Abstract
Fibrillin-1 is a microfibrillar extracellular matrix protein that was described to be a ligand for α8 integrin. α8 integrin is a matrix receptor specifically expressed in mesangial and smooth muscle cells of the kidney. In previous studies we detected glomerular expression of fibrillin-1. Moreover, fibrillin-1 promoted adhesion, migration, and proliferation of mesangial cells. We hypothesized that fibrillin-1 and α8 integrin might interact in the glomerulus, and thus, regulate mesangial cell properties. Our studies showed that fibrillin-1 and α8 integrin colocalize in the glomerular mesangium. Induction of experimental glomerulonephritis led to an increase of both fibrillin-1 and α8 integrin expression. In vitro studies revealed that mesangial cells deficient for α8 integrin adhere weaker to fibrillin-1 and migrate more easily on fibrillin-1 than wild-type mesangial cells. Baseline proliferation on fibrillin-1 is higher in α8 integrin-deficient mesangial cells, but the induction of proliferation is not different in α8 integrin-deficient and wild-type mesangial cells. We conclude that fibrillin-1 and α8 integrin interact, and thus, regulate mesangial cell adhesion and migration. The concomitant induction of both fibrillin-1 and α8 integrin in a self-limited model of glomerular injury points to a protective role of the interaction of fibrillin-1 with α8 integrin in the glomerulus resulting in reduced damage of the glomerular tuft as a consequence of firm adhesion of mesangial cells.
Keywords: α8 integrin-deficient mice, cell adhesion, fibrillin-1, integrins, mesangial cells, migration, glomerulus, glomerulonephritis
Abbreviations
- α8−/−
alpha8 integrin-deficient
- BSA
bovine serum albumin
- FCS
fetal calf serum
- FN
fibronectin
- sd
standard deviation
- wt
wild-type
Introduction
Fibrillin-1 is a component of the microfibrillar network of the extracellular matrix serving together with elastin elastic properties in various tissues, including skin, lung, and vasculature.1,2 Fibrillin-1 has an important function in conveying compliance of the vasculature, preventing aortic dissection and rupture, as exemplified in individuals with Marfan syndrome caused by mutations in the fibrillin-1 gene which results in increased risk of aortic aneurysms.3 In the kidney, fibrillin-1 is expressed by mesangial cells and is incorporated in the elastin-free glomerular mesangial matrix.4 Adhesion of mesangial cells to fibrillin-1 regulates mesangial cell attachment, migration and proliferation in vitro.5 Moreover, in an experimental model of acute glomerulonephritis, fibrillin-1 expression was induced arguing for a contribution of fibrillin-1 to the regulation of cell adaptations to this glomerular disease.5 Attachment of cells to fibrillin-1 is mediated via the integrin receptors αvβ3, α5β1, and αvβ6.6-8 Recently, α8β1 integrin was also described to interact with fibrillin-1.9
In the kidney, the α8 integrin chain is expressed on vascular smooth muscle cells and mesangial cells.10,11 After dimerization with the β1 integrin chain, it serves as a receptor for fibronectin, vitronectin, osteopontin, tenascin C and nephronectin.12-14 α8 integrins contribute to the regulation of various cell functions, including adhesion, migration and proliferation.15 Studies with α8 integrin-deficient mice revealed that loss of α8 integrin leads to a delay in recovering from experimental glomerulonephritis.16 We therefore hypothesized that fibrillin-1 might convey its effects on mesangial cells via α8 integrin. It became apparent that interactions of α8 integrin and fibrillin-1 contribute to mesangial cell adhesion and prevent them from migrating.
Results
In the glomerular mesangium fibrillin-1 and α8 integrin were coexpressed and colocalized (Fig. 1A, B, C). After induction of an acute model of mesangioproliferative glomerulonephritis (Thy1.1 nephritis) characterized by mesangial expansion at day 7, both fibrillin-1 and α8 integrin were more abundant in the glomerulus but still colocalized (Fig. 1D–F). Concordant with these findings, cortical mRNA expression levels of fibrillin-1 and α8 integrin were increased at day 7 of disease (Fig. 2), which confirms previous data obtained in Thy1.1 nephritis.5,11
Figure 1.
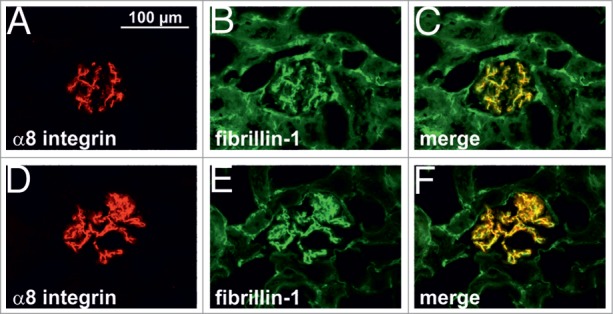
Double immunofluorescence staining of fibrillin-1 (green) and α8 integrin (red). A, B, C kidney section from control rat. D, E, F kidney section from rat after 7 d of Thy1.1 nephritis. Colocalization is indicated by yellow color.
