Abstract
Somatostatin subtype-4 receptors (sst4) inhibit L-type calcium channel currents (ICa) in retinal ganglion cells (RGCs). Here we identify the signaling pathways involved in sst4 stimulation leading to suppression of ICa in RGCs. Whole cell patch clamp recordings were made on isolated immunopanned RGCs using barium as a charge carrier to isolate ICa. Application of the selective sst4 agonist, L-803 (10 nM), reduced ICa by 41.2%. Pretreatment of cells with pertussis toxin (Gi/o inhibitor) did not prevent the action of L-803, which reduced ICa by 34.7%. To determine the involvement of Gβγ subunits after sst4 activation, depolarizing pre-pulse facilitation paradigms were used to remove voltage-dependent inhibition of calcium channels. Pre-pulse facilitation did not reverse the inhibitory effects of L-803 on ICa (8.4 vs. 8.8% reductions, ctrl vs. L-803); however, pharmacologic inhibition of Gβγ reduced ICa suppression by L-803 (23.0%, P < 0.05). Inhibition of PKC (GF109203X; GFX) showed a concentration-dependent effect in preventing the action of L-803 on ICa (1 μM GFX, 34.3%; 5 μM GFX, 14.6%, P < 0.05). When both PKC and Gβγ were inhibited, the effects of L-803 on ICa were blocked (1.8%, P < 0.05). These results suggest that sst4 stimulation modulates RGC calcium channels via Gβγ and PKC activation. Since reducing intracellular Ca2+ is known to be neuroprotective in RGCs, modulating these sst4 signaling pathways may provide insights to the discovery of unique therapeutic targets to reduce intracellular Ca2+ levels in RGCs.
Keywords: G-protein coupled receptor, Sst4, GPCR, SRIF, L-803, 087
Introduction
Somatostatin, also known as somatotropin release inhibiting factor (SRIF), is a neuropeptide transmitter mediating biological actions throughout the nervous system.1 Many biological actions regulated by SRIF are in the endocrine system,2 such as hypothalamic SRIF release inhibiting growth hormone secretion from the pituitary gland,3 or pancreatic SRIF inhibiting insulin and glucagon secretion.4 However, there are also significant effects of SRIF in the retina.5
In order to produce effects in a cell, SRIF must stimulate one of its 5 G-protein coupled receptor subtypes, sst1-5, which are distributed throughout the nervous system.2,5,6 Previous studies have shown that selective stimulation of somatostatin receptor subtype 4 (sst4) reduces calcium influx through voltage-gated calcium channels,7,8 and that in the retina, sst4 is expressed exclusively in retinal ganglion cells (RGCs).7 Since RGC degeneration due to Ca2+ dysregulation can be a significant contributor to vision loss, understanding the mechanisms of sst4 stimulation may aid in the development of cell-selective neuroprotective therapies.
L-803,087 (L-803), a selective agonist for sst4, was shown to modulate Ca2+ influx via inhibition of L-type calcium channels, but not N-type calcium channels in RGCs.7 The intracellular signaling mechanisms through which sst4 modulates these channels has yet to be determined. Most sst subtypes have been shown to couple to the inhibitory GTP-binding protein Gi/o,9 suggesting that sst4 stimulation could modulate calcium channels through inhibition of cAMP-dependent actions. Gαi inhibits adenylyl cyclase, reducing levels of cAMP and thus diminishes the activation of PKA, which may in turn lead to a reduction of ICa.10 Another mechanism through which some sst subtypes have been reported to act is via Gαq.11 When a Gq coupled receptor is activated, Gαq stimulates phospholipase C (PLC) resulting in increased levels of DAG and PKC activity, which can also lead to reduction of ICa.6,12 GTP-binding proteins also contain βγ subunits, which may directly inhibit calcium channels13 or activate other signaling molecules such as PLC.14,15 Voltage-dependent Gβγ inhibition of calcium channels can be identified by applying strong depolarizing voltage prepulses that briefly displace the Gβγ inhibition and show an increase, or facilitation, of ICa. There are also voltage-independent Gβγ interactions with calcium channels, which can be blocked using pharmacological agents.16,17 Thus, Gi/o, Gq and Gβγ are potential signaling pathways that sst4 may utilize to inhibit ICa in RGCs.
The purpose of this study is to identify the intracellular signaling pathways through which selective sst4 stimulation reduces Ca2+ influx through calcium channels. We performed whole cell patch clamp experiments on isolated rat retinal ganglion cells to identify the cytoplasmic components of sst4 signaling. This report demonstrates that calcium channel inhibition in RGCs results from sst4 acting not through a cAMP-mediated process, but rather through a combination of Gαq activation of the PKC pathway and a voltage-independent Gβγ interaction causing direct inhibition of the calcium channels.
Results
The aim of the present study was to determine the intracellular signaling molecules involved in the sst4-mediated inhibition of L-type voltage-gated calcium channels in isolated retinal ganglion cells (RGCs). Figure 1A shows examples of voltage-clamp recordings from an acutely isolated adult rat RGC in the absence (black trace) and presence (red trace) of the selective sst4 agonist (L-803, 10 nM). The voltage ramp protocol (−60 mV to +40 mV; 1 Hz) used to depolarize the cell membrane is shown below. Similar to our previous findings in cultured neonatal rat RGCs,7 mean data show that L-803 (10 nM, 2 min.) significantly reduced ICa in acutely isolated adult rat RGCs (Fig. 1B; *P < 0.05, 2-way RM ANOVA vs. control). In isolated adult rat RGCs stimulation of sst4 by L-803 caused an average reduction in ICa of approximately 40% (Fig. 7).
Figure 1.
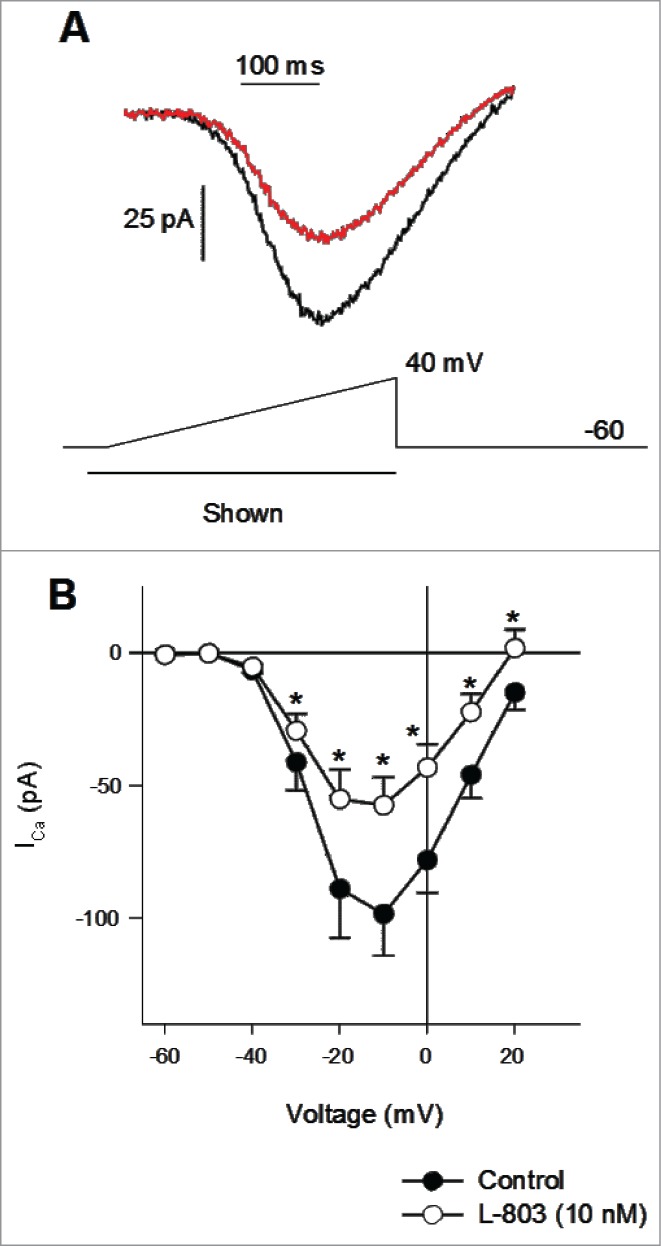
Calcium channel currents reduced by L-803,087 in isolated adult retinal ganglion cells (RGCs). (A) Calcium channel currents recorded during a voltage ramp from an isolated RGC in the absence (black) and presence of L-803,087 (gray, 10 nM). (B) The mean data show that L-803,087 produced a significant reduction of calcium channel current (n = 27; *P < 0.05, 2-way RM ANOVA vs. control).
