Abstract
Cell differentiation is associated with the functional differentiation of the nucleus, in which alteration of the expression profiles of transcription factors occurs to destine cell fate. Nuclear transport machineries, such as importin-α, have also been reported as critical factors that induce cell differentiation. Using various fluorescence live cell imaging methods, including time-lapse imaging, FRAP analysis and live-cell imaging associated correlative light and electron microscopy (Live CLEM) of Tetrahymena, a unicellular ciliated protozoan, we have recently discovered that type switching of the NPC is the earliest detectable event of nuclear differentiation. Our studies suggest that this type switching of the NPC directs the fate of the nucleus to differentiate into either a macronucleus or a micronucleus. Our findings in this organism may provide new insights into the role of the NPC in controlling nuclear functions in general in eukaryotes, including controlling cell fate leading to cell differentiation in multicellular metazoa.
Keywords: ciliate, germline, nuclear pore complex, nuclear differentiation, soma, Tetrahymena thermophila
In multicellular organisms, including humans, cells differentiate into various cell types to constitute different tissues and organs during early developmental stages. Such cell differentiation is governed by epigenetic chromatin remodeling involving histone modification1 and DNA methylation2 to establish cell linage-specific gene expression without changing the genomic sequence. Thus, cell differentiation is destined by the functional differentiation of the nucleus.
Several factors are known to be involved in the nuclear differentiation that in turn causes cell differentiation. One set of such factors are transcription factors. It is known that expression of a specific set of transcription factors can reprogram the differentiation state of fully differentiated cells obtained from an adult animal back to a pluripotent state;3 Oct4 (Pou5f1), Sox2, c-Myc, and Klf4 have been identified as key factors that cause nuclear reprogramming in mice and humans.3,4 Thus, transcription factors act as a molecular switch to change the cellular state of differentiation.
Because such transcription factors have to be transported into the nucleus, the nuclear transport machinery that transports transcription factors into the nucleus is likely to be another factor involved in nuclear differentiation. In support of this premise, it has been reported that subtype switching of importin-α leads to cellular differentiation of embryonic stem (ES) cells into neuronal cells;5 importin-α is an adaptor protein that connects the cargo protein to the transporter protein, called importin-β, by binding to the amino acid residues known as the nuclear localization signal (NLS) of the cargo protein. Distinct subtypes of importin-α that transport a distinct set of neural transcription factors into the nucleus cause neural differentiation in ES cells.6
Another factor that participates in the nuclear transport of transcription factors, and consequently likely to be involved in nuclear differentiation, is the nuclear pore complex (NPC). All nuclear proteins, including transcription factors, must travel from the cytoplasm through the NPC to reach the nucleus. The NPC structure is well conserved among the eukaryotes.7-11 It is composed of multicopies of almost 30 kinds of the protein components, called nucleoporins (Nup). Recent studies have revealed that the composition of NPC components varies between various cell lines that have a different state of cell differentiation and also changes during cell differentiation.12-16 However, it remains unclear whether such differences in NPC composition is a cause or a consequence of cell differentiation, because no experimental system that directly links active changes of NPC composition to cell differentiation exists.
Tetrahymena is a fascinating model organism that has 2 functionally and structurally distinct nuclei, the macronucleus (MAC) and the micronucleus (MIC), within a single cell. The MAC is a somatic nucleus, in which gene expression is fully active during all life cycle stages. The MIC is a germline nucleus, which is used for sexual reproduction; gene expression in the MIC is kept inactive in all life cycle stages.17 The MAC and MIC are generated from a single zygotic nucleus that originates from the MIC. Thus, in Tetrahymena the mitotic daughters of a single nucleus differentiate into 2 functionally and structurally distinct nuclei.