Figure 2.
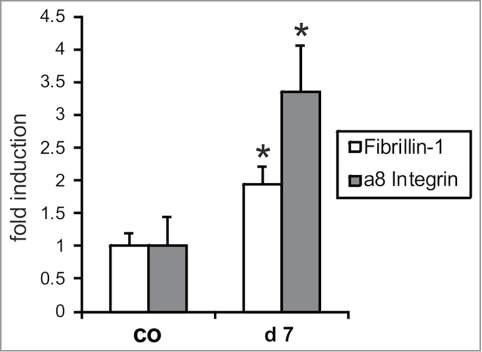
Real-time RT-PCR expression analysis of fibrillin-1 and α8 integrin in the renal cortex of control rats (co) and rats after 7 days of Thy1.1 nephritis (d7) Data are means ± sd. * P < 0.05 vs. respective control.
Attachment of mesangial cells isolated from wild type and α8 integrin-deficient mice was compared after seeding on fibrillin-1. One hour after seeding, the percentage of attached as well as spread mesangial cells was assessed. The percentage of adhered and spread α8 integrin-deficient mesangial cells was significantly lower compared with wild type mesangial cells (Fig. 3). Incubation with a blocking antibody to αv integrin further considerably reduced attachment of α8 integrin-deficient mesangial cells, while attachment of wild type mesangial cells was not affected (Fig. 4), arguing for an important contribution of α8 integrin to the adhesion of mesangial cells on fibrillin-1. Moreover, staining of wild type mesangial cells adhered to fibrillin-1 with an antibody to α8 integrin resulted in positive immunoreactivity of focal contacts (Fig. 5A) which was not seen on bovine serum albumin (BSA) as a control (Fig. 5C). As additional controls, mesangial cells seeded on fibronectin and on collagen I were evaluated (Fig. 5B and D). Focal contacts stained positive for α8 integrin on the α8 integrin ligand fibronectin (Fig. 5B), but did not stain positive on collagen I, which is not a ligand for α8 integrin (Fig. 5D). As expected, in α8 integrin-deficient mesangial cells positive staining for α8 integrin was never observed (not shown). Compared with wild type mesangial cells, α8 integrin-deficient mesangial cells migrate more easily on fibrillin-1, indicating that interactions between fibrillin-1 and α8 integrins inhibit migration (Fig. 6). Loss of α8 integrin led to a higher proliferative activity of mesangial cells on fibrillin-1 under serum-starved conditions (Fig. 7A). Stimulation of α8 integrin-deficient mesangial cells with 2% fetal calf serum (FCS) resulted in increased proliferation, which was similar to the increase seen in wild type mesangial cells: In both cell types there was a 100% increase in the proliferation rate in response to stimulation with FCS (Fig. 7A). This is in contrast to mesangial cell proliferation on fibronectin (Fig. 7B), where basal proliferation rates of both cell types are low and not different from each other, resulting in a more prominent induction of proliferation of α8 integrin-deficient mesangial cells on fibronectin after stimulation with FCS (Fig. 7B).
Figure 3.
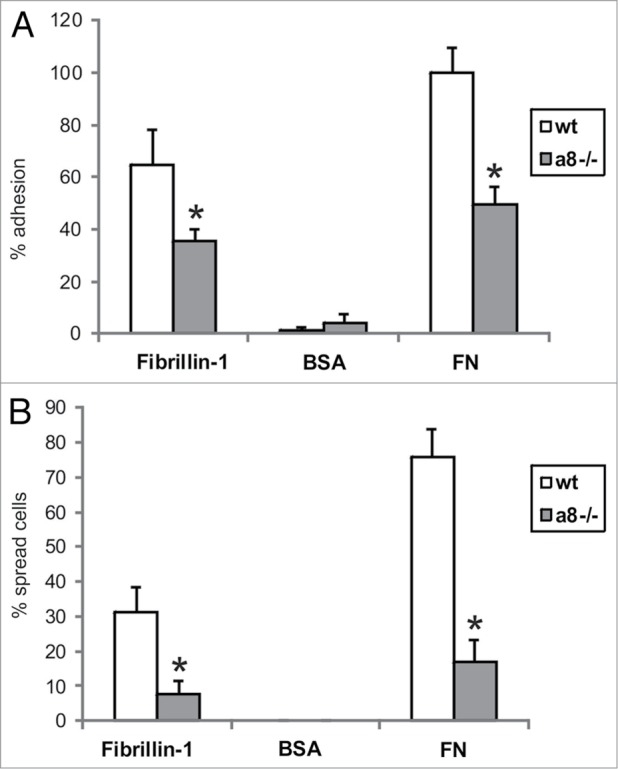
Attachment of wild type (wt) and α8 integrin-deficient (a8−/−) mesangial cells on fibrillin-1 fragment. A: evaluation of cells adhered to fibrillin-1. B: evaluation of cells spread on fibrillin-1. Coating with bovine serum albumin (BSA) served as a negative control, coating with fibronectin (FN) served as a positive control. Results are representative for 3 independent experiments. Data are means ± sd. * P < 0.05 vs. wt.
