Abstract
Snail, a zinc-finger transcription factor, induces epithelial-mesenchymal transition (EMT), which is associated with increased cell migration and metastasis in cancer cells. Rac1 is a small G-protein which upon activation results in formation of lamellipodia, the first protrusions formed by migrating cells. We have previously shown that Snail promotes cell migration through down-regulation of maspin tumor suppressor. We hypothesized that Snail's regulation of cell migration may also involve Rac1 signaling regulated by PI3K/AKT and/or MAPK pathways. We found that Snail overexpression in LNCaP and 22Rv1 prostate cancer cells increased Rac1 activity associated with increased cell migration, and the Rac1 inhibitor, NSC23766, could inhibit Snail-mediated cell migration. Conversely, Snail downregulation using shRNA in the aggressive C4–2 prostate cancer cells decreased Rac1 activity and cell migration. Moreover, Snail overexpression increased ERK and PI3K/AKT activity in 22Rv1 prostate cancer cells. Treatment of Snail-overexpressing 22Rv1 cells with LY294002, PI3K/AKT inhibitor or U0126, MEK inhibitor, decreased cell migration significantly, but only LY294002 significantly reduced Rac1 activity, suggesting that Snail promotes Rac1 activation via the PI3K/AKT pathway. Furthermore, 22Rv1 cells overexpressing Snail displayed decreased maspin levels, while inhibition of maspin expression in 22Rv1 cells with siRNA, led to increased PI3K/AKT, Rac1 activity and cell migration, without affecting ERK activity, suggesting that maspin is upstream of PI3K/AKT. Overall, we have dissected signaling pathways by which Snail may promote cell migration through MAPK signaling or alternatively through PI3K/AKT-Rac1 signaling that involves Snail inhibition of maspin tumor suppressor. This may contribute to prostate cancer progression.
Keywords: cell migration, MAPK pathway, PI3K/AKT pathway, Rac1, snail
Abbreviations
- EMT
epithelial to mesenchymal transition
- Rac1
Ras-related C3 botulinum toxin substrate 1
- CaP
prostate cancer
- PI3K/AKT pathway
Phosphoinositide 3-kinases/Protein Kinase B
- ERK
Extracellular signal-regulated kinases
- P-ERK
Phospho-ERK
- P-AKT
Phospho-AKT
Introduction
Prostate Cancer (CaP) is the second leading cause of cancer death in males in the United States.1 In 2014, it is estimated that 29, 480 men will die from CaP and that there will be 233,000 new cases in the United States.2 In CaP, as in many other types of cancer, metastasis of the primary tumor is the main cause of cancer related death. About 90% of cancers, including prostate cancer, originate from the epithelial tissue.3 In the initial tumorigenesis stages, the tumor cells alter their properties from highly differentiated epithelial morphology to a migratory and invasive phenotype.3 This phenomenon is referred as epithelial to mesenchymal transition (EMT). It is therefore important to find therapeutics targeted at halting or slowing down prostate cancer metastasis.
Snail is a zinc-finger transcription factor that binds to E-box consensus sequence of their target genes.4 There are 3 family members of Snail; Snail1, Snail2/ Slug, and Snail3.5 We are interested in studying Snail1 or Snail as it is more commonly known. Snail is a master regulator of EMT known to have both repressive and activating effects on gene transcription. For example, it suppresses the transcription of epithelial genes and enhances transcription of mesenchymal genes.4 Snail acts as a regulator of EMT through its role of down-regulating E-cadherin expression, a cell adhesion molecule,6 which leads to loss of cell-cell adhesion and increased cell motility.3,7 Snail has been shown to be up-regulated in prostate cancer resulting in increased cell invasion.7,8
Ras-related C3 botulinum toxin substrate 1 (Rac1) is a member of the Rho subfamily of small guanosine triphosphate (GTP)-binding proteins. There are 5 family members, Rac1, Rac1b, Rac2, Rac3, and RhoG.9-11 Rac proteins are activated by upstream signaling from tyrosine kinase, integrins, and G protein coupled receptors (GPCRs).11 Small GTPases act as molecular switches in which the GTP-bound form is active and guanosine diphosphate (GDP)-bound form is inactive.12 Rac1 activation is facilitated by the activity of guanine nucleotide exchange factors (GEFs) such as Tiam1, which allow for the exchange of GDP bound to GTP bound.13 On the other hand, Rac1 inactivation requires the activity of a GTPases activating proteins (GAPs) such as ArhGAP15, to hydrolyze the GTP to GDP inactive form.14 When Rac1 is in the GTP-bound, active form, it can interact with its effectors such as Riam (an adaptor that binds to actin regulators) in order to regulate downstream cellular functions.15 Some of the roles of Rac proteins include, actin reorganization and cell motility through the formation of membrane ruffles and lamellipodia,16 phagocytosis, endocytosis17 and cell adhesion.18 During cell migration, the formation of lamellipodia is important as they are the first protrusions formed by migrating cells as the move forward.19 Rac1 has been reported to be activated in breast cancer cells leading to increased cell invasion.20 Therefore, activated Rac1 levels are associated with more aggressive cancers.
The PI3K/AKT pathway is activated via the stimulation of the receptor tyrosine kinase (RTK) by its ligand(s) and phosphorylation of its cytoplasmic tail.21 This leads to the recruitment of PI3K (p85-p110 complex) to the active RTK receptor and its activation. PI3K then phosphorylates phosphatidylinositol-4,5-bisphosphate (PtdIns (4,5) P2) to produce PtdIns (3,4,5) P3 or PIP3 which then acts as a second messenger that binds to domains of its downstream targets recruiting them to the membrane.22 Production of PIP3 leads to recruitment of AKT where 3-phosphoinositide- dependent protein kinase-1 (PDK1) phosphorylates AKT.21 The PI3K/AKT pathway is usually aberrantly expressed in cancers such as ovarian, breast, digestive tract and thyroid.21 This pathway is known to regulate cell growth, translation, metabolism and proliferation.22 Activation of PI3K has been shown to activate GEFs for Rac1.23 Therefore, Rac1 seems to be a downstream target of PI3K signaling pathway.
