Abstract
The endosomal pathway constitutes a highly dynamic intracellular transport system, which is composed of vesicular and tubular compartments. Endosomal tubules enable geometry-based discrimination between membrane and luminal content. Extended tubular endosomes were suggested to deliver a steady stream of membrane proteins to one location more reliable and effective than vesicular endosomes. Recently, we demonstrated that human dendritic cells (DCs) form a large elongated tubular endosomal network, e.g. ETEN, upon distinct triggers. LPS-stimulation triggered late endosomal tubulation. Additional clustering of class I MHC and ICAM-1 by a cognate interaction between antigen-laden DC and antigen-specific CD8+ T-cells induces formation of transferrin-positive tubules emanating from the endosomal recycling compartment (ERC). We here discuss cell-biological mechanisms that are involved in membrane bending and possibly underlie initiation, elongation, and stabilization of ETEN in human DCs. Using a knock-down approach we demonstrate that MICAL-L1 is necessary for ETEN remodeling originating from ERC in human DCs.
Keywords: elongated tubular endosomal network (ETEN), MICALL1, endosomal recycling, membrane remodeling
Introduction
The endosomal pathway is a highly dynamic membrane system composed of vesicular and tubular lipid bodies, which is involved in intracellular transport. Endosomal compartments are best known for incorporating membrane molecules derived from the cell surface, and sorting these molecules for either degradation or recycling back to the cell surface. Within seconds after uptake of cell-surface molecules through endocytosis, these molecules are targeted to Early Endosomes (EEs). Components that are destined for degradation are enriched in membrane subdomains that are remodelled to form intra-luminal vesicles. As time passes by, more intra-luminal vesicles accumulate in this compartment, while simultaneously the vesicles mature into a Late Endosomal compartment (LE). Eventually, LE can fuse with lysosomes upon which the luminal content is degraded. In parallel, cargo may be rescued from this degradation pathway by sorting vesicles toward tubular transport intermediates that direct cargo to the trans-Golgi network, or recycles these back to the cell surface either directly or via the perinuclear endocytic recycling compartment (ERC).1
It was estimated that cells internalize the equivalent of their cell surface one to 5 times per hour,2 demonstrating the importance of endosomal recycling to normal cellular function. Recycling is under tight structural and motor control by a variety of proteins that include microtubules, motor proteins, SNARE proteins, and various small GTPases. Multiple recycling pathways co-exist in parallel; including the Rab22a, Rab8-EHD1, Rab35-EHD1, and Rab11-Rab4-SNX4 as previously reviewed by us and others.3,4
We recently demonstrated that LPS stimulation of human dendritic cells (DCs) drives LE (LDL+) endosomes remodelling into elongated tubular structures leading to formation of an elongated tubular endosomal network, e.g., ETEN.5 This corroborates earlier studies in murine DCs,6-8 thereby showing conservation of this mechanism between mice and man. Electron microscopic studies showed that tubules emanating from LEs extend toward the periphery with small transport vesicles protruding toward the plasma membrane.9 These data suggest that activation-induced LE tubules represent transport intermediates for cell surface-directed transport.
However, the vast majority of endosome-cell surface transport occurs normally via aforementioned ERC, which is known to form short tubular transport intermediate structures.10 One apparent question was whether ETEN is derived from ERC-derived membranes. Therefore, we investigated whether the characteristic recycling marker transferrin (Tfn) may also be recycled via ETEN.5 Tfn+ ETEN in human DCs did not arise upon LPS stimulation as in mouse DCs, but did so upon subsequent cognate interaction with antigen-specific CD8+ T cells. These Tfn+ elongated endosomal tubules emanate from a juxta-nuclear region where the ERC is located. Further support that ERC contributes to ETEN came from use of the recycling inhibitor primaquine: Tfn+ tubules were lost when human DCs are briefly treated with a low dose of primaquine at 30 minutes prior to T cell contact. These data suggested that these induced tubular structures are indeed involved in cell-surface directed transport, which is crucial for DC function as antigen presenting cells. We underscored the importance of this tubular cell-surface directed transport to adaptive immune activation by showing decreased antigen-specific CD8+ T cell activation upon treatment of DCs with either primaquine or nocodazole (disruptor of microtubules).5 Below we will discuss possible requirements and underlying mechanisms involved in ETEN formation.