Figure 4.
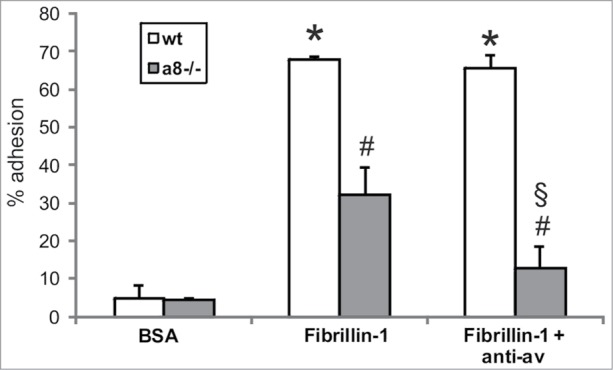
Attachment of wild type (wt) and α8 integrin-deficient (a8−/−) mesangial cells on fibrillin-1 fragment after blocking of αv integrin. Coating with bovine serum albumin (BSA) served as a negative control. Results are representative for 3 similar experiments. Data are means ± sd. * P < 0.05 vs. BSA control, # P < 0.05 vs. wt, § P < 0.05 vs. a8−/− on fibrillin-1.
Figure 5.
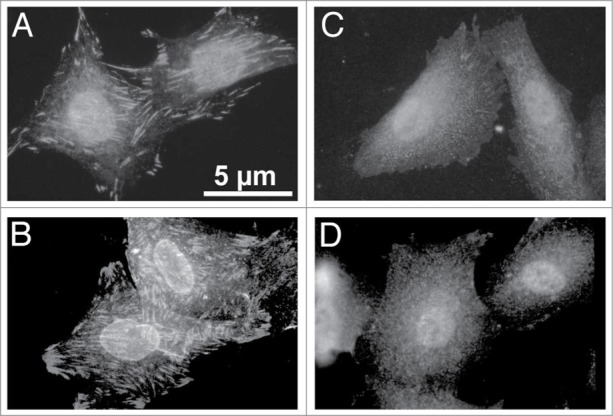
Staining of α8 integrin in wild type mesangial cells attached to fibrillin-1 (A), to fibronectin (α8 integrin ligand) as a positive control (B) to bovine serum albumin (C) as a negative control, and to collagen I (not a ligand for α8 integrin; D).
Figure 6.
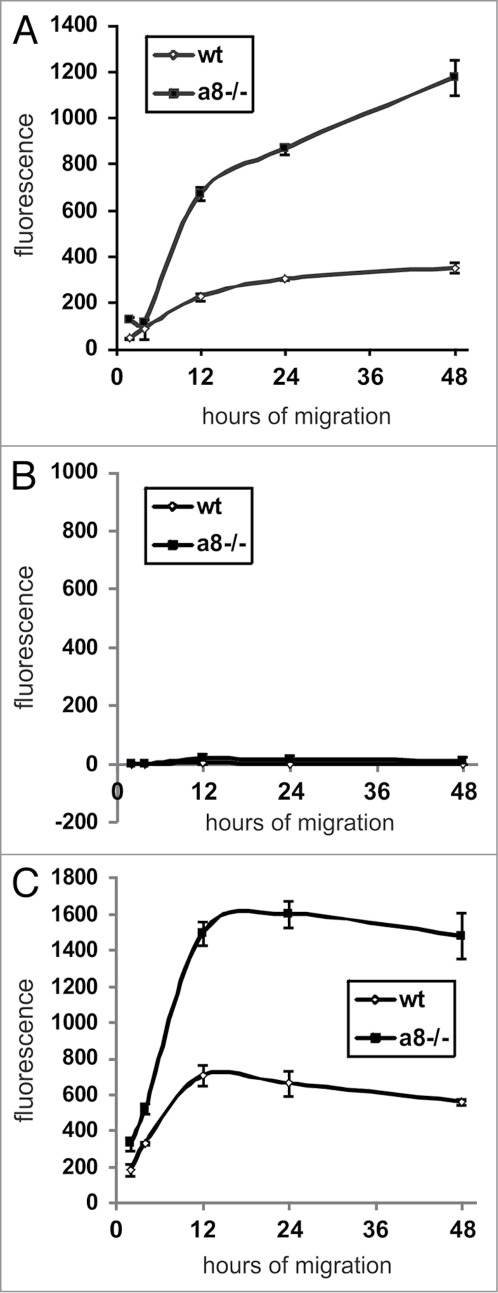
Migration of wild type (wt) and α8 integrin-deficient (a8−/−) mesangial cells on fibrillin-1 fragment (A). B: Migration on BSA as a negative control. C: Migration on fibronectin (FN) as a positive control. Results are representative for 3 similar experiments. Data are means ± sd.