Maspin (mammary serine protease inhibitor) is a tumor suppressor that is down-regulated in prostate cancer.24 It is a serine protease inhibitor that has been shown to inhibit Rac1 activity and subsequently PAK activity leading to decreased cell migration in breast cancer cells.25 Snail transcription factor has been shown to bind to maspin promoter and negatively regulate it in human prostate cancer cells resulting in decreased cell migration and invasion.26
Mitogen- activated protein kinase/ Extracellular regulated kinases (MAPK/ERK) pathway is activated by various factors such as G-protein coupled receptors (GPCR), Receptor tyrosine kinases (RTK), integrins, and ion channels.27,28 It is important during differentiation, cell growth and survival and often aberrantly activated in human cancers.29-31 ERK has been shown to play a role during cell migration by suppressing integrins (which are receptors which mediate cell-matrix attachments) from binding to their extracellular matrix ligands thus controlling their activation.32 The role of the ERK pathway during tumor migration has been reported and shown to involve in part, Rac1.33
Snail, PI3K/AKT pathway, and Rac1 have been shown to be aberrantly expressed in human cancers7,8,11,12,22 and Rac1 and PI3K/AKT pathway suggested as good therapeutic targets for cancer.22,34 One of the mechanisms for Snail regulating tumor progression is through AKT upregulating NF-κB which transcriptionally increases Snail expression and results in more EMT.35 Recently, Snail has been shown to increase Rac1 activity in regulating cell migration.36 However, the signaling pathway by which Snail may regulate Rac1 has not been shown. Moreover, the regulation of Rac1 by maspin has also never been shown in CaP. Here we show for the first time that Snail, which is upregulated in prostate cancer,7 can increase cell migration through MAPK signaling or PI3K/AKT-mediated Rac1 activation through repression of maspin tumor suppressor. We also show that the PI3K/AKT pathway but not the ERK pathway, is involved in Snail-Rac1 mediated cell migration. Put together, we show for the first time that Snail regulates cell migration during CaP through inactivation of maspin tumor suppressor which subsequently allows for recruitment of PI3K/AKT and Rac1 signaling pathways.
Results
Snail overexpression is associated with increased cell migration and Rac1 activity in LNCaP and 22Rv1 prostate cancer cells
Previously we generated LNCaP and 22Rv1 cell lines that overexpress the Snail gene and showed that this increases cell migration.26,37 We confirmed these results; as shown by western blotting, LNCaP and 22Rv1 cells transfected stably with Snail display increased expression of Snail protein (Fig. 1A). Furthermore, the 22Rv1 and LNCaP Snail overexpressing cell lines showed increased cell migration compared to the Neo vector only control (Fig. 1B). Recently, it has been shown that Snail-induced migration and scattering was mediated by Rac1 small GTPase in pancreatic cancer cells.36 We hypothesized that similarly, Snail would regulate Rac1activity in prostate cancer cells. Since Rac1 activity has been shown to control cell migration through formation of lamellipodia,38 we determined the Rac1-GTP levels in LNCaP and 22Rv1 overexpressing Snail. Our results showed that activated Rac1 levels were increased in the 22Rv1 and in LNCaP cells overexpressing Snail as shown using the G-LISA method (Fig. 1C). Therefore, Snail overexpression results in higher cell migration and Rac1 activity in prostate cancer cells.
Figure 1.
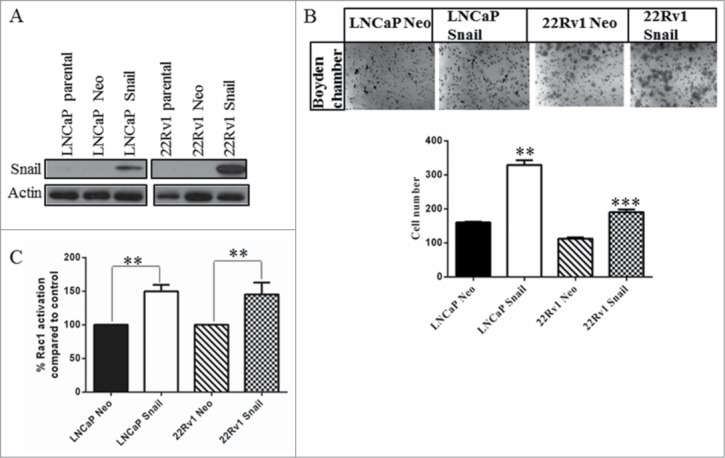
Snail overexpression increases cell migration and Rac1 activity in prostate cancer cells. (A) LNCaP and 22Rv1 prostate cancer cells stably transfected with empty vector (Neo) or constitutively active Snail cDNA using lipofectamine 2000 were tested by protein gel blot to assess the Snail protein levels. (B) A cell migration assay was done using the LNCaP and 22Rv1 cells overexpressing Snail or Neo control using a boyden chamber. 5 × 104 cells were plated in the upper chamber of inserts coated with collagen I. Cells that migrated through the pores to the underside of the insert were stained with crystal violet, imaged, as well as counted and graphed. (C) Rac1 activity assay was performed using G-LISA Rac1 activity assay followed by analysis at O.D. 590 nm and the values used to calculate the percent Rac1 activation compared to Neo vector control. Actin was utilized as a loading control. Results are representative of 2 independent experiments done in triplicates and western blots done as 3 independent experiments. Statistical significance was assessed using GraphPad Prism software by paired Student's t-test compared to Neo-transfected control cells (*P < 0.05, **P < 0.01, ***P < 0.001).
Knockdown of Snail results in reduced cell migration and Rac1 activity in the aggressive C4–2 prostate cancer cells
We next used C4–2 with stable knockdown of Snail (C4–2 Snail shRNA) to observe their effect on cell migration and Rac1 activity. We observed that Snail expression was decreased upon Snail knockdown as compared to non-silencing control (Fig. 2A) which was accompanied by decreased cell migration (Fig. 2B). In addition, the Rac1 activity was significantly reduced in C4–2 Snail shRNA expressing cells (Fig. 2C). Therefore, we provide further proof that the Snail gene is important for cell migration in C4–2 cells, as previously reported,39 possibly through Rac1 activation.
Figure 2.
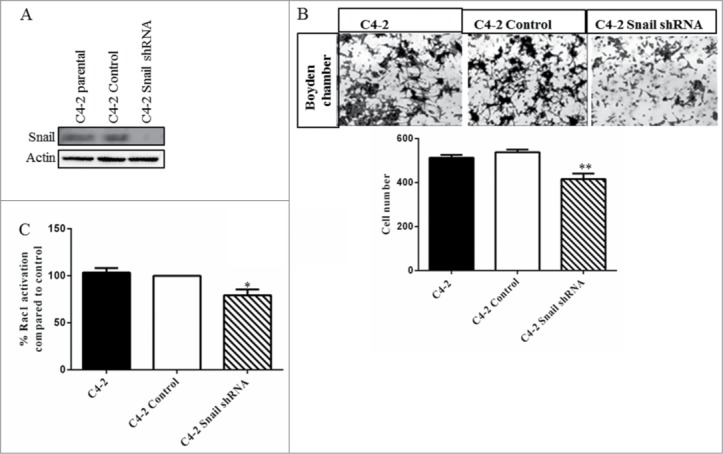
Knockdown of Snail results in reduced cell migration and Rac1 activity in C4–2 prostate cancer cells. (A) Western blot analysis was done to confirm that Snail protein levels were decreased in C4–2 cells with stable knockdown of Snail using shRNA as compared to control (non-silencing shRNA-expressing C4–2 cells). (B) Cell migration through collagen I matrix was performed using the boyden chamber where cells were allowed to migrate for 24 hrs. Representative images were taken by light microscopy and cell numbers of migrated cells were counted and graphed. (C) Rac1 activity was assayed using G-LISA assay in C4–2 parental, C4–2 control and C4–2 Snail shRNA cells. Results are reported as mean ± SD from 2 independent experiments done in triplicates and protein gel blots done as 3 independent experiments.Statistical significance was assessed using GraphPad Prism software by paired Student's t-test compared to C4–2 control cells expressing non-silencing shRNA (*P < 0.05, **P < 0.01).