Figure 7.
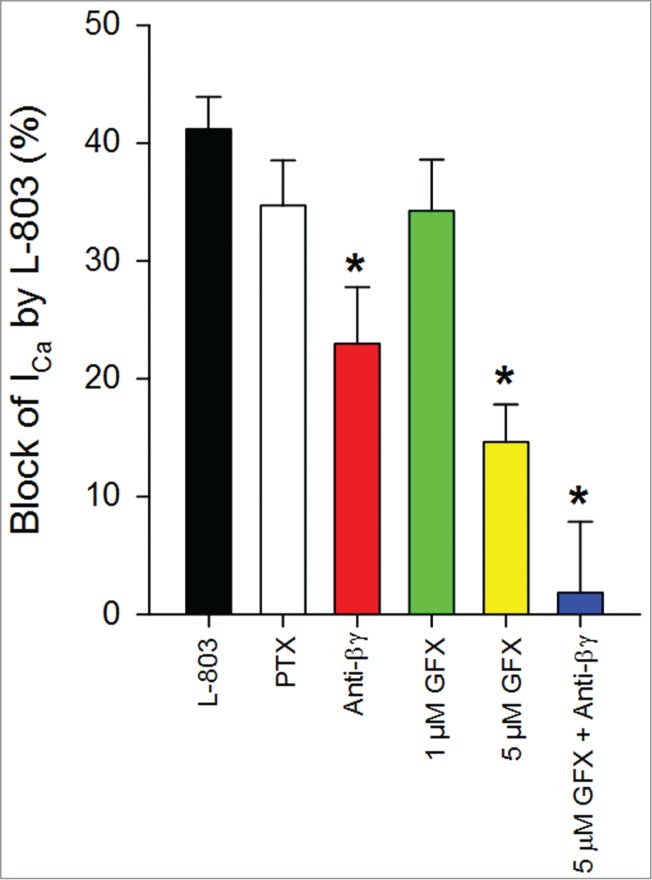
Summary of effects of signaling pathway modulators administered with L-803,087. L-803 reduced ICa in isolated RGCs (n = 27; 41.2 +/− 2.7% vs. control). Pre-treatment with pertussis toxin (PTX) had no effect on the L-803-mediated calcium channel current reduction (n = 10; 34.7 +/− 3.8%). The non-selective PKC antagonist, GF109203X had a concentration dependent effect on L-803-mediated current reduction (1 μM: n = 11; 34.3 +/− 4.4%; 5 μM: n = 8; 14.6 +/− 3.2%; *P < 0.05 vs. L-803 alone). Pre-treatment of cells with anti-β gamma (Anti-Gßγ) peptide impaired the ability of L-803 to reduce calcium channel current (n = 10; 23.0 +/− 4.8%; *P < 0.05 ANOVA vs. L-803 alone). Combination treatment with 5 μM GFX and Anti-Gßγ peptide abolished the effects of L-803 (n = 6; 1.8 +/− 6.0%; *P < 0.05 ANOVA vs. L-803 alone).
To determine whether the sst4-mediated reduction in ICa involved a pertussis toxin-sensitive G-protein (Gi/o), RGCs were incubated with pertussis toxin (PTX; 0.5 μg/mL overnight; as per ref. 18) to inactivate toxin-sensitive G-proteins. Figure 2A shows an example of ICa recordings from an isolated RGC pretreated with PTX in the absence (black trace) and presence (red trace) of L-803 (10 nM). Pretreatment with PTX did not affect ICa amplitudes when compared to control (no PTX pretreatment) recordings (data not shown). Mean data show that L-803 (10 nM, 2 min) significantly reduced ICa in RGCs that had been pretreated with PTX (Fig. 2B; *P < 0.05, 2-way RM ANOVA vs. PTX pretreatment). When compared to RGCs that had not been pretreated with PTX, L-803 administration showed similar reductions in ICa (Fig. 7; 34.7 ± 3.8% vs. 41.2 ± 2.7%, PTX pre-treated vs. L-803 alone). Thus, sst4-mediated reduction of ICa in RGCs does not require the activation of a pertussis-sensitive G-protein.
Figure 2.
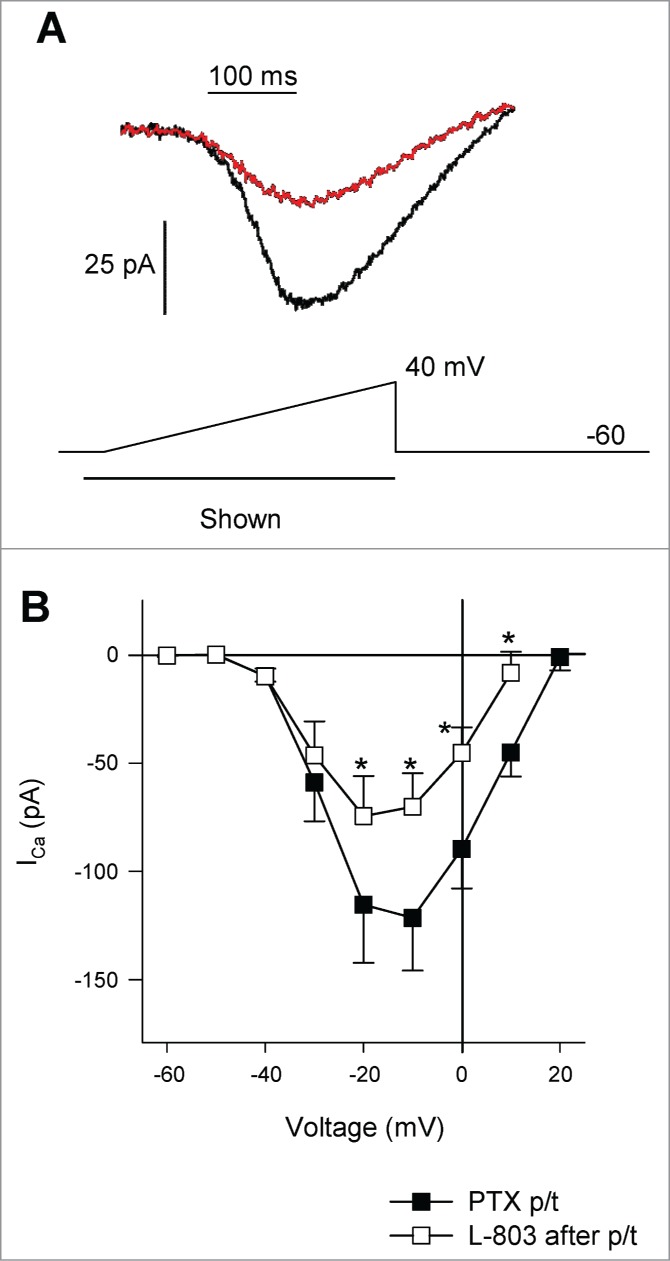
Pre-treatment of RGCs with pertussis toxin (PTX; Gi/o inhibitor) does not prevent the inhibitory actions of L-803,087 on calcium channel currents. (A) Calcium channel current in an isolated RGC pre-treated (p/t) with PTX. Administration of L-803,087 (gray, 10 nM) reduced calcium channel current compared to control (black trace). (B) The mean data show that L-803,087 significantly reduced calcium channel current(n = 10; *P < 0.05, 2-way RM ANOVA vs. PTX p/t).