Figure 7.
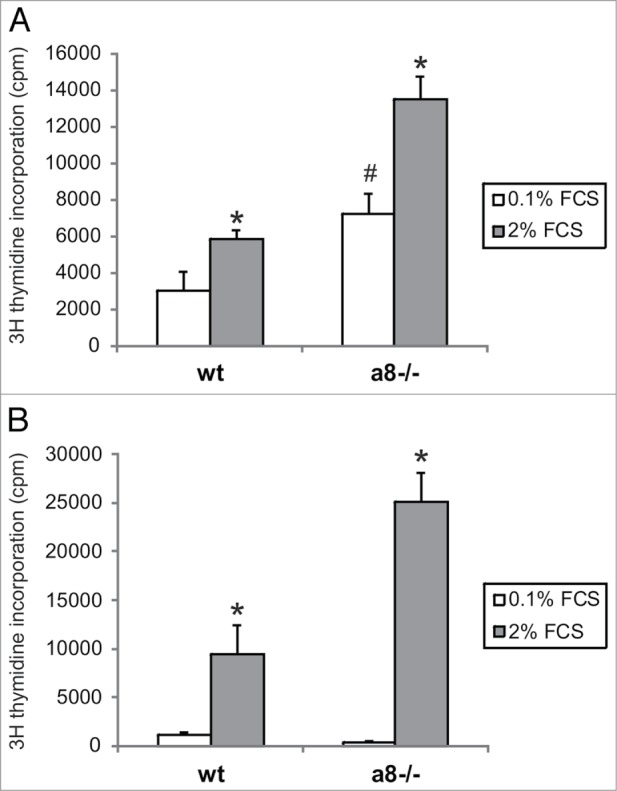
Proliferation of wild type (wt) and α8 integrin-deficient (a8−/−) mesangial cells seeded on fibrillin-1 fragment (A). Cells were serum starved in medium containing 0.1% fetal calf serum (FCS). Proliferation was stimulated with addition of 2% fetal calf serum. B: Proliferation on fibronectin was evaluated as a positive control. Data are means ± sd. * P < 0.05 vs. respective serum starved control (0.1% FCS). # P < 0.05 vs. serum starved wt. Results are representative for 3 similar experiments.
Discussion
The overall findings of the study are that in mesangial cells α8 integrin and fibrillin-1 interact, arguing for glomerular fibrillin-1 to act as a ligand for α8 integrin. On fibrillin-1 50% of mesangial cell adhesion seems to account for α8 integrin. This interaction has an inhibitory effect on mesangial cell migration and growth.
Our results show that fibrillin-1 and α8 integrin colocalize in the glomerular mesangium and that there is a coordinate increase in fibrillin-1 and α8 integrin expression in nephritic glomerular disease, which is supportive of the notion that both might interact in the glomerulus under physiological and pathological conditions. Interaction of α8 integrin with fibrillin-1 was demonstrated in cardiac fibroblasts.9 This is in accordance with our studies using mesangial cells which show that α8 integrin conveys adhesion while reducing migration and some basal proliferation on fibrillin-1. These findings argue for a beneficial role of the interactions of fibrillin-1 with α8 integrin in tissue homeostasis during acute glomerulonephritis, resulting in firm adhesion and quiescence of mesangial cells. This notion is also supported by the finding that in α8 integrin-deficient mice glomerular injury leads to a more severe disruption of the integrity of the glomerular tuft compared with wild type mice.17 On the other hand, mice underexpressing fibrillin-1 seem to be protected from glomerular injury in chronic renal disease.18 Wild type mesangial cells adhere, migrate and proliferate on fibrillin-1 efficiently.5 These effects of fibrillin-1, however, are not only conveyed by interactions with α8 integrin, but also with αv or α5 integrin. As a consequence, fibrillin-1 could contribute to pathological changes, like mesangial hypercellularity in chronic renal disease. Thus, it seems conceivable that fibrillin-1 overexpression during glomerular injury can be beneficial or detrimental to glomerular integrity. Besides its putative protective interactions with α8 integrin in mesangial cells, fibrillin-1 might increase compliance in mechanically stressed glomeruli. Interactions of fibrillin-1 with LTBP-1, the latency-associated TGFβ binding protein, are known to regulate the activity of profibrotic TGFβ 19, which is known to be relevant in the vasculature, as Marfan patients which frequently suffer from aortic aneurysms, were shown to have a dysregulation of TGFβ activation.20 Coexpression of LTBP-1 and fibrillin-1 in the glomerulus and induction of both in Thy1.1 nephritis was also demonstrated,21 arguing for a role of fibrillin-1 in the regulation of TGFβ activity in the glomerulus. Fibrillin-1 might therefore serve antifibrotic functions by reducing profibrotic active TGFβ.