Rac1 inhibitor antagonizes Snail-mediated cell migration
NSC23766, a Rac1 inhibitor, has been shown to decrease Rac1 activity.40 We treated 22Rv1 cells overexpressing Snail with this Rac1 inhibitor for 24 hrs and showed that there was a dose-dependent decrease in Rac1 activity (Fig. 3A). The Rac1 inhibitor at lower concentrations (50–100 μM) did not significantly affect Rac1, Snail, maspin, PI3K/AKT activity (p-AKT) or MAPK activity (p-ERK) (Fig. 3B). Interestingly, the highest dose (200 μM) appeared to decrease Rac1 and Snail protein expression, but did not affect PI3K/AKT, MAPK activities or maspin protein levels (Fig. 3B). We also performed a migration assay following treatment of 22Rv1 Snail cells with Rac1 inhibitor and observed significantly decreased cell migration especially with the 200 μM dose (Fig. 3C). Put together, Rac1 inhibitor antagonizes Snail-mediated cell migration without affecting PI3K/AKT, MAPK activities or maspin protein level. These data show that Rac1 activity is essential for Snail-mediated cell migration in 22Rv1 cells.
Figure 3.
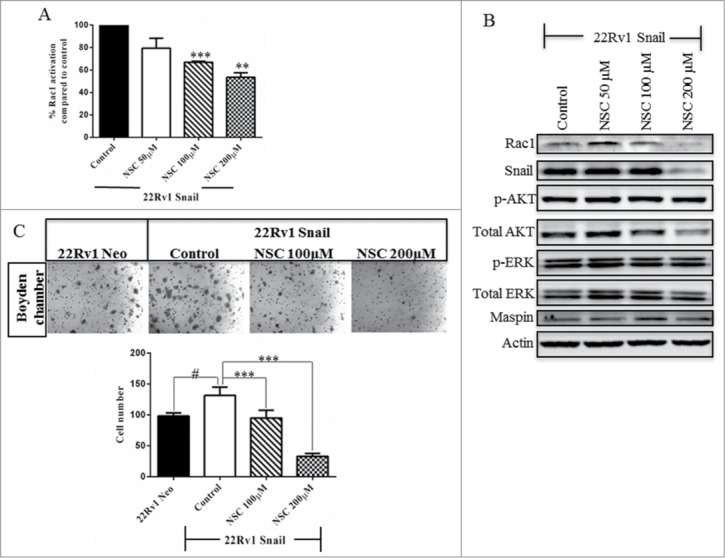
Rac1 inhibitor antagonizes Snail-mediated cell migration. 22Rv1 Snail-overexpressing cells were treated with NSC23766, Rac1 inhibitor, at 50 µM, 100 µM and 200 µM for 24 hrs. (A) Rac1 activity was assayed using G-LISA assay and (B) western blot analysis was performed to show the protein levels of Rac1, Snail, maspin, total and phospho-AKT and ERK and actin as a loading control. (C) Cell migration through collagen was performed using the boyden chamber for 24 hrs and represented as images taken by light microscopy as well as graphing of cell numbers following counting. Results are reported as mean ± SD from 2 independent experiments done in triplicates and protein gel blots done as 3 independent experiments. Statistical significance was assessed using GraphPad Prism software by paired Student's t-test compared to 22Rv1 Snail control cells treated with ddH2O (#,*P < 0.05, **P < 0.01, ***P < 0.001).
The ERK pathway regulation of Snail-mediated cell migration does not involve Rac1 activity
Next, we wanted to find out if Snail and Rac1 regulate cell migration through the ERK or the AKT signaling pathway. To test this, we first looked at the basal protein activity for AKT and ERK. LNCaP and C4–2 cells appeared to have constitutively high levels of phospho-AKT (p-AKT) even before overexpression of Snail or after Snail knockdown, which is expected as LNCaP and C4–2 cells have mutated PTEN gene (Fig. 4A).41 However, Snail overexpression in 22Rv1 cells resulted in greater AKT activity as compared to Neo control (Fig. 4A). Additionally, phospho-ERK (p-ERK) levels increased with overexpression of Snail in 22Rv1 but did not change significantly in LNCaP cells overexpressing Snail compared to parental cells or C4–2 cells with Snail knockdown (Fig. 4A). We next tested whether Rac1 activity increased by Snail is mediated via the MAPK pathway by utilizing the MEK inhibitor, U0126. By protein gel blotting, we observed that the p-ERK but not p-AKT levels were diminished within 30 min up to 24 hrs, as a result of treating 22Rv1 cells overexpressing Snail with the U0126 inhibitor for various time points as expected (Fig. 4B). In addition, Snail levels decreased briefly by 30 min, then increased after 24 hrs time-point (Fig. 4B). On the other hand, maspin protein levels seemed to increase between 2–2 4 hrs while Rac1 levels were unaffected (Fig. 4B). Overall, it seems that treatment of 22Rv1 overexpressing Snail cells with MEK inhibitor resulted in reduced Snail and increased maspin expression. These results show that the ERK pathway may be involved in the Snail regulation of maspin in 22Rv1 cells. Interestingly, the Rac1 activity levels did not change significantly with MEK inhibitor treatment (Fig. 4C), while cell migration was decreased compared to control (Fig. 4D). Therefore, we concluded that the ERK pathway is not involved in the Snail regulation of Rac1 activity in 22Rv1 cells but it can regulate Snail-mediated cell migration by an alternate pathway.
Figure 4.