It is also possible Gβγ proteins may be involved in the inhibition of L-type calcium channels in rat RGCs. Previous studies have shown voltage-dependent and -independent Gβγ interactions with Cav2 calcium channels that result in reduced ICa.19,20 To determine whether voltage-dependent Gβγ interactions with L-type (Cav1) calcium channels were involved in the sst4-mediated reduction in ICa, a pre-pulse facilitation voltage paradigm was designed. It has been shown previously that a short, strong depolarizing voltage step causes a conformational change in the N-type calcium channel which causes dissociation of the Gβγ protein from the channel resulting in a temporary increase in ICa.21 Figure 4A shows an example paired pre-pulse recording from an isolated RGC in the presence of L-803 (10 nM, 2 min). Prior to the depolarizing step, ICa may be influenced by Gβγ; however, after the strong depolarization, ICa is increased due to the dissociation of Gβγ from the channels. Pre-pulse facilitation of ICa was measured as the increase in ICa after the depolarizing step (120 mV, 50 ms) as a percentage of the current prior to the pre-pulse. When comparing paired recordings in the absence and presence of L-803, the mean data show no significant change in pre-pulse facilitation (Fig. 4B). These results show that while pre-pulse facilitation of calcium channels occurs in RGCs, voltage-dependent Gβγ interactions with calcium channels are not involved in the L-803-mediated reduction in ICa in these cells.
Figure 4.
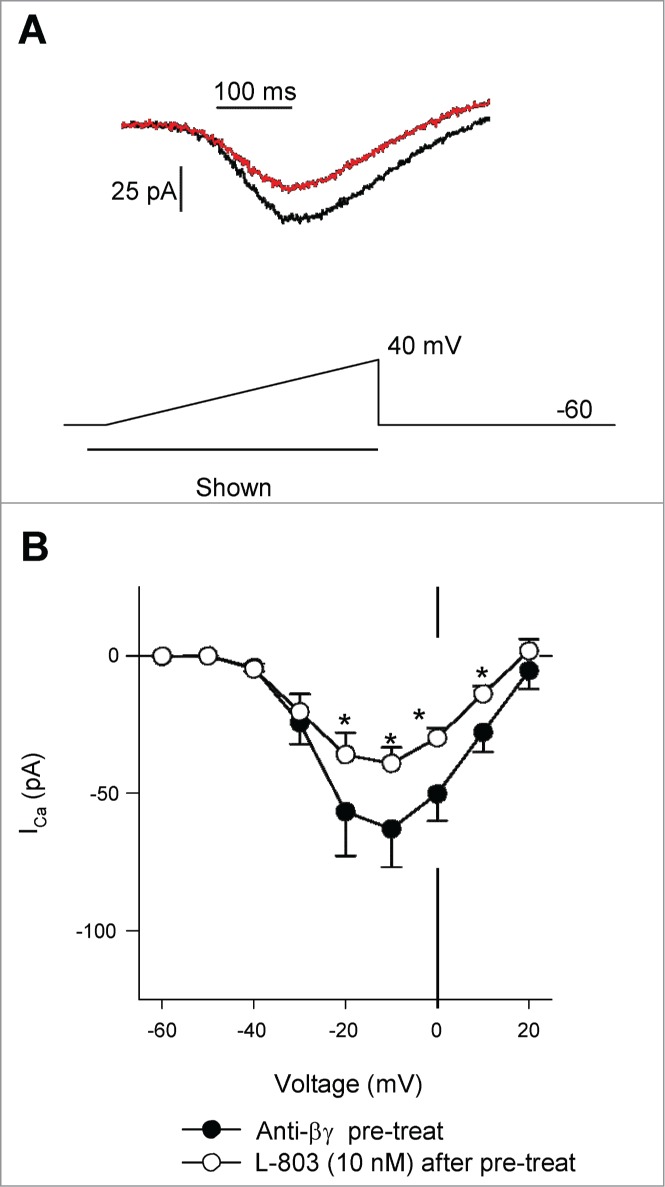
Pre-treatment of isolated RGCs with anti-Gβγ peptide reduced calcium channel suppression by L-803,087. (A) Calcium channel currents recorded from an isolated RGC pretreated with a combination of anti-Gβγ peptide (1 μM) in the absence (black) and presence of L-803,087 (gray, 10 nM). (B) The mean data show that L-803,087 reduced calcium channel current in cells pre-treated with anti-Gβγ peptide (n = 10, *P < 0.05, 2 way RM ANOVA).
Next, to determine whether Gβγ plays other roles in sst4 signaling (e.g., via voltage-independent interactions between Gβγ and calcium channels or stimulation of other signaling molecules), an inhibitor of Gβγ proteins was incubated with isolated RGCs prior to recording. Anti-βγ peptide is a small membrane permeable peptide that interacts with Gβγ, preventing its dissociation from the G-protein trimeric complex after receptor stimulation.16,17 Figure 5A shows a voltage-clamp recording from an isolated RGC after pre-treatment with anti-βγ peptide (1 μM, 15 min.) before (black trace) and after L-803 administration (red trace). Stimulation of sst4 by L-803 (10 nM, 2 min.) significantly reduced ICa in RGCs pre-treated with anti-βγ peptide (Fig. 5B, *P < 0.05, 2-way RM ANOVA vs. Anti-βγ peptide pretreatment). However, the inhibitory effect of L-803 was less than that observed in cells that have not been pretreated (Fig. 7; 23.0 ± 4.8% vs. 41.2 ± 2.7%, anti-βγ peptide pretreatment vs. L-803 alone; *P < 0.05, ANOVA). Thus, these data suggest a role of Gβγ signaling in the sst4-mediated inhibition of L-type calcium channels.
Figure 5.