Tubular Transport Intermediates in Endosomal Transport
Endosomal tubulation is a phenomenon known for many years, with the vast majority of reported endosomal tubules having a size maximal up to 1.5 μm in length. In contrast, tubule length of ETEN can extend up to 15 μm in DCs.5,6 The short tubules that are more established form the tubular endosomal network (TEN) or tubular sorting endosomes (TSEs)1,10,11 and account for about 2-thirds of the surface area of an endosome, but only one-third of its volume.12 This high surface-to-volume ratio enables a geometry-based discrimination between membrane and luminal content, by which mainly lipids and membrane-bound cargo, but not luminal content, are exchanged between endosome and receiving organelle. It also suggests that endosomal tubules are designated to transport mainly lipids and membrane proteins. Indeed, many surface receptors are shown to be transported via tubular transport intermediates. Examples include transferrin receptors, class I MHC molecules, β-integrins, and several more.10,13
Endosomal tubules may be a more reliable transport intermediate than vesicular endosomes in terms of delivering high amount of cargo to one target membrane location, as a tubule may deliver the same amount of transmembrane cargo in a single package as what would be transported by multiple distinct endosomes. Moreover, efficient endosomal trafficking relies on collaboration of multiple motor proteins. Kinesin and myosin-V were shown to enhance each other's processivity in vitro.14 During endosomal transport across the cytoskeletal meshwork, proper function of motor proteins requires their temporal detachment of a particular cytoskeletal element and attachment to another cytoskeletal element. Therefore endosomal tubules may also be a more reliable transport intermediate as they should allow for more interaction with motor proteins connected over an extended distance.
Another important feature of tubules is compartmentalization of endosomal signaling. High membrane curvature in endosomal tubules can induce sorting of lipids and proteins. Lipids may respond to membrane curvature by concentrating into lipid microdomains induced by the curvature, as was demonstrated by lipid segregation into tubules during tubule-pulling experiments.15 In support, endosomal tubules have a lipid composition significantly different from the compartment from which they originate.16 In its turn, proteins may be directed to endosomal tubules by recognizing these sorted lipids and/or the high curvature.17
Recently the importance of endosomal tubules in compartmentalization of endosomal signaling was demonstrated in vivo.18 Nakamura et al., demonstrated that TLR-stimulated ETEN emanating from LE are a preferred compartment for bacterial sensing and NOD2 signaling in vivo.18
A final consideration regarding endosomal transport and compartment morphology, is that the endosomal membrane is a typical lipid bilayer, in which the tendency to be continuous and avoiding edges outcompetes the resistance to bending by intramolecular interaction between the lipid molecules. The same fundamental rules steer the spherical shapes of vesicular endosomes. Tubules are simply not the energetically favored conformation of lipid bilayers in soluble surroundings. However, we have shown that both LDL+ and Tfn+ ETEN are stable for at least 6 hours in human DCs, thus there should be a concerted action of several factors that model and stabilize these endosomal tubules. Such factors are subject of the next paragraphs.
Membrane Remodeling Mechanisms
Initiation of tubules
Changing the energy-favorable spherical shape of endosomes in order to initiate tubular remodeling requires changes of the bilayer structural properties thereby making it asymmetric, or to apply force to the bilayer surface that provides sufficient energy proportional on the extent of deformation.19
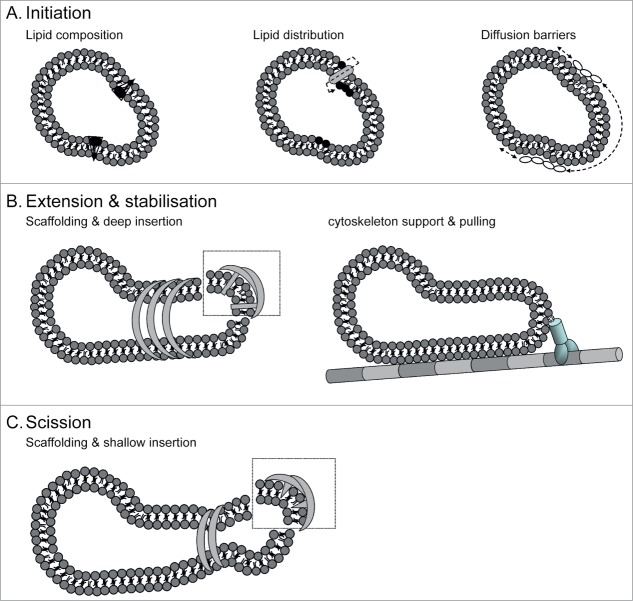
As schematically depicted in Figure 1A, bilayer asymmetry can be achieved by altering the lipid composition and/or different number of lipids per layer in the bilayer.20 For example, flippases facilitate translocation of phospholipids from one side of a membrane to the other. As a consequence, a transmembrane asymmetrical lipid distribution is established. Every inserted lipid molecule lowers the energy required for bending the lipid bilayer. Hence, insertion of even a small proportion of lyso-PC into a Giant Unilamellar vesicle (GUV) is accompanied by the formation of a single bud.21 Another approach to asymmetrically displace lipids in the outer leaflet, is the shallow insertion of short hydrophobic or amphipathic protein domains in only the outer lipid monolayer, such as achieved by BAR domain proteins.22
Figure 1.