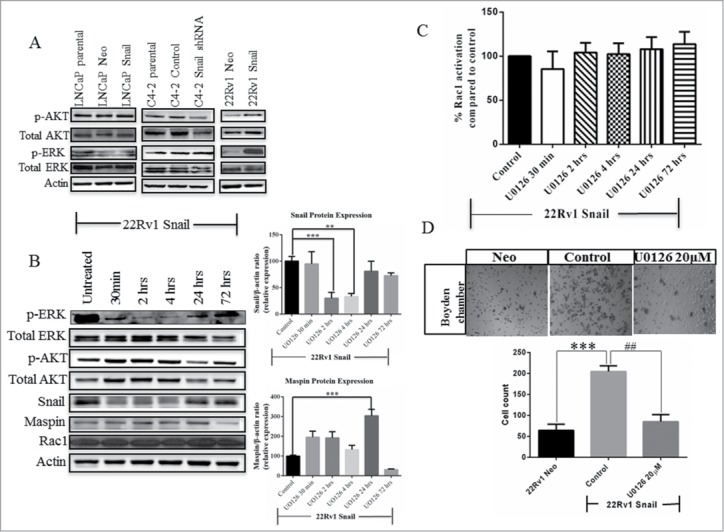
Snail-Rac1 signaling is not regulated by the ERK pathway in 22Rv1 cells. (A) Western blot analysis was performed using LNCaP and 22Rv1 Neo and Snail over-expressing cells or C4–2 cells with stable Snail knockdown, to assess the levels of total and phospho-AKT (p-AKT) and total and phospho-ERK (p-ERK). (B) 22Rv1 Snail-overexpressing cells were treated with 20 µM U0126, MEK inhibitor, for various time points and a western blot analysis performed to measure the total and p-ERK and AKT levels, Snail, maspin and Rac1 protein levels. Western blot data for Snail and maspin was quantified using Image J Software from NIH. (C) Rac1 activity assay was performed following treatment of 22Rv1 Snail-overexpressing cells with UO126, using the G-LISA Rac1 activity assay. (D) A migration assay was also performed after 24 hrs treatment of 22Rv1 Snail-overexpressing cells with the U0126. Actin was used as a loading control. Results are reported as mean ± SD from 3 independent experiments. Statistical significance was assessed using GraphPad Prism software by paired Student's t-test compared to 22Rv1 Snail control cells treated with DMSO (##,**P < 0.01, ***P < 0.001).
The PI3K/AKT pathway regulates the Snail- Rac1 mediated cell migration
LY294002 has been shown to inhibit the PI3K/AKT signaling pathway.42 We wanted to determine if the Snail regulation of Rac1 activity and cell migration is through PI3K/AKT signaling. We therefore treated 22Rv1 Snail-overexpressing cells with LY294002 inhibitor for various time-points, followed by western blotting, Rac1 activity assay and migration assay. Following treatment with LY294002, as expected, p-AKT decreased within 30 min while p-ERK levels did not decrease (Fig. 5A). Snail expression decreased within 30 min but returned by 24 hrs (Fig. 5A). On the other hand, the maspin protein levels seemed to increase slightly by 4 hrs up to 24 hrs while Rac1 levels did not significantly change (Fig. 5A). In addition, Rac1 activity decreased significantly within 30 minutes, up to 24 hrs (Fig. 5B). Concomitantly, the cell migration decreased significantly after the 24 hrs (Fig. 5C). Therefore, the PI3K/AKT signaling pathway is involved in the Snail- Rac1 mediated cell migration and may act upstream of Rac1 as we saw a decrease in Rac1 activity after treatment of 22Rv1 Snail-overexpressing cells with the PI3K/AKT inhibitor.
Figure 5.
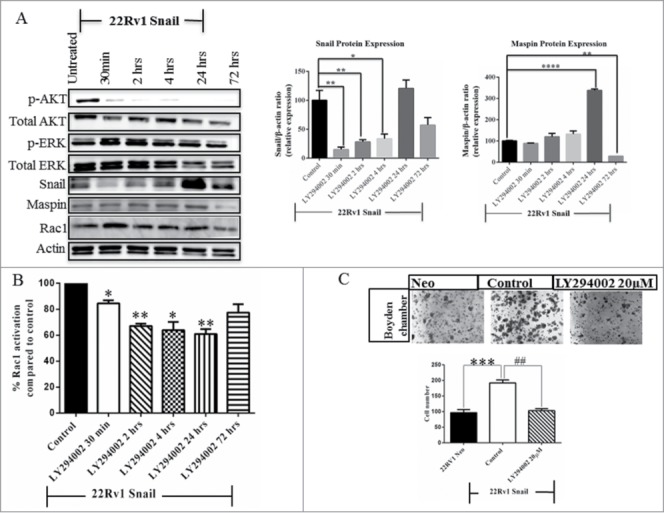
The PI3K/AKT pathway regulates the Snail-Rac1 mediated cell migration in 22Rv1 cells. (A) 22Rv1 Snail-transfected cells were treated with 20 µM of LY294002, PI3K/AKT inhibitor, for various time points followed by protein gel blot analysis to assess total and p-AKT and ERK, Snail, maspin and Rac1 protein levels. Western blot data for Snail and maspin was quantified using Image J Software from NIH. (B) Rac1 activation was determined using G-LISA assay following treatment of 22Rv1 Snail-transfected cells with LY294002 for various time points. (C) A migration assay was also performed after 24 hrs treatment of 22Rv1 Snail-overexpressing with the LY294002 inhibitor; cells that had migrated were fixed, stained, counted and graphed. Actin was used as a loading control. Results are reported as mean ± SD from 3 independent experiments. Statistical significance was assessed using GraphPad Prism software by paired Student's t-test compared to 22Rv1 Snail control cells treated with DMSO (*P < 0.05,##,**P < 0.01, ***P < 0.001, ****P < 0.0001).
Maspin suppresses Rac1 activity via inhibition of PI3K/AKT activity
Since Snail is known to transcriptionally downregulate maspin26 and maspin has been previously shown to repress Rac1 activity in breast cancer cells,25 we wanted to see whether maspin could be a possible link between Snail and Rac1 activity. To do this, we first confirmed that Snail overexpression in 22Rv1 cells leads to downregulation of maspin as compared to Neo control cells (Fig. 6A). Subsequently, we transiently knocked down maspin in 22Rv1 Neo cells with maspin siRNA then tested for maspin and PI3K/AKT activity by protein gel blot analysis. Interestingly, when we knocked down maspin, p-AKT increased while p-ERK was not affected (Fig. 6B). In addition, we performed a Rac1 activity assay to assess if the levels of activated Rac1 changed after treatment with the maspin siRNA. We observed that Rac1 activity increased following maspin knockdown, P value = 0.068 (Fig. 6C). This observation is indicative of the normal role of maspin as repressing Rac1 activity hence when maspin is knocked down, the Rac1 activity levels increased. We also did a migration assay after knocking down maspin in 22Rv1 Neo cells and observed a significant increase in cell migration (Fig. 6D). Therefore, Snail may lead to suppression of maspin which results in activation of AKT and Rac1 leading to increased cell migration in 22Rv1 prostate cancer cells.
Figure 6.