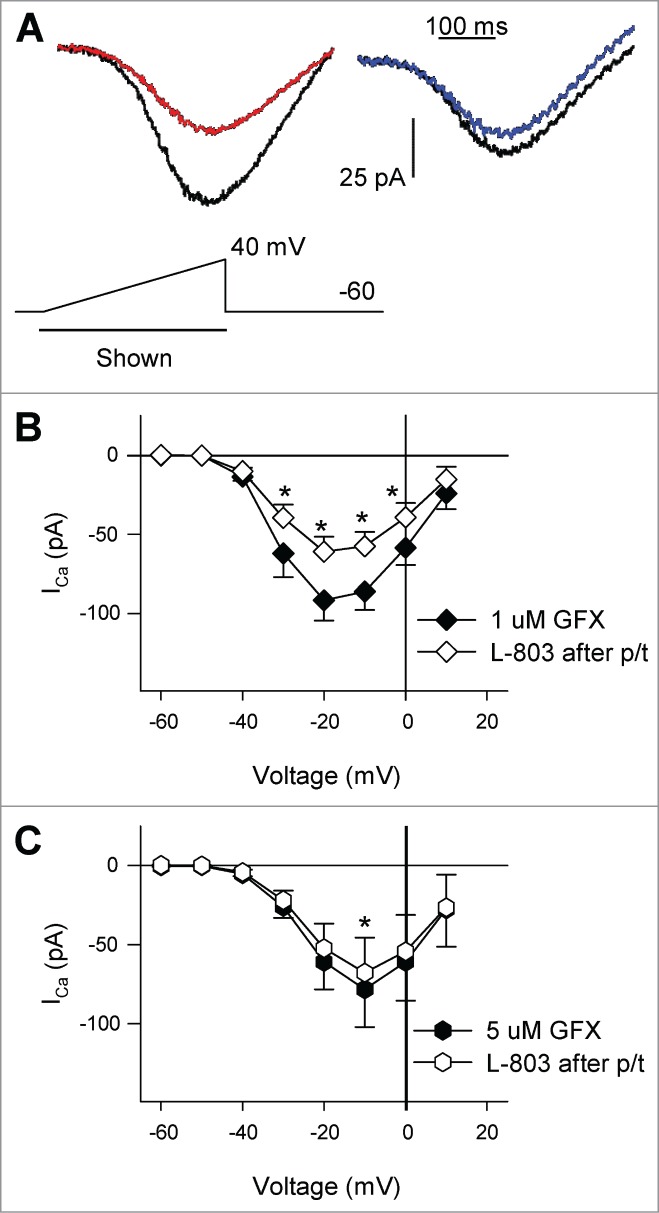
Pre-treatment of RGCs with PKC antagonist GF109203X reduced the actions of L-803, 087. (A) Calcium channel currents in isolated RGCs pretreated (p/t) with GFX (left: 1 μM, right: 5 μM). Administration of L-803,087 (left: gray; right: bold) reduced calcium channel currents compared to pre-treatment conditions (regular trace). (B) The mean data show that after cells were pre-treated with 1 μM GFX, L-803,087 reduced calcium channel current (n = 11; *P < 0.05, 2-way RM ANOVA vs. 1 μM GFX p/t). C. Pre-treatment of cells with 5 μM GFX reduced the effect of L-803,087 on calcium channel current (n = 8; *P < 0.05, 2-way RM ANOVA vs. 5 μM GFX p.t).
Several other intracellular signaling molecules could be responsible for the reduction in ICa observed after stimulation of the sst4 receptor in RGCs. Previous studies have shown that sst4 stimulation activates phospholipase C (PLC),6 which is an intermediate in the Gq signaling pathway resulting in the activation of protein kinase C (PKC). In addition, studies have shown that Gq and PKC are involved in the inhibition of L-type calcium channels in response to the stimulation of other G-protein coupled receptors.22-24 Thus, to determine whether PKC activation was required to reduce ICa during sst4 stimulation, isolated RGCs were pretreated with the PKC antagonist GF109203X (GFX). First, isolated RGCs were incubated with a moderate concentration of GFX (1 μM, 30 min.) prior to voltage-clamp recordings. Fig. 3A (left panel) shows an example of a recording from an isolated RGC pretreated with 1 μM GFX in the absence (black trace) and presence (red trace) of L-803 (10 nM). RGCs pretreated with 1 μM GFX showed a significant reduction in ICa in the presence of L-803 (Fig. 3B; *P < 0.05, 2-way RM ANOVA vs. 1 μM GFX pretreatment). The effect of L-803 in cells that had been treated with 1 μM GFX was similar to that of non-pretreated cells (Fig. 7; 34.3 ± 4.4% vs. 41.2 ± 2.7%, 1 μM GFX pre-treatment vs. L-803 alone).
Figure 3.
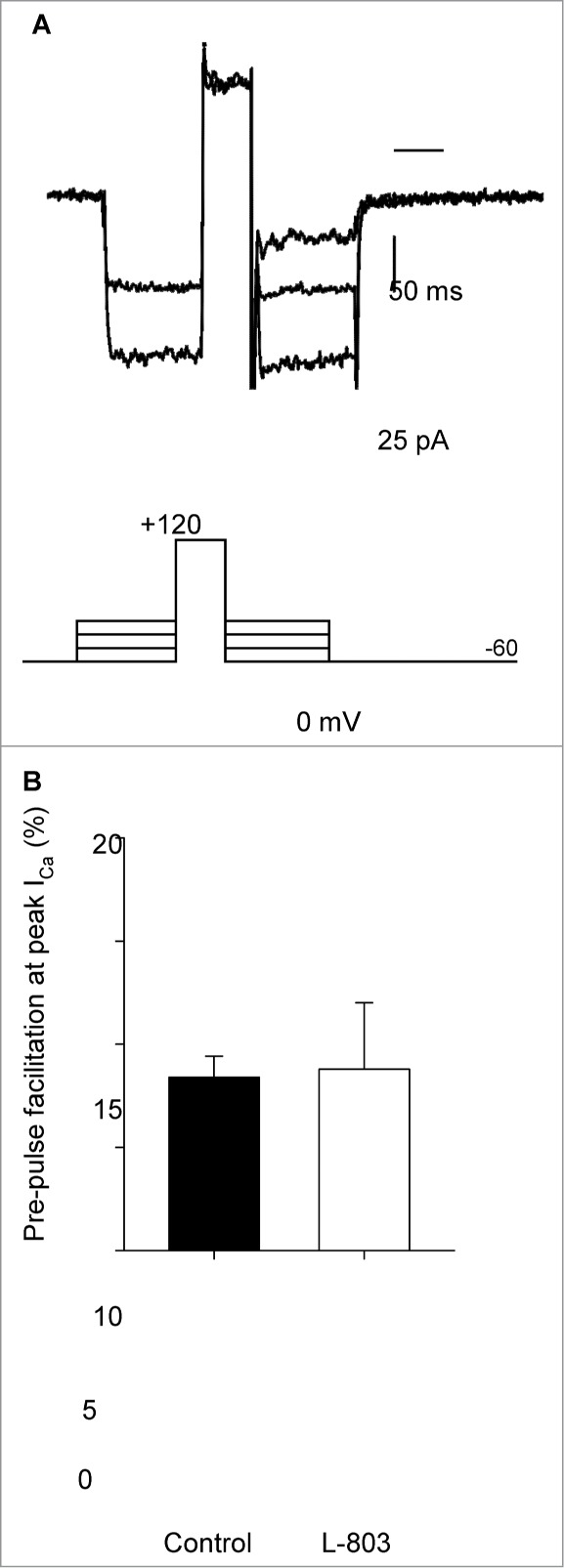
Depolarizing pre-pulse facilitates calcium channel current but has no influence on the suppression by L-803,087. (A) Calcium channel current recorded using a pre-pulse facilitation voltage command protocol (shown below current traces) in an isolated RGC in the presence of L-803. (B) Pre-pulse facilitation was measured as the percentage increase in calcium channel current after the depolarizing step (120 mV, 50 ms). The mean facilitation in the absence (control) and presence of L-803 was the same showing that pre-pulse facilitation did not reverse the suppression of calcium channel current caused by L-803 (n = 5; 8.4 +/− 1.0 vs. 8.8 +/− 3.2% ctrl vs. L-803, paired t-test).