Candidate molecular mechanisms involved with endosomal tubulation. (A) Formation of tubules is triggered by the initial bending of membranes by either (from left to right) altering membrane lipid composition, inducing asymmetrical lipid distribution between juxtapositioned monolayers, or creating domains by establishing diffusion barriers. (B) Tubules are extended and stabilized by (from left to right) membrane-bound scaffolds that either imprint their shape into the membrane, deeply insert amphipathic helices, or simply by protein crowding, or by support or pulling forces enabled by the cytoskeleton. (C) Membrane fission mediated in ATP-dependent and independent manner by members of the dynamin-super family.
The lipid composition may be altered by chemical modification of lipids, or by displacement of lipids themselves, by de novo generation, retention, selection, or recruitment from elsewhere. As lipids can diffuse fast within membrane planes, local generation would be insufficient unless lipid diffusion can be deterred. Indeed, actin alone or in combination with Ezrin/Radixin/Moesin proteins can limit lipid diffusion.23 Phosphatidic acid (PA) is an example of such a generated lipid. It has an intrinsic negative curvature and is synthesized by phospholipase D. Adding PA to v-SNARE vesicles increases the rate of fusion with targeted t-SNARE containing vesicles. In contrast, addition of PA to t-SNARE vesicles inhibits SNARE-mediated fusion.24 As PA also affects other unrelated molecular mechanisms in a similar manner,25 it is believed that PA promotes fusion via a biophysical mechanism, probably with its negative curvature. Thus, lipid-lipid interactions such as PA present in outer leaflet of an endosome may contribute to (limited) bending of membranes.
Alternatively, presence of PA may act as a signpost for the recruitment of proteins and complexes that can apply mechanical forces required for membrane tubulation.26 This corroborates with the notion that lipid headgroups commonly serve as attachment sites for peripheral membrane proteins, thereby recruiting proteins necessary for the generation of membrane curvature. Especially, phosphoinositides (PtdIns) seem pivotal for recruitment of curvature generating proteins as their inositol headgroup can easily reversibly phosphorylated and thereby acting as chemical switch.
Elongation or stabilization of tubules
An example of such PtdIns-binding proteins, are some members of the Bin/Amphiphysin/Rvs (BAR) domain protein superfamily. While this BAR domain protein superfamily lacks a consensus sequence motif, all members contain the banana-shaped BAR domain that typically form homo or heterodimers with the aid of complementary positioned charged residues in the hydrophobic dimerization pocket. This BAR domain has an arced structure with positively charged residues at its concave side that associates with membranes. The membrane-associated BAR domains can oligomerize into stable helices. In vitro studies on liposomes show that the arced structure allows them to bind curved membrane. By insertion of amphipathic helices,22 scaffolding,27 or crowding28 (Fig. 1B), BAR domain protein can stabilize pre-existing and/or induce extensive membrane curvatures.29
The BAR protein Endophilin A1 induces different membrane shapes by changing its conformation upon phosphorylation. Endophilin A asymmetrically displaces lipids from the outer leaflet by shallow insertion of its amphipathic helices, thereby forming small vesicles with high membrane curvature. Accordingly, deep insertion of the amphipathic helices and close interaction of membrane and BAR domain drives tubule formation.22 Of note, BAR domains appear somewhat flexible: the SNX9-BAR domain was shown to adopt 2 different arced structures in solution.29 Whether this means that BAR domain proteins are less-specific in recognizing and binding distinct curved membranes is yet unknown.