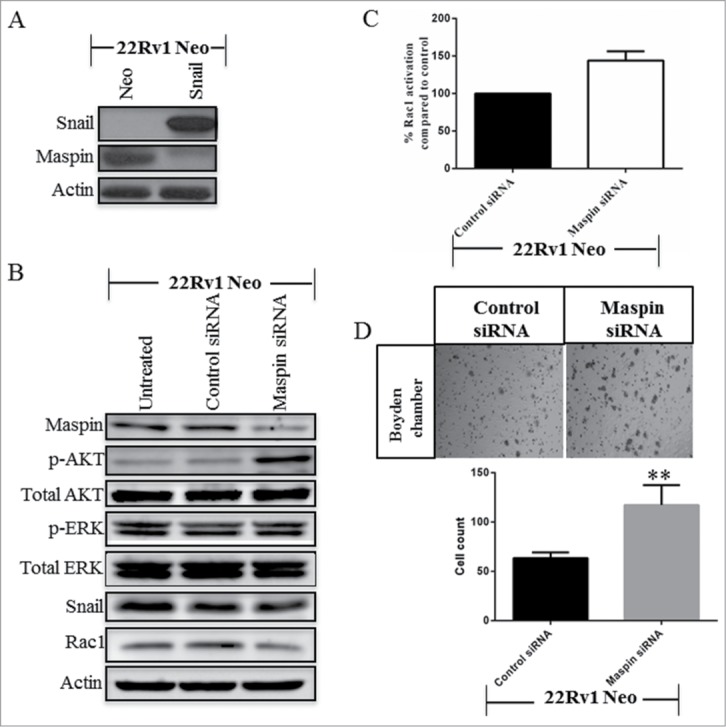
Maspin suppresses PI3K/AKT and Rac1 signaling in 22Rv1 cells. (A)The levels of Snail and maspin were determined by western blot analysis using 22Rv1 Neo- or Snail-transfected cells. 22Rv1 Neo control cells were treated with maspin siRNA for 72 hrs followed by (B) protein gel blot analysis for maspin, p-AKT, Total-AKT, p-ERK, Total-ERK, Snail and Rac1, and (C) analysis of Rac1 activity using G-LISA Rac1 activity assay (P value = 0.068). (D) 22Rv1 Neo control cells were treated with maspin siRNA for 72 hrs followed by a migration assay using the boyden chamber for an additional 24 hrs. Cells that had migrated are represented as images taken by light microscopy as well as graphing of cell numbers following counting. Actin was used as a loading control. Results are reported as mean ± SD from 3 independent experiments. Statistical significance was assessed using GraphPad Prism software by paired Student's t-test compared to control siRNA (**p < 0.01).
Discussion
Tumor metastasis is a complex process that involves increased cell motility, recruitment of cellular components such as matrix metalloproteases to degrade the extracellular matrix, cell proliferation, among other features.43 One of the early steps during the metastatic process is EMT, during which epithelial cells lose their adhesions to neighboring cells and acquire migratory capabilities.44 Snail is known to regulate EMT through its role in downregulating E-cadherins.6 To further elucidate the role of Snail during CaP progression, we overexpressed Snail in androgen-dependent, LNCaP and 22Rv1 cell lines. We have previously shown that Snail overexpression in prostate cancer cells induces EMT associated with increased migration, invasion and tumorigenicity.37,45,46 We have also shown previously that Snail may promote cell migration and invasion through repression of maspin tumor suppressor by binding to E-boxes located in the promoter region.26 Rac1 activity is involved in cell migration and maspin has been shown to repress Rac1 activity in breast cancer cells.25 Moreover, Snail has been shown to promote cell migration and scattering in pancreatic cancer cells by activation of Rac1 although the signaling mechanism was not delineated.36 Therefore, we hypothesized that Snail may regulate Rac1activity and cell migration in prostate cancer cells via PI3K/AKT and/or MAPK signaling pathway, which has never been reported. We found that Snail-overexpressing LNCaP and 22Rv1 cells displayed increased migration and Rac1 activity. Next, utilizing C4–2 cells with stable knockdown of Snail, we observed that these cells had decreased cell migration, as previously reported,39 and reduced Rac1 activity. This supports the conclusion that Snail may mediate cell migration partly through increased Rac1 activity in prostate cancer cells. We further observed that NSC23766, a Rac1 inhibitor, could significantly decrease Snail-mediated cell migration at a dose of 100–200 μM. Interestingly, treatment with the Rac1 inhibitor reduced Snail protein levels which suggests that although Snail can increase Rac1 activity, this may further aid in maintaining Snail protein levels by a positive feedback loop. Indeed, expression of Rac1b, a splice variant of Rac1, in SCp2 mouse mammary epithelial cells has been shown to increase reactive oxygen species which led to increased expression of Snail and EMT.47
Although MAPK pathway has been implicated in cell migration32 and has been found to regulate Rac1,33 our data suggested that Snail-mediated Rac1 activation in 22Rv1 cells is not mediated by MAPK pathway, as the MEK inhibitor did not affect Rac1 activity. However, the MEK inhibitor still resulted in decreased cell migration, suggesting that the MAPK pathway can utilize Rac1-independent pathways to regulate cell migration. Indeed, it has been shown that ERK-dependent Fra-1 activation promotes cell motility by inactivation β-1 integrin levels and decreasing RhoA activity.33
PI3K/AKT has been reported to be activated during EMT through the regulation of E-cadherin48 and Rac1 has been shown to be a downstream target of this pathway.23 We found that LY294002, a PI3K/AKT inhibitor, led to a decline in both Snail-mediated Rac1 activity and cell migration. This supports the conclusion that Rac1 is downstream of the PI3K/AKT pathway and that this pathway cooperates with Snail and Rac1 to mediate cell migration during tumor progression. We noted that although Snail decreased only transiently following inhibitor treatments, maspin increase was more sustained and migration occurred later after 24 hrs. We believe that Snail is an immediate early gene that may exert its effect transiently, especially when treated with the inhibitors. Its expression decreases only transiently, but enough to affect other effector genes such as maspin that then increase but stay on longer and may then exert effects such as decreased cell migration at later time points such as 24 h, when by then Snail has already recovered.
We speculated on a mechanism by which Snail would be able to increase Rac1 activity. Since we had recently published that Snail can decrease the promoter activity of maspin tumor suppressor resulting in decreased maspin protein expression and cell migration,26 and previous studies had shown that maspin can repress Rac1 activity,25 it seemed logical to test whether maspin could be a link between Snail and Rac1. We did indeed confirm that maspin was repressed by Snail and that alternatively if we inhibited maspin expression with siRNA in maspin expressing 22Rv1 Neo cells, then this resulted in higher Rac1 activity. This would suggest that Snail repression of maspin may lead to increased Rac1 activity. Interestingly, maspin inhibition led to increased PI3K/AKT activity suggesting that maspin may repress Rac1 activity by repressing PI3K/AKT activity. Nam et al., have shown that maspin transfection could significantly reduce AKT phosphorylation in NCI-H157 lung cancer cells, whereas, maspin knockdown increased AKT phosphorylation.49 Currently, we do not know how maspin can regulate AKT activity. Since 22Rv1 cells contain intact PTEN which normally suppresses PI3K/AKT signaling, one possibility is that maspin may inactivate PTEN and increase PI3K/AKT activity. PTEN has been shown to bind with p53 to maspin promoter and increase its expression,50 however, it has never been shown that maspin can regulate PTEN. Therefore, this suggests that Snail may regulate Rac1 activity through maspin-dependent regulation of PI3K/AKT activity in 22Rv1 cells. The fact that Rac1 inhibitor did not affect maspin levels or AKT activity supports the fact that Rac1 activity must be downstream of maspin and AKT.