Next, a higher concentration of GFX (5 μM, 30 min.) was used to pretreat RGCs. Fig. 3A (right panel) shows ICa recorded from an isolated RGC which had been pretreated with 5 μM GFX before (black trace) and after administration of L-803 (blue trace). The mean data show that L-803 caused a modest reduction in ICa in RGCs pretreated with the high concentration of PKC antagonist (5 μM GFX; Fig. 3C). Pre-treatment of RGCs with 5 μM GFX significantly reduced the effects of L-803 compared to cells that had not been pretreated (Fig. 7; 14.6 ± 3.2% vs. 41.2 ± 2.7%, 5 μM GFX pre-treatment vs. L-803 alone; *P < 0.05, ANOVA). These data suggest that PKC is involved in the sst4-mediated reduction in ICa in RGCs.
Finally, to determine whether inhibition of both PKC and Gβγ could completely abolish the reduction of ICa by L-803, isolated RGCs were pretreated with both GFX (5 μM) and anti-βγ peptide (1 μM). Figure 6A shows an example of a voltage-clamp recording from an isolated RGC pretreated with GFX and anti-βγ peptide in the absence (black trace) and presence of L-803 (red trace; 10 nM, 2 min). The mean data show no change in ICa after L-803 administration in cells that had been pre-treated with the combination of GFX (5 μM) and anti-βγ peptide (1 μM; Fig. 6B). Blocking both PKC activation and Gβγ interactions with calcium channels abolished the sst4-mediated reduction in ICa (Fig. 7; 1.8 ± 6.0% vs. 41.2 ± 2.7%, GFX + anti-βγ peptide pre-treatment vs. L-803 alone; *P < 0.05, ANOVA). These results suggest additive roles for Gβγ and PKC in the sst4-mediated inhibition of L-type calcium channels.
Figure 6.
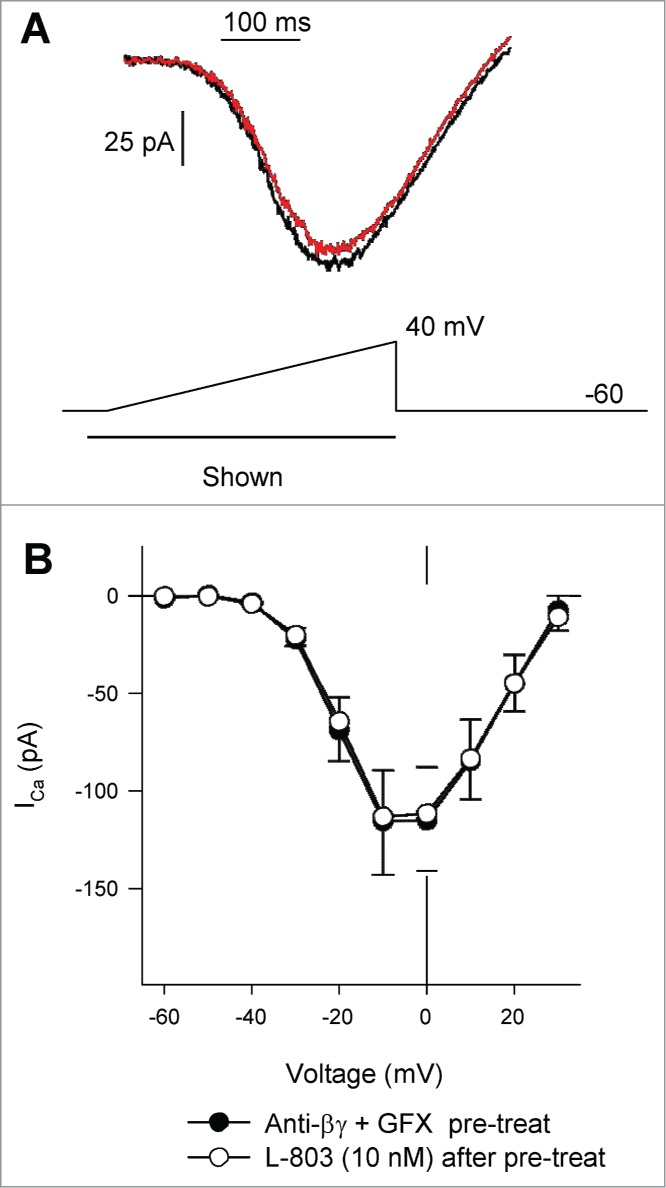
Pre-treatment of isolated RGCs with anti-Gßγ peptide and GFX blocked L-803,087 induced suppression of calcium channel current. (A) Calcium channel currents recorded from an isolated RGC pre-treated with a combination of anti-Gßγ peptide (1 μM) and GFX (5 μM) in the absence (black) and presence of L-803,087 (gray, 10 nM). (B) The mean data show that L-803,087 had no effect on calcium channel current in pre-treated cells (n = 6, NS 2 way RM ANOVA).
Discussion
The present study characterized the intracellular signaling pathway of sst4-mediated inhibition of L-type calcium channels in RGCs. Activation of sst4 in these cells caused activation of PKC via a pertussis-toxin insensitive G-protein (Gq) signaling cascade, which resulted in the inhibition of calcium channels. In addition, sst4-activated Gβγ proteins caused further inhibition of ICa via a voltage-independent mechanism. Together, these results demonstrate the roles of 2 potential intracellular targets for the selective modulation of L-type calcium channels in RGCs.
An earlier report7 showed that sst4 stimulation selectively reduced ICa via L-type calcium channels in neonatal rat RGCs. In the present study we have extended our experimental model by using acutely isolated adult rat RGCs. In adult RGCs, we show selective sst4 stimulation using a non-peptide agonist (L-803,087, 10 nM) caused a significant reduction in ICa, similar to findings in neonatal RGCs where L-803 (10 nM) caused an average reduction in ICa of 32.5%.7 In fact, when the effect of L-803 in these 2 groups was compared directly (percent block of ICa by L-803; adult vs. neonatal RGCs, ANOVA), no statistical difference was found between adult and neonatal cells. Thus, these findings indicate that, at least in RGCs, the sst4-mediated reduction of ICa does not change throughout development.
The present study aimed to characterize the G-protein coupled intracellular signaling pathway mediating the sst4-mediated inhibition of L-type calcium channels. As the net effect of sst4 stimulation is a reduction in ICa, we began by investigating G-proteins known to cause inhibitory responses. Several studies report that all sst subtypes couple to Gi/o proteins, inhibiting cAMP formation.25-27 However, in the present study, isolated RGCs pretreated with pertussis toxin (0.5 μg/mL, 2.5–24 hr) to inhibit Gi/o proteins showed significant reductions in ICa after L-803 administration. It is possible that the concentration of PTX was insufficient to completely inhibit the G-proteins involved in the sst4-mediated response. However, an identical PTX pre-treatment protocol (0.5 μg/mL, 18 hr) in isolated RGCs showed coupling of a glutamate receptor subtype to calcium channels via a PTX-sensitive G-protein.16 A significant inhibition of the glutamate-mediated response (a well characterized Gi-coupled receptor) in the presence of PTX was demonstrated; thus this pre-treatment protocol was deemed to be sufficient to inhibit Gi/o in the present study. The finding that L-803 reduced ICa in cells that had been pre-treated with PTX suggests that the sst4-mediated reduction in ICa involves a PTX-insensitive G-protein.