Based on the fact that wild type mesangial cells effectively adhere, migrate and proliferate on fibrillin-1,5 combined with our present findings, we speculate that adhesion of wild type mesangial cells to fibrillin-1 is largely due to interactions of fibrillin-1 with α8 integrin. On the other hand migration and proliferation on fibrillin-1 as shown previously5 is likely mediated by interactions with αv or α5 integrins which are both abundant on mesangial cells and upregulated in Thy1.1 nephritis.22 Both integrins are known to propagate migration or proliferation not only of mesangial cells,22 but also of other cell types adhering to fibrillin-1, such as endothelial cells.23 Thus, the increased migratory and proliferative activity of mesangial cells lacking α8 integrin argues for a counter regulatory effect of α8 integrin activity, which reduces wild type mesangial cell migration and basal proliferation rates. Previous studies employing α8 integrin-deficient mesangial cells revealed equal amounts of cell surface expression of αv and α5 integrins when compared with wild type mesangial cells.15 This precludes that the increased migration and basal proliferation rates of α8 integrin-deficient mesangial cells grown on fibrillin-1 is due to changes in αv and α5 integrin abundance and not to the lack of α8 integrin. Fibronectin is another ligand for α8, αv and α5 integrins. Mesangial cells lacking α8 integrin adhere weaker but migrate more efficiently on fibronectin, which is comparable to the effects seen on fibrillin-1. In this respect, interactions of α8 integrin with fibrillin-1 do not seem to be the only interactions regulating mesangial cell migration in vivo.
Adhesion of mesangial cells to fibrillin-1 could be largely contributed to α8 integrin. A blocking antibody to αv integrin only reduced attachment of α8 integrin-deficient mesangial cells, not wild type mesangial cells. Thus, at least in cell culture, α8 integrin seems to be more important for mesangial cell adhesion to fibrillin-1 than αv integrin. The contribution of α5 integrin could not be tested, because the antibodies available to us did not block α5 integrin function in our hands. However, in α8 integrin-deficient mesangial cells after blocking αv integrin the percentage of attached cells on fibrillin-1 was only marginally higher than on control BSA coating, arguing against a prominent role for α5 integrin.
In glomerulonephritis, however, other ligands of α8 integrin, like fibronectin,11 may be increased as well. Therefore, interactions of α8 integrin with other ligands might well have strong effects on α8 integrin-regulated disease mechanisms during glomerular injury, which will be difficult to be dissected from the mechanisms mediated by the interaction of fibrillin-1 with α8 integrin in vivo.
Taken together, our data suggest that α8 integrin interacts with fibrillin-1 in the glomerular mesangium and thus might play a protective role for glomerular integrity by conveying firm adhesion and quiescence of mesangial cells, while interactions of αv or α5 integrins with fibrillin-1 might primarily result in increased cell migration and proliferation.
Materials and Methods
Induction of anti-Thy1.1 nephritis
All animal procedures were done in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Committee on the Ethics of Animal Experiments of the government of Mittelfranken and local government authorities (AZ: # 621–2531.31–11/02). All efforts were made to minimize suffering. Male Sprague-Dawley rats (150 to 200 g) were obtained from Charles River Deutschland. Anti-Thy1.1 nephritis was induced by a single intravenous injection of 1 mg/kg body weight anti-Thy1.1 antibody into the tail vein in light isoflurane anesthesia. Controls received solvent only. The monoclonal antibody against Thy1.1 (ER4) was from Antibody Solutions. Anti-Thy1.1 nephritis is an acute mesangioproliferative glomerulonephritis with mesangial expansion peaking at day 7 of disease. Five animals per group were sacrificed under sodium pentobarbital anesthesia on day 7 after induction of nephritis and renal tissue was obtained for further preparation. The kidneys were decapsulated and immediately snap-frozen in liquid nitrogen for RNA extraction or preparation of cryostat sections.