Collectively, our data supports a pathway in which Snail can promote cell migration via multiple pathways in prostate cancer cells; Snail activation of MAPK pathway can promote cell migration, while Snail repression of maspin tumor suppressor can lead to PI3K/AKT activation resulting in Rac1 activation (Fig. 7). Interestingly, inhibition of MAPK or PI3K/AKT pathway decreased Snail levels suggesting a positive feedback loop may exist from these pathways to maintain Snail levels; however, the mechanism of how this would occur remains unclear. In summary, our study shows that Snail-mediated cell migration acts in part via MAPK signaling and in part via maspin suppression and PI3K/AKT- Rac1 activation during CaP progression.
Figure 7.
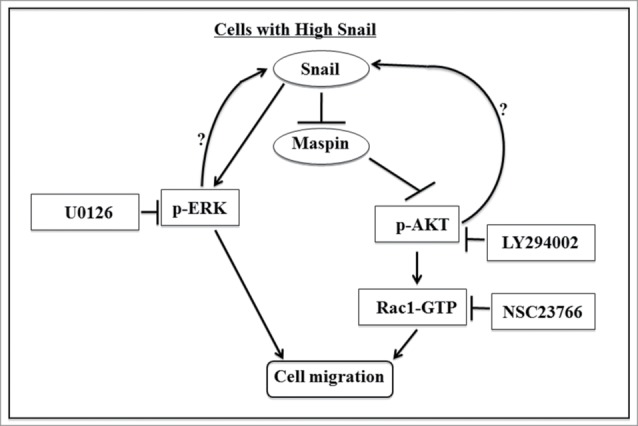
Overview of Snail signaling pathway that regulates cell migration in prostate cancer cells. Our data supports a signaling pathway in which Snail promotes cell migration via repression of maspin expression which allows for PI3K/AKT activation and subsequently Rac1 activation. An alternate pathway involves Snail activation of MAPK signaling pathway that leads to increased cell migration independent of Rac1. There may also be a positive feedback loop by which PI3K/AKT and MAPK activity can increase Snail expression.
Materials and Methods
Reagents and antibodies
RPMI medium was purchased from Lonza, Waltersville, MD and penicillin/streptomycin was purchased from Cell Gro, Manassas, VA. Rat tail collagen type I used for migration assay was obtained from BD Biosciences, Bedford, MA. The protease inhibitor cocktail was from Roche Molecular Biochemicals, Indianapolis, IN. Mouse monoclonal anti-human maspin antibody was from BD Transduction Laboratories, Lexington, KY. G418 and anti-human actin antibodies were from Sigma-Aldrich, Inc.., St Louis, MO. Rabbit monoclonal anti-human Snail antibody, Rabbit monoclonal anti-human phospho-AKT, phospho-ERK antibodies and HRP-conjugated goat anti-rat antibody were from Cell Signaling Technology, Inc.., Danvers, MA. HRP-conjugated sheep anti-mouse antibody and HRP-conjugated donkey anti-rabbit were purchased from Amersham Biosciences, Buckingham, England. Enhanced chemiluminescence (ECL) prime western blotting detection reagent was purchased from Thermo Fisher Scientific Inc.., Waltham, MA. Fetal bovine serum (FBS) was from PAA laboratories Inc.., Dartmouth, MA. The Charcoal/dextran treated FBS (DCC-FBS) was from Hyclone, South Logan, UT. Rac1 antibody and Rac1 activation assay kit was purchased from Upstate (Millipore), Billerica, MA. The G-LISA Rac1 Activation Assay Biochem Kit (Absorbance Based) was purchased from Cytoskeleton, Inc., Denver, CO. PI3K/AKT inhibitor (LY294002) and Rac1 inhibitor (NSC23766) were purchased from EMD Millipore, Billerica, MA. MEK inhibitor (U0126) was purchased from Sigma- Aldrich corp., St. Louis, MO, USA.
Cell culture
The human prostate cancer cell lines, LNCaP, and 22Rv1 were obtained from ATCC, Manassas, VA. LNCaP and 22Rv1 cells stably overexpressing Snail utilized in these experiments were previously generated.26,37 C4–2 cells were a kind gift from Dr. Leland Chung (Cedar Sinai Medical Center, Los Angeles, CA). C4–2 cells with stable knockdown of Snail using shRNA were previously generated.39 Cells were grown in RPMI medium supplemented with 10% fetal bovine serum and 1 × penicillin-streptomycin (plus 400 μg/ml G418 for Snail overexpressing cells), at 37 °C with 5% CO2 in a humidified incubator.
Western blot analysis
Confluent cells were lysed in a modified RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.02% NaN3, 0.1% SDS, 1% NP-40, 0.5 % sodium deoxycholate) containing 1.5 × protease inhibitor cocktail, 1 mM phenylmethylsufonyl fluoride, and 1 mM sodium orthovanadate. The cell lysates were centrifuged, and supernatants collected and quantified using a micro BCA assay. 30–50 μg of cell lysate was resolved on a 10–13% SDS PAGE, followed by transblotting onto nitrocellulose membrane from Schleicher & Schuell, Keene, NH. The membranes were blocked in TBS-T (Tris Buffer Saline with 0.05% Tween-20) containing 3% milk, and subsequently incubated with diluted antibody in blocking buffer. After washing, the membranes were incubated in peroxidase-conjugated sheep anti-mouse, donkey anti-rabbit, or goat anti-rat IgG, then washed, and visualized using ECL prime reagent. The membranes were stripped using stripping buffer (Pierce Biotechnology, Inc., Rockford, IL) prior to re-probing with a different antibody.
siRNA Transfection
22Rv1 Neo cells (5 × 105 cells per well) were plated in 6-well plates in growth media and left overnight for attachment. The next day, maspin siRNA (Human SERPINB5, Dharmacon, Inc..) transfections were performed according to manufacturer instructions. The maspin siRNA were pooled from 4 On-Target plus SMARTpool siRNA with the following identities and target sequences; J-019684-08, target sequence: GAAGAAAUUUCCUGAAUCA, J-019684-07, target sequence: AAUCUAGGGCUGAAACAUA, J-019684-06, target sequence: CGAAAGGUCAGAUCAACAA, J-019684-05, target sequence: UGGGAAACAUUGACAGUAU. Briefly, the growth media was removed from cells and the cells washed with sterile Phosphate Buffered saline (1 × PBS). Control and maspin siRNA transfection reagents were prepared in phenol and serum-free RPMI at a concentration of 200 nM, and then added to their corresponding wells in the 6-well plates. For the untreated control cells, only media was added to wells. The cells were incubated at 37°C, 5% CO2 for 5 hours after which the media was replaced with 2 ml of phenol-free charcoal stripped media (5% DCC) followed by incubation at 37°C, 5% CO2 for 72 hours. Cell lysates were then harvested after the given time period.