Inhibitory effects of Gβγ subunits on N-type calcium channels have been described previously.19-21 However, such an inhibitory role of Gβγ subunits in interactions with L-type calcium channels is not seen.20,28 The physical interaction between the Gβγ subunit and the N-type calcium channel stabilizes a closed state of the channel and smaller currents cross the membrane.20 Studies have shown that strong depolarization of the cell can cause a conformational change in the N-type channel resulting in dissociation of the Gβγ protein, facilitating an increase in the net current through the channels.19,21,29,30 In the present study, we examined the possibility that this type of voltage-dependent Gβγ interaction may be involved in the L-803-mediated reduction in L-type ICa. Indeed, when large depolarizing voltage steps were applied, ICa increased, showing a transient pre-pulse facilitation (Fig. 3). However, the extent of facilitation was the same in cells before and after L-803 administration; thus, a direct voltage-dependent interaction between Gβγ proteins and the L-type channel was not responsible for the inhibition of ICa observed with sst4 stimulation. Although this result suggested no voltage-dependent role of Gβγ, previous reports describe a voltage-independent component of Gβγ inhibition of calcium channels19,29 while others demonstrate Gβγ activation of PLC,14,15 a known modulator of calcium channels.12 This led us to further examine the potential involvement of Gβγ in the sst4 signaling pathway. The anti-βγ peptide used in the present study (Anti-BetaGamma MPS-phosducin-like protein C terminus peptide) has been shown previously to bind to and inactivate Gβγ signaling.16,17 Indeed, pretreatment of RGCs with anti-βγ peptide reduced the effects of L-803 on ICa (Fig. 4), although not entirely. Thus, these results corroborate those of previous reports that there can be voltage-independent Gβγ signaling which influences calcium channels.12,14,15,19,29 In addition, these results demonstrate a role of Gβγ in the sst4-mediated inhibition of ICa in RGCs; however, further studies in native cell models are required to elucidate the precise Gβγ mechanism involved.
While pretreatment of RGCs with anti-βγ peptide reduced the effects of sst4 stimulation; there was still a significant reduction of ICa after L-803 administration, suggesting involvement of another intracellular signaling molecule. There is evidence that protein kinase C (PKC) phosphorylates calcium channels, which can result in either reduction or enhancement of current amplitude.31,32 Specifically, phosphorylation of L-type channels by PKC has been shown to reduce ICa,31 thus, it seemed possible that PKC was involved in the sst4 signaling pathway resulting in inhibition of L-type calcium channels in the retina. Indeed, results of the present study showed a significant block of the effects of L-803 in the presence of a non-selective PKC antagonist. Since there are several isoforms of PKC, we chose an antagonist (GF109203X, GFX) with activity against all of the known PKC isoforms. In addition, GFX (1 μM) has been shown to completely inhibit PKC activity involved in cytoskeleton dynamics in salamander retina.33 In the present study, we observed that GFX had a concentration-dependent effect on the L-803 mediated ICa reduction (Fig. 5), with a significant block of L-803 activity at 5 μM GFX. These results demonstrate that PKC is involved in the sst4-mediated reduction of ICa and suggest that PKC isoforms may be selectively involved in different cellular processes within the retina. In addition, the role of PKC is complex and can be significantly altered by changing a single amino acid in the target protein.32 Thus, further studies would be required to elucidate which PKC isoform is involved in the sst4 signaling pathway in RGCs. This would be a significant challenge using a native cell model, such as the one used in the present study, as many antagoinsts are not specific enough to distinguish between PKC isoforms. However, results of the present study demonstrate a clear role for PKC in the modulation of retinal calcium channels via the sst4 signaling pathway.
Finally, we treated RGCs with both anti-βγ peptide and PKC antagonist (GFX, 5 μM) to determine whether blockade of both aspects of the signaling pathway would completely prevent the effect of L-803 on ICa (Fig. 6). Studies have shown that there is significant cross-talk between PKC and Gβγ signaling pathways. A competitive interaction between PKC and Gβγ pathways has been shown where phosphorylation of N-type calcium channels by PKC prevented the interaction of Gβγ with the ion channel resulting in a net positive ionotropic effect of PKC.32 Results of the present study showed an additive effect of blocking 2 different signaling molecules (Gβγ and PKC) that contributes to the inhibition of the L-type calcium channel in isolated RGCs. Administration of anti-βγ peptide and PKC antagonist together completely abolished the effects of L-803 compared to cells that had not been pretreated with antagonists (Fig. 7). While there have been multiple reports of the competitive signaling between PKC and Gβγ,32,34,35 the present study is the first to report these signaling molecules acting in parallel to inhibit L-type calcium channels. These results suggest that cells may have signaling pathway redundancies to prevent over or under stimulation of cellular targets. Although these results add to our understanding of convergent signaling pathways, additional investigations will provide improved understanding of how these pathways integrate and function in this physiological network.
Results of the present study show that it is possible to selectively modulate L-type calcium channels via Gβγ and PKC by stimulating the sst4 receptor. Since sst4 is exclusively expressed in RGCs in the retina, selective modulation of this receptor may be a useful therapeutic target to regulate intracellular Ca2+ levels in RGCs. Further studies are warranted to determine whether sst4 modulation or manipulation of the downstream signaling pathways described here may provide neuroprotection in the setting of retinal injury or disease.
Methods
Purified retinal ganglion cell isolation
Experimental protocols were approved by the Dalhousie University Committee on Laboratory Animals and performed in accordance with the Canadian Council on Animal Care Guide to the Care and Use of Experimental.
Ganglion cells were isolated and purified using a modified panning procedure.36 Briefly, young adult (6–8 weeks old) Long-Evans rats (Charles River, strain code 006) were exposed to an overdose of isofluorane prior to enucleation of the eyes. Retinas were dissected in Hibernate-A (Gibco, Life Technologies, catalog # A12475-01) and transferred to papain solution containing: Leibovitz (L-15) media (without glutamine; Lonza, catalog # 12-700F), 20 U/mL papain, 1 mM cysteine, 0.5 mM EDTA (Worthington, catalog # LK003178), 50 U/mL DNase (Worthington, catalog # LK003172; 37°C, pH ∼7.4). Retinas were dissociated for 5–15 minutes (37°C); the papain solution was then removed and replaced with enzyme inhibitor solution containing: L-15 media, 0.5 mg/mL ovomucoid, 50 U/mL DNase (Worthington, catalog # LK003182; 37°C, pH ∼7.4). The tissue was incubated in enzyme inhibitor solution for 5 minutes at room temperature (RT) prior to trituration in fresh enzyme inhibitor solution. The retinal cell suspension was transferred to a conical tube and centrifuged (2 × 1000 rpm, RT, L-15 media), resuspending the pellet in supplemented media containing: L-15 media, 2% B-27 (Gibco, Life Technologies, catalog #0080085SA), 200 mM L-glutamine, 10,000 U/mL penicillin, 10 mg/mL streptomycin (SigmaAldrich, catalog #G1146), 50 U/mL DNase (37°C, pH ∼7.4). The retinal cell suspension was then incubated (37°C, 1 hr) in cell culture dishes containing glass coverslips (Fisher Scientific, catalog # 12-545-82) coated with antibody against the RGC protein marker Thy-1 (goat anti-mouse IgM; Jackson ImmunoResearch, catalog #115-005-020; 4°C, overnight; mouse anti-Thy-1; produced from cell line T11D7e2, ATCC catalog #TIB-103; 37°C, 5% CO2/O2, 2 hrs). Coverslips were washed with L-15 media (37°C, pH ∼7.4) to remove non-adherent cells and maintained at 37°C in supplemented L-15 media until electrophysiology recordings were made.