We used Tetrahymena to study the relationship between nuclear structure and function and to investigate the possibility that the NPC may be involved in nuclear differentiation. The NPC is composed of distinct sets of nucleoporins in the MAC and MIC. The well-conserved NPC component Nup98 has 4 homologs in Tetrahymena thermophila.18,19 Two of the 4 Nup98 homologs are specifically localized in the MAC NPC and the other 2 are specifically localized in the MIC NPC.18,20 The MAC-specific Nup98s possess Gly-Leu-Phe-Gly (GLFG) repeats in their N-terminal regions, while the 2 MIC-specific Nup98s possess Asn-Ile-Phe-Asn (NIFN) repeats in their corresponding N-terminal regions.18 These distinct repeat regions of the Nup98s function in the transport of nucleus-specific linker histones exclusively to the correct nucleus.18 Thus, the MAC-type NPC and the MIC-type NPC can direct nuclear transport of particular proteins to either the MAC or the MIC in Tetrahymena.
To delineate the process of nuclear differentiation, we observed conjugating Tetrahymena cells using various fluorescence live-cell imaging methods, including time-lapse observation, FRAP analysis and Live CLEM (live-cell imaging associated correlative light and electron microscopy).21 We found that biased assembly of the MAC-type NPCs occurs immediately after the last post-zygotic division, which generates anterior-posterior polarized nuclei. MAC-specific NPCs assemble in anterior nuclei (presumptive MACs), but not in posterior nuclei (presumptive MICs) (Fig. 1). MAC-specific NPC assembly in the anterior nuclei occurs much earlier than transport of Twi1p,21 which is required for MAC genome rearrangement.22 This result indicates that type switching of the NPC is the first event to determine/direct the fate of the nucleus.
Figure 1.
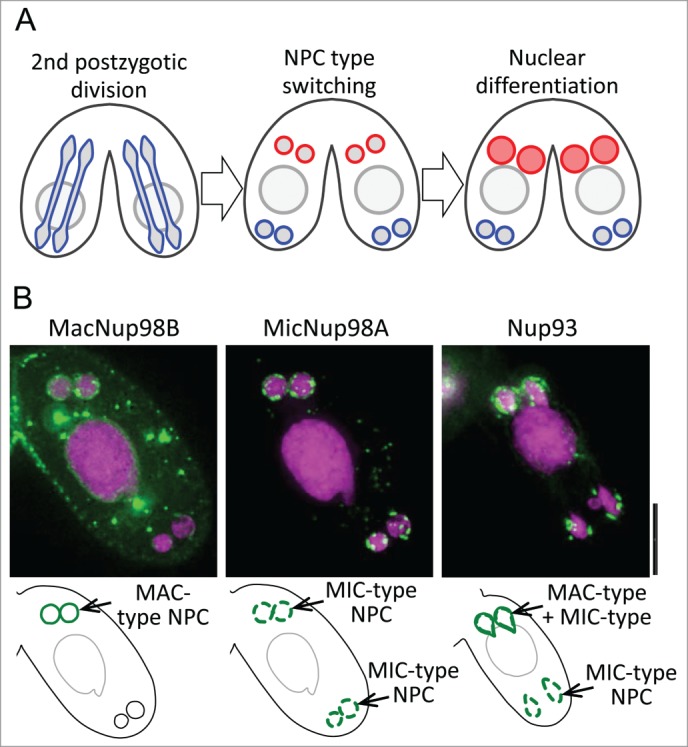
De novo assembly of the macronuclear NPC occurs only in presumptive new macronuclei. (A) Schematic representation of nuclear differentiation of Tetrahymena. NPC type switching from MIC-type (represented by blue) to MAC-type (represented by red) occurs only in the two anterior-located daughter nuclei of the second postzygotic division (PZD) of the MIC-like zygotic nucleus in each mating partner. The two nuclei that acquire MAC-type NPCs differentiate to new macronuclei. (B) Localization of fluorescent protein-tagged nucleoporins early in nuclear differentiation. Methanol-fixed mating pairs were observed. MacNup98B (representing the MAC-type NPC) appeared only in the anterior daughter nuclei of the second PZD (left panel). The pre-existing MicNup98A (representing the MIC-type NPC) is evenly segregated to both daughter nuclei (center panel). Nup93 that commonly exists in all NPCs (representing both types of NPC) exhibits biased distribution to the anterior nuclei (right panel). Left and center panels show the same mating partner of the pair expressing GFP-MacNup98B and mCherry-MicNup98A. mCherry fluorescence in the center panel is shown in green. In the right panel, the single partner of the GFP-Nup93-expressing pair is different from that shown in the other panels. In all panels, the upper side is the anterior of the cell and magenta represents DAPI staining. Bar is 10 mm.