Inhibitor treatment
22Rv1 Snail-transfected cells were plated at 1.25 × 106 cells in T-75 flasks and treated with 20 µM LY294002 or U0126 inhibitors for time points, 30 min, 2 hrs, 4 hrs, 24 hrs, 72 hrs and controls were treated with DMSO. Treatment with NSC23766 at 50 µM, 100 µM and 200 µM was performed for 24 hrs, using double distilled water as a control. All treatments were performed using RPMI phenol-free supplemented with 5% DCC-FBS.
In vitro cell migration assay
We utilized Costar 24-well plates containing a polycarbonate filter insert (BD Biosciences, Franklin Lakes, NJ) with an 8-μm pore size, coated with rat tail collagen I on the outside for migration assays. 50,000 cells were plated in the upper chamber containing 0.1% fetal bovine serum (FBS) while the lower chamber contained 10% FBS. 24 hrs later, cells that had migrated to the bottom of the insert was fixed with 10% formalin, stained with crystal violet, and counted to obtain the relative cell migration.
Rac1 activity assay
Cells were allowed to grow to 70–80% confluency before the protein lysates were collected. In order to analyze Rac1 activity we utilized a Rac1 G-LISA kit. The Rac1 G-LISA kit has a Rac1-GTP-binding protein linked to a 96 well plate.51,52 Hence active GTP-bound Rac1 in the protein lysates will bind to the wells. The bound Rac1-GTP can then be detected with a Rac1 antibody. Briefly, 50 µg of protein lysates were allowed to bind to the 96 well plate. The samples were then washed and incubated with Rac1 primary antibody. After washing, the secondary antibody was added followed by HRP solution at room temperature. The plate was then read at OD 490 nm and the Rac1 signal values used to calculate the percent Rac1 activation compared to control treatment.
Statistical analysis
Results are reported as mean ±SD from 2–3 experiments. Statistical significance was assessed using GraphPad Prism software by paired Student's t-test and considered significant at P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by: NIH grants 1P20MD002285 (VOM), G12MD007590 (VOM).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11-30; PMID:23335087; http://dx.doi.org/ 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9-29; PMID:24399786; http://dx.doi.org/ 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 3.Christofori G. New signals from the invasive front. Nature 2006; 441:444-50; PMID:16724056; http://dx.doi.org/ 10.1038/nature04872 [DOI] [PubMed] [Google Scholar]
- 4.De Craene B, Berx G. Snail in the frame of malignant tumor recurrence. Breast Cancer Res 2006; 8:105; PMID:16834762; http://dx.doi.org/ 10.1186/bcr1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katoh M. Identification and characterization of human SNAIL3 (SNAI3) gene in silico. Int J Mol Med 2003; 11:383-8; PMID:12579345 [PubMed] [Google Scholar]
- 6.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000; 2:76-83; PMID:10655586; http://dx.doi.org/ 10.1038/35000025 [DOI] [PubMed] [Google Scholar]
- 7.Heeboll S, Borre M, Ottosen PD, Dyrskjot L, Orntoft TF, Torring N. Snail1 is over-expressed in prostate cancer. Apmis 2009; 117:196-204; PMID:19245592; http://dx.doi.org/ 10.1111/j.1600-0463.2008.00007.x [DOI] [PubMed] [Google Scholar]
- 8.De Craene B, Gilbert B, Stove C, Bruyneel E, van Roy F, Berx G. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res 2005; 65:6237-44; PMID:16024625; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-3545 [DOI] [PubMed] [Google Scholar]
- 9.Haataja L, Groffen J, Heisterkamp N. Characterization of RAC3, a novel member of the Rho family. J Biol Chem 1997; 272:20384-8; PMID:9252344; http://dx.doi.org/ 10.1074/jbc.272.33.20384 [DOI] [PubMed] [Google Scholar]
- 10.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 2000; 19:3013-20; PMID:10871853; http://dx.doi.org/ 10.1038/sj.onc.1203621 [DOI] [PubMed] [Google Scholar]
- 11.Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Sosa MS, Kazanietz MG. Rac signaling in breast cancer: a tale of GEFs and GAPs. Cell Signal 2012; 24:353-62; PMID:21893191; http://dx.doi.org/ 10.1016/j.cellsig.2011.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci 2003; 116:435-40; PMID:12508104; http://dx.doi.org/ 10.1242/jcs.00238 [DOI] [PubMed] [Google Scholar]
- 13.Adithi M, Venkatesan N, Kandalam M, Biswas J, Krishnakumar S. Expressions of Rac1, Tiam1 and Cdc42 in retinoblastoma. Exp Eye Res 2006; 83:1446-52; PMID:17027002; http://dx.doi.org/ 10.1016/j.exer.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 14.Seoh ML, Ng CH, Yong J, Lim L, Leung T. ArhGAP15, a novel human RacGAP protein with GTPase binding property. FEBS Lett 2003; 539:131-7; PMID:12650940; http://dx.doi.org/ 10.1016/S0014-5793(03)00213-8 [DOI] [PubMed] [Google Scholar]
- 15.Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell 2004; 7:585-95; PMID:15469846; http://dx.doi.org/ 10.1016/j.devcel.2004.07.021 [DOI] [PubMed] [Google Scholar]
- 16.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992; 70:401-10; PMID:1643658; http://dx.doi.org/ 10.1016/0092-8674(92)90164-8 [DOI] [PubMed] [Google Scholar]
- 17.Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci 2004; 117:1259-68; PMID:14996945; http://dx.doi.org/ 10.1242/jcs.00997 [DOI] [PubMed] [Google Scholar]
- 18.Kiosses WB, Shattil SJ, Pampori N, Schwartz MA. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat Cell Biol 2001; 3:316-20; PMID:11231584; http://dx.doi.org/ 10.1038/35060120 [DOI] [PubMed] [Google Scholar]
- 19.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. Faseb J 2002; 16:1195-204; PMID:12153987; http://dx.doi.org/ 10.1096/fj.02-0038com [DOI] [PubMed] [Google Scholar]
- 20.Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF. Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Res 2005; 7:R965-74; PMID:16280046; http://dx.doi.org/ 10.1186/bcr1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2:489-501; PMID:12094235; http://dx.doi.org/ 10.1038/nrc839 [DOI] [PubMed] [Google Scholar]
- 22.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 2005; 4:988-1004; PMID:16341064; http://dx.doi.org/ 10.1038/nrd1902 [DOI] [PubMed] [Google Scholar]
- 23.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 1998; 279:558-60; PMID:9438848; http://dx.doi.org/ 10.1126/science.279.5350.558 [DOI] [PubMed] [Google Scholar]