Cultivation of mouse mesangial cells
Mesangial cells were isolated from kidneys of wild type or α8 integrin-deficient mice (obtained from U. Müller, San Diego, USA24) by the sieving method15 using 63, 75 and 38 μm grid sieves. Cultured wild type and α8 integrin-deficient mesangial cells were characterized as described.15 Mesangial cells were grown in Dulbecco‘s modified Eagle's Medium (DMEM; PAA Laboratories GmbH) containing 10% FCS, 5 μg/ml insulin, 5 μg/ml plasmocin (TEBU) and 2 mM L-glutamine (Sigma) in a 95% air – 5% CO2 humidified atmosphere at 37 °C. Mesangial cells were used for experiments in passages 5–10.
Isolation of mRNA and real-time PCR
To evaluate mRNA expression levels, total RNA was obtained from renal cortical tissue by extraction with RNeasy® Mini columns (Qiagen). First-strand cDNA was synthesized with TaqMan reverse transcription reagents (Applied Biosystems) using random hexamers as primers. Final RNA concentration in the reaction mixture was adjusted to 0.1 ng/μL. Reactions without Multiscribe reverse transcriptase were used as negative controls for genomic DNA contamination. PCR was performed with an ABI PRISM 7000 Sequence Detector System and SYBR Green or TaqMan reagents (Applied Biosystems) according to the manufacturer's instructions. The relative amount of the specific mRNA was normalized with respect to 18S rRNA. Primers used for amplification of 18S cDNA were forward 5′ TTGATTAAGTCCCTGCCCTTTGT 3′ and reverse 5′ CGATCCGAGGGCCTCACTA 3′. For amplification of the rat fibrillin-1 cDNA, the forward primer was 5′ TGCTCTGAAAGGACCCAATGT 3′ and the reverse primer was 5′ CGGGACAACAGTATGCGTTATAAC 3′. For amplification of the rat α8 integrin cDNA, the forward primer was 5′ CCTTGGGAACCCGATGGT 3′, the reverse primer was 5′ TCTCAAGACGAGGAACAGCAAA 3′ and the TaqMan probe was 5′ TGGAACGAATTTTTCTCTGGGCCTCC 3′. All samples were analyzed in triplicates.
Immunohistochemistry
For localization of fibrillin-1 and α8 integrin in the kidney, double immunofluorescence was performed on cryostat sections of snap-frozen tissue. The goat polyclonal antibody to α8 integrin was from R&D Systems and used in a concentration of 1:500. The rabbit antibody to fibrillin-1 was used as described previously.5 Primary antibodies were applied simultaneously overnight at 4 °C. After washing, sections were incubated with secondary antibodies for 2 h. CY3-labeled mouse anti-goat and CY2-labeled donkey anti-rabbit IgG (both from Dianova) were used simultaneously.
Immunocytochemistry
Mesangial cells were seeded in DMEM on glass 8-well chamber slides blocked with 2% bovine serum albumin (BSA). Cells were allowed to adhere for 24 h. Then, the media were removed, adherent cells were rinsed 3x with PBS and fixed in 3% paraformaldehyde for 20 min. After blockade of free aldehyde groups with 50 mM ammonium chloride, cells were permeabilized by 1% Triton X-100 and nonspecific binding was blocked using 100% FCS. Cells were incubated with the primary α8 integrin antibody (generous gift from U. Müller, San Diego, USA25) overnight, followed by a CY3-labeled goat anti-rabbit immunoglobulin G (Dianova) as secondary antibody and embedding in Tris-buffered Mowiol, pH 8,6 (Hoechst).
Coating of plates and chamber slides with fibrillin-1
The recombinant C-terminal half of fibrillin-1 (rF6H) containing the only RGD site was used for coating.5 This fibrillin-1 fragment was chosen for experiments, because adhesion, migration and proliferation of mesangial cells on this fragment were comparable to full-length fibrillin-1.5 The coating concentration used was 20 μg/ml according to previous experiments determining optimal concentrations for attachment of mesangial cells.5 Coating with BSA was used as a negative control. Coating with 10μg/ml fibronectin was used as a positive control.
Adhesion assay
An attachment assay was used based on the microscopic evaluation of the number of adherent cells and spread cells,5 as described. Cells were allowed to attach for one hour. To block adhesion of mesangial cells via αv integrins, a blocking antibody to αv integrin (H9 2B8, BD PharMingen) was applied at a dilution of 1:200 one hour before seeding the cells on fibrillin-1. Attachment was assessed after seeding of 5000 mesangial cells per chamber slide. After washing and staining with hematoxilin, adhered and spread cells were counted in 9 medium power views (x 200) per chamber slide.