Tetrahymena may be regarded as an extreme and singular representative of an organism in which the NPC is involved in the nuclear differentiation process. However, the involvement of the NPC in nuclear differentiation is difficult to detect in typical mononucleated cells since nuclear transport is only directed to a single nucleus. Interestingly, Nup210 (also called gp210) is required for myogenic and neuronal differentiation16 and Nup133 is required for neural differentiation,13 suggesting that a role of the NPC in controlling nuclear differentiation may be common in mammalian cells. In addition, tissue-specific expression of the nucleoporins12 and disease related mutations of the nucleoporins14 also support our idea that the NPC is one of determinants controlling the state of nuclear differentiation. Thus, our finding in Tetrahymena, revealing a role of the NPC and/or nucleoporins in nuclear differentiation, opens the possibility that the NPC is a master switch in determining the fate of cells during differentiation generally in multicellular eukaryotes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. David B. Alexander for critical reading of the manuscript.
Funding
This work was supported by grants from JST [to T.H.] and from MEXT [grant numbers 23128514, 24570227 to M.I., 26251037, 26116511 to Y.H. and 21370094, 23114724, 25116006, 26291007 to T.H.].
References
- 1.Barth TH, Imhof A. Fast signals and slow marks: the dynamics of histone modifications. Trends Biochem Sci 2010; 35:618-26; PMID:20685123; http://dx.doi.org/ 10.1016/j.tibs.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Lee HJ, Hore TA, Reik W. Reprogramming the methylome: erasing memory and creating diversity. Cell Stem Cell 2014; 14:710-9; PMID:24905162; http://dx.doi.org/ 10.1016/j.stem.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663-76; PMID:16904174; http://dx.doi.org/ 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 4.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 2007; 448:313-7; PMID:17554338; http://dx.doi.org/ 10.1038/nature05934 [DOI] [PubMed] [Google Scholar]
- 5.Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, Oe S, et al.. Triggering neural differentiation of ES cells by subtype switching of importin-α. Nat Cell Biol 2007; 9:72-9; PMID:17159997; http://dx.doi.org/ 10.1038/ncb1521 [DOI] [PubMed] [Google Scholar]
- 6.Yasuhara N, Yamagishi R, Arai Y, Mehmood R, Kimoto C, Fujita T, et al.. Importin alpha subtypes determine differential transcription factor localization in embryonic stem cells maintenance. Dev Cell 2013; 26:123-35; PMID:23906064; http://dx.doi.org/ 10.1016/j.devcel.2013.06.022 [DOI] [PubMed] [Google Scholar]
- 7.Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol 2000; 148:635-52; PMID:10684247; http://dx.doi.org/ 10.1083/jcb.148.4.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol 2002; 158:915-27; PMID:12196509; http://dx.doi.org/ 10.1083/jcb.200206106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeGrasse JA, DuBois KN, Devos D, Siegel TN, Sali A, Field MC, et al.. Evidence for a shared nuclear pore complex architecture that is conserved from the last common eukaryotic ancestor. Mol Cell Proteomics 2009; 8:2119-30; PMID:19525551; http://dx.doi.org/ 10.1074/mcp.M900038-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I. Identification and characterization of nuclear pore complex components in Arabidopsis thaliana. Plant Cell 2010; 22:4084-97; PMID:21189294; http://dx.doi.org/ 10.1105/tpc.110.079947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asakawa H, Yang HJ, Yamamoto TG, Ohtsuki C, Chikashige Y, Sakata-Sogawa K, et al.. Characterization of nuclear pore complex components in fission yeast Schizosaccharomyces pombe. Nucleus 2014; 5:149-62; PMID:24637836; http://dx.doi.org/ 10.4161/nucl.28487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsson M, Schéele S, Ekblom P. Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp Cell Res 2004; 292:359-70; PMID:14697343; http://dx.doi.org/ 10.1016/j.yexcr.2003.09.014 [DOI] [PubMed] [Google Scholar]
- 13.Lupu F, Alves A, Anderson K, Doye V, Lacy E. Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev Cell 2008; 14:831-42; PMID:18539113; http://dx.doi.org/ 10.1016/j.devcel.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho AR, Yang KJ, Bae Y, Bahk YY, Kim E, Lee H, et al.. Tissue-specific expression and subcellular localization of ALADIN, the absence of which causes human triple A syndrome. Exp Mol Med 2009; 41:381-6; PMID:19322026; http://dx.doi.org/ 10.3858/emm.2009.41.6.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asally M, Yasuda Y, Oka M, Otsuka S, Yoshimura SH, Takeyasu K, et al.. Nup358, a nucleoporin, functions as a key determinant of the nuclear pore complex structure remodeling during skeletal myogenesis. FEBS J 2011; 278:610-21; PMID:21205196; http://dx.doi.org/ 10.1111/j.1742-4658.2010.07982.x [DOI] [PubMed] [Google Scholar]
- 16.D'Angelo MA, Gomez-Cavazos JS, Mei A, Lackner DH, Hetzer MW. A change in nuclear pore complex composition regulates cell differentiation. Dev Cell 2012; 22:446-58; PMID:22264802; http://dx.doi.org/ 10.1016/j.devcel.2011.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orias E, Cervantes MD, Hamilton EP. Tetrahymena thermophila, a unicellular eukaryote with separate germline and somatic genomes. Res Microbiol 2011; 162:578-86; PMID:21624459; http://dx.doi.org/ 10.1016/j.resmic.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamoto M, Mori C, Kojidani T, Bunai F, Hori T, Fukagawa T, et al.. Two distinct repeat sequences of Nup98 nucleoporins characterize dual nuclei in the binucleated ciliate Tetrahymena. Curr Biol 2009; 19:843-7; PMID:19375312; http://dx.doi.org/ 10.1016/j.cub.2009.03.055 [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto M, Asakawa H, Hiraoka Y, Haraguchi T. Nucleoporin Nup98: a gatekeeper in the eukaryotic kingdoms. Genes Cells 2010; 15:661-9; PMID:20545767; http://dx.doi.org/ 10.1111/j.1365-2443.2010.01415.x [DOI] [PubMed] [Google Scholar]
- 20.Malone CD, Falkowska KA, Li AY, Galanti SE, Kanuru RC, LaMont EG, et al.. Nucleus-specific importin alpha proteins and nucleoporins regulate protein import and nuclear division in the binucleate Tetrahymena thermophila. Eukaryot Cell 2008; 7:1487-99; PMID:18676955; http://dx.doi.org/ 10.1128/EC.00193-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwamoto M, Koujin T, Osakada H, Mori C, Kojidani T, Matsuda A, et al.. Biased assembly of the nuclear pore complex is required for somatic and germline nuclear differentiation in Tetrahymena. J Cell Sci 2015; 128:1812-23; PMID:25788697; http://dx.doi.org/ 10.1242/jcs.167353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochizuki K, Fine NA, Fujisawa T, Gorovsky MA. Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in Tetrahymena. Cell 2002; 110:689-99; PMID:12297043; http://dx.doi.org/ 10.1016/S0092-8674(02)00909-1 [DOI] [PubMed] [Google Scholar]