Electrophysiological recordings
Extracellular solution (containing: (mM) 137 NaCl, 5 KCl, 1 MgCl2, 2.5 CaCl2, 22.2 glucose, and 5 HEPES, adjusted to pH 7.4 with NaOH) was perfused over isolated RGCs throughout all voltage-clamp experiments. Calcium channel currents (ICa) were isolated by switching the extracellular solution immediately surrounding the cell of interest using a rapid perfusion system (VC8-S, ALA Scientific) containing (in mM): 110 choline chloride, 5 KCl, 1 MgCl2 7 BaCl2, 15 TEACl, 0.1 4-aminopyridine, 20 glucose, 10 HEPES and 1 μM tetrodotoxin, adjusted to pH 7.4 with CsOH. Bath applied drugs were diluted in this barium choline extracellular solution and administered to RGCs using the ALA perfusion system (21–25°C). Recordings were taken before (control recording), during and after bath applied drug administration; however, only recordings after steady-state had been reached (2–3 min. after drug application) were used in comparisons.
All electrophysiology recordings were made in whole-cell patch clamp mode, using borosilicate electrodes (5–10 MΩ; Sutter Instruments; catalog #BF150-86-10). The intracellular pipette solution contained (mM): 128 CsCl, 2 MgCl2, 1 CaCl2, 10 EGTA, 10 HEPES, 2 Mg2+-ATP, 0.4 Na+-GTP, and 10 phosphocreatine, adjusted to pH 7.2 with CsOH.
The bath reference electrode consisted of an agar bridge with an AgCl wire. An Axopatch-1D amplifier (Molecular Devices) was used to control membrane voltage and adjust whole-cell capacitance and series resistance. Voltage command protocols were initiated using pClamp 10 software (Molecular Devices). The current signal was filtered at 0.5 Hz (Ithaco 4302 Dual 24dB/octave filter, Ithaca, NY) and digitized at 1 kHz with Digidata 1440 (Molecular Devices).
Drugs and chemicals
Routine chemicals and reagents were obtained from Sigma Aldrich Canada and Cedarlane Laboratories. L-803,087 (catalog #1979), GF109203X (GFX; catalog #0741) and pertussis toxin (PTX; catalog #3097) were purchased from R&D Systems. Tetrodotoxin citrate (TTX; catalog #T-550) was purchased from Alomone Labs. Anti-BetaGamma MPS-phosducin-like protein C terminus peptide (Anti-βγ peptide, catalog #24178) was obtained from AnaSpec.
L-803,087 (0.001%) and GFX (0.01–0.05%) were prepared as DMSO stock solutions, frozen at -20°C and thawed immediately before experiments (numbers in parenthesis indicate percentage of DMSO in the final working solution). These levels of DMSO do not influence ICa. Pertussis toxin stock solution was stored at 4°C for no longer than 6 weeks. All other drugs and reagents were prepared in double distilled water as stock solutions (stored at −20°C) and warmed to room temperature immediately before performing experiments.
Drug pretreatments were performed by administering drugs directly to the cell culture wells during incubation (37°C, supplemented L-15 media).
Data analysis
All data are reported as the means ± SEM. Data analysis was performed using Clampfit 10 software (Molecular Devices). Graphing and statistical analyses were performed using SigmaPlot 11.0 (Systat Software Inc.). Specific statistical tests used are noted in the figure legend in the appropriate results sections. P values of less than 0.05 were considered statistically significant. All pair-wise multiple comparison procedures were performed in SigmaPlot, using the Holm-Sidak method.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Support for these studies came from a Canadian Institute of Health Research and Nova Scotia Health Research Foundation Regional Partnership Program grant (SB), a National Science and Engineering Research Council Discovery Award (SB), a Plum Foundation Award (SB), NIH EY04067 (NCB), and a Veterans Administration (VA) Merit Review (NCB). Part of this work was made possible by a contract agreement awarded to NCB and administered by the US. Army Medical Research & Materiel Command and the Telemedicine & Advanced Technology Research Center at Fort Detrick, MD under Contract Number: W81XWH-10-2-0077. NCB is a VA Senior Career Research Scientist.
References
- 1. Møller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta 2003; 1616:1-84; PMID:14507421; http://dx.doi.org/ 10.1016/S0005-2736(03)00235-9 [DOI] [PubMed] [Google Scholar]
- 2. Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2003; 2:999-1017; PMID:14654798; http://dx.doi.org/ 10.1038/nrd1255 [DOI] [PubMed] [Google Scholar]
- 3. Bilezikjian LM, Vale WW. Stimulation of adenosine 3′,5′-monophosphate production by growth hormone-releasing factor and its inhibition by somatostatin in anterior pituitary cells in vitro. Endocrinology 1983; 113:1726-31; PMID:6194979; http://dx.doi.org/ 10.1210/endo-113-5-1726 [DOI] [PubMed] [Google Scholar]
- 4. Strowski MZ1, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 2000; 141:111-7; PMID:10614629 [DOI] [PubMed] [Google Scholar]
- 5. Brecha NC. Peptide and peptide receptor expression and function in the vertebrate retina. In: Visual System. Cambridge, MA: MIT Press, 2003; p. 3-28. [Google Scholar]
- 6. Lahlou H, Guillermet J, Hortala M, Vernejoul F, Pyronnet S, Bousquet C, Susini C. Molecular signaling of somatostatin receptors. Ann N Y Acad Sci 2004; 1014:121-31; PMID:15153426; http://dx.doi.org/ 10.1196/annals.1294.012 [DOI] [PubMed] [Google Scholar]
- 7. Farrell SR, Raymond ID, Foote M, Brecha NC, Barnes S. Modulation of voltage-gated ion channels in rat retinal ganglion cells mediated by somatostatin receptor subtype 4. J Neurophysiol 2010; 104:1347-54; PMID:20573967; http://dx.doi.org/ 10.1152/jn.00098.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gorham L, Just S, Doods H. Somatostatin 4 receptor activation modulates G-protein coupled inward rectifying potassium channels and voltage stimulated calcium signals in dorsal root ganglion neurons. Eur J Pharmacol 2014; 736:101-6; PMID:24769416; http://dx.doi.org/ 10.1016/j.ejphar.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 9. Law SF, Zaina S, Sweet R, Yasuda K, Bell GI, Stadel J, Reisine T. Gi alpha 1 selectively couples somatostatin receptor subtype 3 to adenylyl cyclase: identification of the functional domains of this alpha subunit necessary for mediating the inhibition by somatostatin of cAMP formation. Mol Pharmacol 1994; 45:587-90; PMID:8183236 [PubMed] [Google Scholar]
- 10. Gomez E, Pritchard C, Herbert TP. cAMP-dependent protein kinase and Ca2+ influx through L-type voltage-gated calcium channels mediate Raf-independent activation of extracellular regulated kinase in response to glucagon-like peptide-1 in pancreatic beta-cells. J Biol Chem 2002; 277:48146-51; PMID:12364324; http://dx.doi.org/ 10.1074/jbc.M209165200 [DOI] [PubMed] [Google Scholar]