Actin and other cytoskeleton components can assist in supporting tubular transport intermediates in various ways. Long actin filaments can stabilize tubular endosomes (Fig. 1C). Indeed, actin-associated β2-adrenergic receptor+ tubular endosomes are much less dynamic than Tfn+ tubules, and actin inhibition decreases TEN tubules by 25%.30 Moreover, the cytoskeleton allows motor proteins to apply mechanical force to pull and extend the membrane, or alternatively drive membrane deformations directly upon polymerization.16 Indeed, an intact cytoskeleton is pivotal to recycling of membrane proteins to the cell surface via ETEN from both LE and ERC.5,31
Scission of endosomal tubular transport intermediates
A second group of proteins that was brought forward to possibly mediate the induction or stabilization of endosomal tubulation is the Eps15 homology domain (EHD) containing protein family. In vitro liposome assays demonstrated that all 4 mammalian EHD proteins are capable of tubule formation by forming oligomeric ring-like structures. EHDs have been linked to a number of Rab proteins through their association with mutual effectors and thereby have a coordinating role in endocytic trafficking.32 EHD proteins harbor a nucleotide-binding domain, which ATPase activity is stimulated by membrane association. ATP activity allows oligomerization and tubule formation in vitro by EHD2 protein.33 However, EHD proteins show ∼70% sequence similarity with Dynamin. Dynamin is known for scission of budding endosomes in ATP-independent manner. Therefore, it was hypothesized that EHD proteins are perhaps involved in membrane fission in vivo. Recently, the ability of each EHD protein to tubulate or vesiculate recycling ETEN was assessed by reconstituting semi-permeabilized cells with purified EHD proteins. These data indeed showed that EHD1, EHD2, and EHD4, but not EHD3, are directly involved in scission of ETEN, similar as Dynamin for budding endosomes during endocytosis.34
Putative mechanisms underlying ETEN formation that emanates from ERC in human DCs
We demonstrated that ETEN arise from the ERC upon cognate interaction between human DC and antigen-specific CD8+ T cells. More specifically, beads cross-linking class I MHC and/or ICAM-1, but not CD45, mimic CD8+ T cells in respect to induction of Tfn+ ETEN.5 It is known that cross-linking ICAM-1 and/or HLA-A2 leads to an increased association between these molecules and recruitment of Src kinases.35 Inactive Src localizes to perinuclear endosomes, whereas active Src localizes to site of stimulated integrin receptor.36 Whereas Tfn is known to recycle via various pathways, recycling of Src kinases is limited to 2 pathways of which only one overlaps with Tfn recycling. This pathway is recently demonstrated by J. Reinecke et al. and involves the molecules MICAL-L1 and EHD1.37
Are MICAL-L1 and EHD1 related to Tfn+ ETEN? First considering EHD1, this protein is best known as a marker for elongated endosomal tubules emanating from the ERC in HeLa cells,38,39 which resemble the Tfn+ ETEN in human DCs.5 Similar to Tfn+ ETEN in human DCs, these EHD1-positive tubules require an intact microtubule cytoskeleton. EHD1-knock out embryonic fibroblasts exhibit delay in Tfn recycling to the plasma membrane with accumulation of Tfn in the ERC, thereby confirming EHD1's role in Tfn recycling. In addition, EHD1 was found to associate with ETEN containing both Arf6 and class I MHC. Overexpression of EHD1 enhances class I MHC recycling.38