- 24.Zou Z, Zhang W, Young D, Gleave MG, Rennie P, Connell T, Connelly R, Moul J, Srivastava S, Sesterhenn I. Maspin expression profile in human prostate cancer (CaP) and in vitro induction of Maspin expression by androgen ablation. Clin Cancer Res 2002; 8:1172-7; PMID:12006534 [PubMed] [Google Scholar]
- 25.Odero-Marah VA, Khalkhali-Ellis Z, Chunthapong J, Amir S, Seftor RE, Seftor EA, Hendrix MJ. Maspin regulates different signaling pathways for motility and adhesion in aggressive breast cancer cells. Cancer Biol Ther 2003; 2:398-403; PMID:14508113; http://dx.doi.org/ 10.4161/cbt.2.4.471 [DOI] [PubMed] [Google Scholar]
- 26.Neal CL, Henderson V, Smith BN, McKeithen D, Graham T, Vo BT, Odero-Marah VA. Snail transcription factor negatively regulates maspin tumor suppressor in human prostate cancer cells. BMC Cancer 2012; 12:336; PMID:22857708; http://dx.doi.org/ 10.1186/1471-2407-12-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seger R, Krebs EG. The MAPK signaling cascade. Faseb J 1995; 9:726-35; PMID:7601337 [PubMed] [Google Scholar]
- 28.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298:1911-2; PMID:12471242; http://dx.doi.org/ 10.1126/science.1072682 [DOI] [PubMed] [Google Scholar]
- 29.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004; 116:855-67; PMID:15035987; http://dx.doi.org/ 10.1016/S0092-8674(04)00215-6 [DOI] [PubMed] [Google Scholar]
- 30.Gioeli D, Mandell JW, Petroni GR, Frierson HF Jr., Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res 1999; 59:279-84; PMID:9927031 [PubMed] [Google Scholar]
- 31.Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, et al.. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene 1999; 18:813-22; PMID:9989833; http://dx.doi.org/ 10.1038/sj.onc.1202367 [DOI] [PubMed] [Google Scholar]
- 32.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci 2004; 117:4619-28; PMID:15371522; http://dx.doi.org/ 10.1242/jcs.01481 [DOI] [PubMed] [Google Scholar]
- 33.Vial E, Sahai E, Marshall CJ. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 2003; 4:67-79; PMID:12892714; http://dx.doi.org/ 10.1016/S1535-6108(03)00162-4 [DOI] [PubMed] [Google Scholar]
- 34.Dokmanovic M, Hirsch DS, Shen Y, Wu WJ. Rac1 contributes to trastuzumab resistance of breast cancer cells: Rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol Cancer Ther 2009; 8:1557-69; PMID:19509242; http://dx.doi.org/ 10.1158/1535-7163.MCT-09-0140 [DOI] [PubMed] [Google Scholar]
- 35.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene 2007; 26:7445-56; PMID:17563753; http://dx.doi.org/ 10.1038/sj.onc.1210546 [DOI] [PubMed] [Google Scholar]
- 36.Shields MA, Krantz SB, Bentrem DJ, Dangi-Garimella S, Munshi HG. Interplay between beta1-integrin and Rho signaling regulates differential scattering and motility of pancreatic cancer cells by snail and Slug proteins. J Biol Chem 2012; 287:6218-29; PMID:22232555; http://dx.doi.org/ 10.1074/jbc.M111.308940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKeithen D, Graham T, Chung LW, Odero-Marah V. Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate 2010; 70:982-92; PMID:20166136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridley AJ. Rho GTPases and cell migration. J Cell Sci 2001; 114:2713-22; PMID:11683406 [DOI] [PubMed] [Google Scholar]
- 39.Neal CL, McKeithen D, Odero-Marah VA. Snail negatively regulates cell adhesion to extracellular matrix and integrin expression via the MAPK pathway in prostate cancer cells. Cell adhesion & migration 2011; 5:249-57; PMID:21478672; http://dx.doi.org/ 10.4161/cam.5.3.15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A 2004; 101:7618-23; PMID:15128949; http://dx.doi.org/ 10.1073/pnas.0307512101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res 1998; 58:2720-3; PMID:9661880 [PubMed] [Google Scholar]
- 42.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 1994; 269:5241-8; PMID:8106507 [PubMed] [Google Scholar]
- 43.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer 2003; 3:55-63; PMID:12509767; http://dx.doi.org/ 10.1038/nrc967 [DOI] [PubMed] [Google Scholar]
- 44.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2:442-54; PMID:12189386; http://dx.doi.org/ 10.1038/nrc822 [DOI] [PubMed] [Google Scholar]
- 45.Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C, Marshall FF, Zhau HE, Chung LW. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res 2008; 18:858-70; PMID:18645583; http://dx.doi.org/ 10.1038/cr.2008.84 [DOI] [PubMed] [Google Scholar]
- 46.Barnett P, Arnold RS, Mezencev R, Chung LW, Zayzafoon M, Odero-Marah V. Snail-mediated regulation of reactive oxygen species in ARCaP human prostate cancer cells. Biochem Biophys Res Commun 2011; 404:34-9; PMID:21093414; http://dx.doi.org/ 10.1016/j.bbrc.2010.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al.. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 2005; 436:123-7; PMID:16001073; http://dx.doi.org/ 10.1038/nature03688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3' kinase/AKT pathways. Oncogene 2005; 24:7443-54; PMID:16288291; http://dx.doi.org/ 10.1038/sj.onc.1209091 [DOI] [PubMed] [Google Scholar]
- 49.Nam E, Park C. Maspin suppresses survival of lung cancer cells through modulation of Akt pathway. Cancer Res Treat 2010; 42:42-7; PMID:20369051; http://dx.doi.org/ 10.4143/crt.2010.42.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eitel JA, Bijangi-Vishehsaraei K, Saadatzadeh MR, Bhavsar JR, Murphy MP, Pollok KE, Mayo LD. PTEN and p53 are required for hypoxia induced expression of maspin in glioblastoma cells. Cell Cycle 2009; 8:896-901; PMID:19221500; http://dx.doi.org/ 10.4161/cc.8.6.7899 [DOI] [PubMed] [Google Scholar]
- 51.Zhou X, Tian F, Sandzen J, Cao R, Flaberg E, Szekely L, Cao Y, Ohlsson C, Bergo MO, Borén J, et al.. Filamin B deficiency in mice results in skeletal malformations and impaired microvascular development. Proc Natl Acad Sci U S A 2007; 104:3919-24; PMID:17360453; http://dx.doi.org/ 10.1073/pnas.0608360104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlegel N, Burger S, Golenhofen N, Walter U, Drenckhahn D, Waschke J. The role of VASP in regulation of cAMP- and Rac 1-mediated endothelial barrier stabilization. Am J Physiol Cell Physiol 2008; 294:C178-88; PMID:17989211; http://dx.doi.org/ 10.1152/ajpcell.00273.2007 [DOI] [PubMed] [Google Scholar]


