 12:367-77; PMID:15157928; http://dx.doi.org/ 10.1016/j.trim.2003.12.016 [DOI] [PubMed] [Google Scholar]
- 41. Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980; 210:908-10; PMID:6776628; http://dx.doi.org/ 10.1126/science.6776628 [DOI] [PubMed] [Google Scholar]
- 42. Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev 2014; 67-68:35-73; PMID:23916992 [DOI] [PubMed] [Google Scholar]
- 43. Giraldo JA, Weaver JD, Stabler CL. Tissue engineering approaches to enhancing clinical islet transplantation through tissue engineering strategies. J Diabetes Sci Technol 2010; 4:1238-47; PMID:20920446; http://dx.doi.org/ 10.1177/193229681000400525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lysy PA, Weir GC, Bonner-Weir S. Concise review: pancreas regeneration: recent advances and perspectives. Stem Cells Transl Med 2012; 1:150-9; PMID:23197762; http://dx.doi.org/ 10.5966/sctm.2011-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]