Determination of cell proliferation
Cell proliferation was estimated after measurement of [3H] thymidine uptake, mesangial cells were serum-starved for 72 h in medium containing 0.1% FCS, seeded into matrix-coated 96-well plates and stimulated with 2% FCS for 14 h. [3H] thymidine uptake was determined as described previously.15
Migration assay
A transmigration assay was applied as described in detail.15 FluoroBlok Inserts (Falcon HTS, Becton Dickinson), containing a proprietary light-opaque membrane to absorb visible light between 490 and 700 nm with 8 μm pores were coated with 20 μg/ml of the fibrillin-1 fragment rF6H and saturated in FCS-free medium containing 1% BSA. Trypsinized mesangial cells were washed twice, labeled with 50 μg/ml DiI (Molecular Probes, Leiden, The Netherlands), a vital lipophilic carbocyanine, for 10 min at 37 °C and seeded into inserts in a volume of 150 μl at a density of 1x106 cells/ml. The inserts were then incubated in 24 Multiwell plates (Becton Dickinson), each well filled with 700μl medium containing 0.1% BSA for several hours. Measurements were performed after 0 (starting point), 2, 4, 12 and 48 h incubation to observe transmigration. Transmigrated mesangial cells were detected with a SPECTRAFluor fluorometer (Tecan).
Statistical analyses
A student's t test was used to test significance of differences between groups. A P value < 0.05 was considered significant. Values are displayed as means ± standard deviation (SD).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Ulrich Muller, San Diego, USA for kindly providing us with α8 integrin-deficient mice and the antibody to α8 integrin. This study was supported by a grant from the Deutsche Forschungsgemeinschaft, Bonn; Sonderforschungsbereich 423, TP A2
References
- 1. Ramirez F, Pereira L. The fibrillins. Int J Biochem Cell Biol 1999; 31:255-9; PMID:10216958; http://dx.doi.org/ 10.1016/S1357-2725(98)00109-5 [DOI] [PubMed] [Google Scholar]
- 2. Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci 2002; 115:2817-28; PMID:12082143 [DOI] [PubMed] [Google Scholar]
- 3. Jones JA, Ikonomidis JS. The pathogenesis of aortopathy in Marfan syndrome and related diseases. Curr Cardiol Rep 2010; 12:99-107; PMID:20425163; http://dx.doi.org/ 10.1007/s11886-010-0083-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sterzel RB, Hartner A, Schlötzer-Schrehardt U, Voit S, Hausknecht B, Doliana R, Colombatti A, Gibson MA, Braghetta P, Bressan GM. Elastic fiber proteins in the glomerular mesangium in vivo and in cell culture. Kidney Int 2000; 58:1588-602; PMID:11012893; http://dx.doi.org/ 10.1046/j.1523-1755.2000.00320.x [DOI] [PubMed] [Google Scholar]
- 5. Porst M, Plank C, Bieritz B, Konik E, Fees H, Dötsch J, Hilgers KF, Reinhardt DP, Hartner A. Fibrillin-1 regulates mesangial cell attachment, spreading, migration and proliferation. Kidney Int 2006; 69:450-6; PMID:16395273; http://dx.doi.org/ 10.1038/sj.ki.5000030 [DOI] [PubMed] [Google Scholar]
- 6. Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth CA, Humphries MJ, Kielty CM. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J Biol Chem 2003; 278:34605-16; PMID:12807887; http://dx.doi.org/ 10.1074/jbc.M303159200 [DOI] [PubMed] [Google Scholar]
- 7. Jovanovic J, Takagi J, Choulier L, Abrescia NG, Stuart DI, van der Merwe PA, Mardon HJ, Handford PA. alphaVbeta6 is a novel receptor for human fibrillin-1. Comparative studies of molecular determinants underlying integrin-rgd affinity and specificity. J Biol Chem 2007; 282:6743-51; PMID:17158881; http://dx.doi.org/ 10.1074/jbc.M607008200 [DOI] [PubMed] [Google Scholar]
- 8. Pfaff M, Reinhardt DP, Sakai LY, Timpl R. Cell adhesion and integrin binding to recombinant human fibrillin-1. FEBS Lett 1996; 384:247-50; PMID:8617364; http://dx.doi.org/ 10.1016/0014-5793(96)00325-0 [DOI] [PubMed] [Google Scholar]
- 9. Bouzeghrane F, Reinhardt DP, Reudelhuber TL, Thibault G. Enhanced expression of fibrillin-1, a constituent of the myocardial extracellular matrix in fibrosis. Am J Physiol Heart Circ Physiol 2005; 289:H982-91; PMID:15849235; http://dx.doi.org/ 10.1152/ajpheart.00151.2005 [DOI] [PubMed] [Google Scholar]