- 11. Van Op den Bosch J, Adriaensen D, Van Nassauw L, Timmermans JP. The role(s) of somatostatin, structurally related peptides and somatostatin receptors in the gastrointestinal tract: a review. Regul Pept 2009; 156:1-8; PMID:19362110; http://dx.doi.org/ 10.1016/j.regpep.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 12. Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ. Stimulation of 5-HT(2) receptors in prefrontal pyramidal neurons inhibits Ca(v)1.2 L type Ca(2+) currents via a LCbetaIP3calcineurin signaling cascade. J Neurophysiol 2002; 87:2490-504; PMID:11976386 [DOI] [PubMed] [Google Scholar]
- 13. Zamponi GW, Currie KP. Regulation of CaV2 calcium channels by G protein coupled receptors. Biochim Biophys Acta 2013; 1828:1629-43; PMID:23063655; http://dx.doi.org/ 10.1016/j.bbamem.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park D, Jhon DY, Lee CW, Lee KH, Rhee SG. Activation of phospholipase C isozymes by G protein beta gamma subunits. J Biol Chem 1993. 268:4573-6; PMID:8383116 [PubMed] [Google Scholar]
- 15. Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature 1992; 360:684-6; PMID:1465133; http://dx.doi.org/ 10.1038/360684a0 [DOI] [PubMed] [Google Scholar]
- 16. Orr AW, Pallero MA, Murphy-Ullrich JE. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J Biol Chem 2002; 277:20453-60; PMID:11923291; http://dx.doi.org/ 10.1074/jbc.M112091200 [DOI] [PubMed] [Google Scholar]
- 17. Morrey C, Estephan R, Abbott GW, Levi R. Cardioprotective effect of histamine H3-receptor activation: pivotal role of Gβγ-dependent inhibition of voltage-operated Ca2+ channels. J Pharmacol Exp Ther 2008; 326:871-8; PMID:18523159; http://dx.doi.org/ 10.1124/jpet.108.137919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robbins J, Reynolds AM, Treseder S, Davies R. Enhancement of low-voltage-activated calcium currents by group II metabotropic glutamate receptors in rat retinal ganglion cells. Mol Cell Neurosci 2003; 23:341-50; PMID:12837619; http://dx.doi.org/ 10.1016/S1044-7431(03)00056-3 [DOI] [PubMed] [Google Scholar]
- 19. Kisilevsky AE, Zamponi GW. D2 dopamine receptors interact directly with N-type calcium channels and regulate channel surface expression levels. Channels (Austin) 2008; 2:269-77; PMID:18719394; http://dx.doi.org/ 10.4161/chan.2.4.6402 [DOI] [PubMed] [Google Scholar]
- 20. Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev 2006; 58:837-62; PMID:17132857; http://dx.doi.org/ 10.1124/pr.58.4.11 [DOI] [PubMed] [Google Scholar]
- 21. Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gbeta subunit. Proc Natl Acad Sci USA 1998; 95:4035-9; PMID:9520488; http://dx.doi.org/ 10.1073/pnas.95.7.4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu K, Gui B, Sun Y, Shi N, Gu Z, Zhang T, Sun X. Inhibition of L-type Ca2 +channels by curcumin requires a novel protein kinase-theta isoform in rat hippocampal neurons. Cell Calcium 2013; 53:195-203; PMID:23261315; http://dx.doi.org/ 10.1016/j.ceca.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 23. Lu Z, Jiang YP, Ballou LM, Cohen IS, Lin RZ. Gq inhibits cardiac L-type Ca2+ channels through phosphatidylinositol 3-kinase. J Biol Chem 2005; 280:40347-54; PMID:16186103; http://dx.doi.org/ 10.1074/jbc.M508441200 [DOI] [PubMed] [Google Scholar]
- 24. Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ. Stimulation of 5-HT2 receptors in prefrontal pyramidal neurons inhibits Cav1.2 L type Ca2+ currents via a PLCbetaIP3calcineurin signaling cascade. J Neurophysiol 2002; 87:2490-504; PMID:11976386 [DOI] [PubMed] [Google Scholar]
- 25. Somvanshi RK, Billova S, Kharmate G, Rajput PS, Kumar U. C-tail mediated modulation of somatostatin receptor type-4 homo- and heterodimerizations and signaling. Cell Signal 2009; 21:1396-414; PMID:19426801; http://dx.doi.org/ 10.1016/j.cellsig.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 26. Bruns C, Raulf F, Hoyer D, Schloos J, Lübbert H, Weckbecker G. Binding properties of somatostatin receptor subtypes. Metabolism 1996; 45:17-20; PMID:8769372; http://dx.doi.org/ 10.1016/S0026-0495(96)90072-4 [DOI] [PubMed] [Google Scholar]
- 27. Patel YC, Greenwood MT, Warszynska A, Panetta R, Srikant CB. All five cloned human somatostatin receptors (hSSTR1-5) are functionally coupled to adenylyl cyclase. Biochem Biophys Res Commun 1993; 198:605-12; http://dx.doi.org/ 10.1006/bbrc.1994.1088 [DOI] [PubMed] [Google Scholar]
- 28. Bourinet E, Charnet P, Tomlinson WJ, Stea A, Snutch TP, Nargeot J. Voltage-dependent facilitation of a neuronal alpha 1C L-type calcium channel. EMBO J 1994; 13:5032-9; PMID:7957069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kammermeier PJ, Ruiz-Velasco V, Ikeda SR. A voltage-independent calcium current inhibitory pathway activated by muscarinic agonists in rat sympathetic neurons requires both Galpha q11 and Gbeta gamma. J Neurosci 2000; 20:5623-9; PMID:10908599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci 1994; 17:531-6; PMID:7532338; http://dx.doi.org/ 10.1016/0166-2236(94)90157-0 [DOI] [PubMed] [Google Scholar]
- 31. Sculptoreanu A, de Groat WC. Protein kinase C is involved in neurokinin receptor modulation of N- and L-Type Ca2+ channels in DRG neurons of the adult rat. J Neurophysiol 2003; 90:21-31; PMID:12660348; http://dx.doi.org/ 10.1152/jn.00108.2003 [DOI] [PubMed] [Google Scholar]
- 32. Hamid J, Nelson D, Spaetgens R, Dubel SJ, Snutch TP, Zamponi GW. Identification of an integration center for cross-talk between protein kinase C and G protein modulation of N-type calcium channels. J Biol Chem 1996; 274:6195-202; http://dx.doi.org/ 10.1074/jbc.274.10.6195 [DOI] [PubMed] [Google Scholar]
- 33. Cristofanilli M, Akopian A. Calcium channel and glutamate receptor activities regulate actin organization in salamander retinal neurons. J Physiol 2006; 575:543-54; PMID:16777935; http://dx.doi.org/ 10.1113/jphysiol.2006.114108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barrett CF, Rittenhouse AR. Modulation of N-type calcium channel activity by G-proteins and protein kinase C. J Gen Physiol 2000; 115:277-86; PMID:10694257; http://dx.doi.org/ 10.1085/jgp.115.3.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cooper CB, Arnot MI, Feng ZP, Jarvis SE, Hamid J, Zamponi GW. Cross-talk between G-protein and protein kinase C modulation of N-type calcium channels is dependent on the G-protein beta subunit isoform. J Biol Chem 2000; 275:40777-81; PMID:11053424; http://dx.doi.org/ 10.1074/jbc.C000673200 [DOI] [PubMed] [Google Scholar]
- 36. Hartwick AT, Lalonde MR, Barnes S, Baldridge WH. Adenosine A1-receptor modulation of glutamate-induced calcium influx in rat retinal ganglion cells. Invest Ophthalmol Vis Sci 2004; 45:3740-8; PMID:15452085; http://dx.doi.org/ 10.1167/iovs.04-0214 [DOI] [PubMed] [Google Scholar]