When considering MICAL-L1 next for its possible relationship to Tfn+ ETEN, it has a calponin homology (CH), LIM, proline-rich (PxxP) region including a NPF motif, and coiled-coil domains. The CH domain of MICAL-L1 shares high similarity to the CH domains identified in various actin-associated proteins. MICAL-L1 interacts with various important regulators of Tfn recycling, including Rab840 and a few BAR domain proteins, such as Syndapin 2 (Synd2). Synd2 has a BAR, NPF, and SH3 domains.39 Recently it was demonstrated that MICAL-L1, Synd2, and EHD1 decorate the same endosomal tubules.39,40 The membrane interaction of both proteins is stabilized by binding to each other and EHD1. Moreover, depletion of EHD1 does not affect tubular localization of MICAL-L1 or Synd2,40 while depletion of MICAL-L1 or Synd2 does decrease tubular localization of the other proteins.39 Thus, MICAL-L1 and/or Synd2 may recruit EHD1 to pre-existing tubules decorated with MICAL-L1 and Synd2. Synd2 harbors a BAR domain, therefore we believe this molecule is driving the elongation of recycling endosomes. In vitro Synd2 has shown to form tubules. Interestingly, Synd2 prefers to tubulate PA-containing liposomes and PA generated by Arf6 is pivotal to Tfn recycling.41 Further investigation shows that Arf6 inhibition decreases MICAL-L1 tubule association.13 The coiled-coil of MICAL-L1 and BAR-domain of Synd2 prefers to associate with PA+ membranes. Therefore, it seems that the local generation of PA by Arf6 may recruit MICAL-L1 and Synd2 to initiate ETEN formation. In its turn, tip-to-tip or wedge loop-mediated lateral interaction would enable Synd2 to induce endosomal tubulation.42
MICAL-L1 Expression Required for Tfn+ Elongated Tubular Endosomal Network (ETEN)
We considered that impairment of Syndapin function interferes with clathrin-dependent endocytosis43 and that Tfn is taken up in a clathrin-dependent manner. As a candidate protein that facilitates the formation of Tfn+ ETEN, we therefore decided to investigate the role of MICAL-L1 and not Syndapin. One way to examine MICAL-L1 function in Tfn ETEN, would be to interfere with MICAL-L1 endogenous function by overexpressing mutant MICAL-L1. However, in a computational model Stachowiak et al. demonstrated that membrane bending can be solely driven by protein crowding via steric pressure.28 Hence, we decided to knock-down the expression of MICAL-L1 by siRNA treatment of human DCs. MICAL-L1 knock-down was shown in HeLa cells to be effective to decrease MICAL-L1 RNA expression previously.40
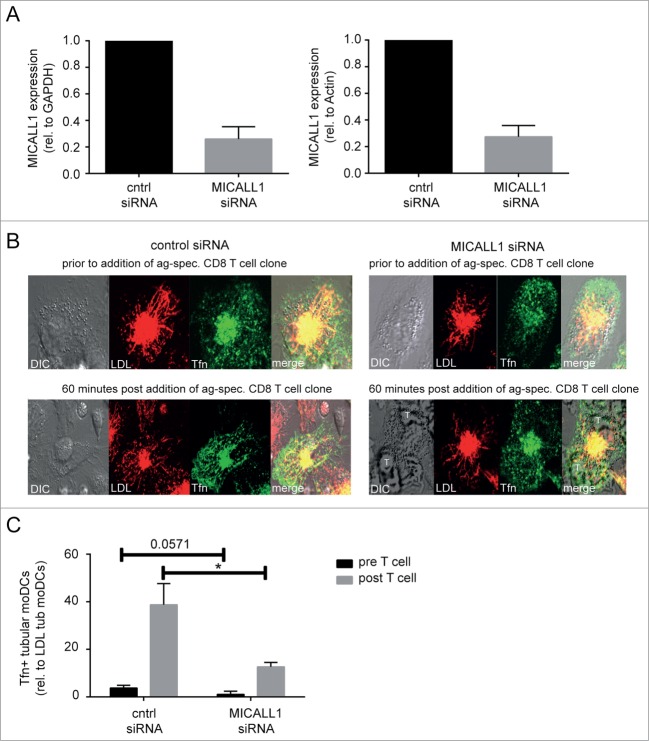
Human monocyte-derived DCs were treated with control or MICAL-L1 targeting siRNA by electroporation at day 4, according to protocol of Hobo et al.44 36 hours later, MICAL-L1 RNA expression was decreased by 75% as determined by quantitative PCR (Fig. 2A).
Figure 2.
MICALL1 is required for Tfn-positive endosomal tubules formation in human dendritic cells. (A) MICAL-L1 RNA expression is efficiently silenced after 36 hrs by 3 unique 27 mer siRNA targeting MICAL-L1 (gray bars) and not by scrambled control siRNA (black bars). (B) Confocal image of stimulated moDCs (200 ng/ml LPS, 5 ug/ml polyI:C, and 3 ug/ml pp65 antigen) 36 hours after 10 nM siRNA treatment (left, control siRNA; right, MICALL1 siRNA). Prior to (upper part) and 60-80 minutes post (lower part) addition of antigen-specific CD8+ T cells. Red depicts LDL, Green Transferrin, and yellow co-localization of LDL and Tfn. (C) The percentage of stimulated moDCs (200 ng/ml LPS, 5 ug/ml polyI:C, and 3 ug/ml pp65 antigen) with LDL+ endosomal tubules expressing tubular Tfn+ endosomes upon antigen-specific CD8+ T cells, 36 hrs after indicated siRNA treatment (10 nM scrambled or MICALL1-targeting siRNA). Data represents mean ± SE of three independent experiments. Two-tailed, Mann-Whitney U test; *P < 0.05.