- 10. Schnapp LM, Breuss JM, Ramos DM, Sheppard D, Pytela R. Sequence and tissue distribution of the human integrin alpha 8 subunit: a beta 1-associated alpha subunit expressed in smooth muscle cells. J Cell Sci 1995; 108:537-44; PMID:7768999 [DOI] [PubMed] [Google Scholar]
- 11. Hartner A, Schöcklmann H, Pröls F, Müller U, Sterzel RB. Alpha8 integrin in glomerular mesangial cells and in experimental glomerulonephritis. Kidney Int 1999; 56:1468-80; PMID:10504498; http://dx.doi.org/ 10.1046/j.1523-1755.1999.00662.x [DOI] [PubMed] [Google Scholar]
- 12. Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J Biol Chem 1995; 270:23196-202; PMID:7559467; http://dx.doi.org/ 10.1074/jbc.270.39.23196 [DOI] [PubMed] [Google Scholar]
- 13. Denda S, Reichardt LF, Müller U. Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol Biol Cell 1998; 9:1425-35; PMID:9614184; http://dx.doi.org/ 10.1091/mbc.9.6.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Müller U, Reichardt LF. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J Cell Biol 2001; 154:447-58; PMID:11470831; http://dx.doi.org/ 10.1083/jcb.200103069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bieritz B, Spessotto P, Colombatti A, Jahn A, Prols F, Hartner A. Role of alpha8 integrin in mesangial cell adhesion, migration, and proliferation. Kidney Int 2003; 64:119-27; PMID:12787402; http://dx.doi.org/ 10.1046/j.1523-1755.2003.00057.x [DOI] [PubMed] [Google Scholar]
- 16. Hartner A, Marek I, Cordasic N, Haas C, Schocklmann H, Hulsmann-Volkert G, Plasa I, Rascher W, Hilgers KF, Amann K. Glomerular regeneration is delayed in nephritic alpha 8-integrin-deficient mice: contribution of alpha 8-integrin to the regulation of mesangial cell apoptosis. Am J Nephrol 2008; 28:168-78; PMID:17951999; http://dx.doi.org/ 10.1159/000110022 [DOI] [PubMed] [Google Scholar]
- 17. Hartner A, Cordasic N, Klanke B, Müller U, Sterzel RB, Hilgers KF. The alpha8 integrin chain affords mechanical stability to the glomerular capillary tuft in hypertensive glomerular disease. Am J Pathol 2002; 160:861-7; PMID:11891185; http://dx.doi.org/ 10.1016/S0002-9440(10)64909-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartner A, Schaefer L, Porst M, Cordasic N, Gabriel A, Klanke B, Reinhardt DP, Hilgers KF. Role of fibrillin-1 in hypertensive and diabetic glomerular disease. Am J Physiol Renal Physiol 2006; 290:F1329-36; PMID:16380460; http://dx.doi.org/ 10.1152/ajprenal.00284.2005 [DOI] [PubMed] [Google Scholar]
- 19. Sinha S, Heagerty AM, Shuttleworth CA, Kielty CM. Expression of latent TGF-beta binding proteins and association with TGF-beta 1 and fibrillin-1 following arterial injury. Cardiovasc Res 2002; 53:971-83; PMID:11922907; http://dx.doi.org/ 10.1016/S0008-6363(01)00512-0 [DOI] [PubMed] [Google Scholar]
- 20. Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 2003; 33:407-11; PMID:12598898; http://dx.doi.org/ 10.1038/ng1116 [DOI] [PubMed] [Google Scholar]
- 21. Porst M, Daniel C, Plank C, Schocklmann HO, Reinhardt DP, Hartner A. Induction and coexpression of latent transforming growth factor beta-binding protein-1 and fibrillin-1 in experimental glomerulonephritis. Nephron Exp Nephrol 2006; 102:e99-104; PMID:16282705; http://dx.doi.org/ 10.1159/000089688 [DOI] [PubMed] [Google Scholar]
- 22. Pröls F, Hartner A, Schöcklmann HO, Sterzel RB. Mesangial cells and their adhesive properties. Exp Nephrol 1999; 7:137-46; PMID:10213867; http://dx.doi.org/ 10.1159/000020594 [DOI] [PubMed] [Google Scholar]
- 23. Mariko B, Ghandour Z, Raveaud S, Quentin M, Usson Y, Verdetti J, Huber P, Kielty C, Faury G. Microfibrils and fibrillin-1 induce integrin-mediated signaling, proliferation and migration in human endothelial cells. Am J Physiol Cell Physiol 2010; 299:C977-87; PMID:20686071; http://dx.doi.org/ 10.1152/ajpcell.00377.2009 [DOI] [PubMed] [Google Scholar]
- 24. Müller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell 1997; 88:603-13; PMID:9054500; http://dx.doi.org/ 10.1016/S0092-8674(00)81903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Müller U, Bossy B, Venstrom K, Reichardt LF. Integrin alpha 8 beta 1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol Biol Cell 1995; 6:433-48; PMID:7626807; http://dx.doi.org/ 10.1091/mbc.6.4.433 [DOI] [PMC free article] [PubMed] [Google Scholar]