To assess MICAL-L1 function in either induction or stabilization of Tfn+ ETEN, we pulsed both scrambled and MICAL-L1-siRNA treated moDCs for 4 h with 3 μg of our model antigen pp65, in the presence of 200 ng/ml LPS and 5 μg/ml poly(I:C). Hereafter LE and ERC (30 min, 37°C) are stained with 20 μg/ml DiI-LDL and 5 μg/ml Alexa Fluor 647-conjugated Tfn, respectively. Vesicle-to-tubule transformation was stimulated by co-culture of antigen-specific (NLVPMVATV) CD8+ T cells for 60–80 minutes.5,45 We next analyzed labeled moDCs for ETEN formation by live cell confocal microscopy prior and post addition of antigen-specific CD8+ T cells. The fraction of moDCs that exhibit late endosomal tubulation (as scored by DiI-LDL fluorescence) showing Tfn+ ETEN were determined by 2 independent investigators as published.5 Representative pictures are shown in Figure 2B.
MICAL-L1 siRNA but not control siRNA treatment of moDCs resulted in a significant reduction in moDCs with Tfn+ ETEN from 40% to approximately 17% (Fig. 2C). Thus, Tfn+ ETEN in human DCs require MICAL-L1 expression in our experimental setup. This suggests that the endosomal Tfn+ tubules emanating from ERC in human DCs are indeed induced or stabilized by MICAL-L1.
As observed,5 most LDL and Tfn tubular structures are overlapping (Fig. 2B, left panel). Therefore, we wondered whether LDL+ and Tfn+ tubules were 2 different compartments or are perhaps 2 distinct tubules walking across the same microtubule. We could not investigate this by confocal microscopy, as the resolving power is insufficient. However, we here show that LDL+ elongated tubules persist while Tfn+ tubules disappear upon depletion of MICAL-L1. Therefore, it seems that both LDL+ and Tfn+ tubules are distinct compartments. Whether both Tfn+ and LDL+ tubules emanate from the same endosomal compartment is still possible, as various studies have shown that distinct populations of membrane tubules may arise from the same membrane.10 This is possible because BAR domain proteins that cannot oligomerize do not colocalize at the same sorting tubule. This corroborates with the observation that Tfn+ and β2-adrenergic receptor+ tubules emanating from the same early endosomal compartment have distinct biochemical and kinetic properties: the β2-adrenergic receptor+ tubular endosomes are more stable than Tfn+ TEN tubules and have endosome-associated actin.30 It is also reported that different BAR domain proteins recruit distinct motor protein complexes.46 Therefore it seems that distinct tubule coats enable the rise of unique tubular transport intermediates, allowing for independent regulation of endosomal cargo transport.
In summary, research on the cell biological processes that underlie endosomal remodeling toward elongated tubular structures is now being applied to human DCs. The consequences of such remodeling to endosomal surface-directed transport of peptide/MHC class II and peptide/MHC class I complexes is not yet fully understood. Mouse work supports a role for late endosomal tubular remodeling in Class II MHC-mediated CD4+ T cell activation,6 work that requires translation into human dendritic cell research. Future directions furthermore should include study of the role of molecules that corroborate ETEN, such as—but not limited to- MICAL-L1 and EHD1, in antigen cross-presentation and CD8+ T cell stimulation. Clarification of endosomal sorting, remodeling and transport mechanisms may prove pivotal to the development of future human dendritic cell-based vaccines.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 2006; 7(8):568-79; PMID:16936697; http://dx.doi.org/ 10.1038/nrm1985 [DOI] [PubMed] [Google Scholar]
- 2. Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol 1983; 96(1):1-27; PMID:6298247; http://dx.doi.org/ 10.1083/jcb.96.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Compeer EB, Flinsenberg TW, van der Grein SG, Boes M. Antigen processing and remodeling of the endosomal pathway: requirements for antigen cross-presentation. Front Immunol 2012; 3:37; PMID:22566920; http://dx.doi.org/ 10.3389/fimmu.2012.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seaman MN. The retromer complex—endosomal protein recycling and beyond. J Cell Sci 2012; 125(Pt 20):4693-702; PMID:23148298; http://dx.doi.org/ 10.1242/jcs.103440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Compeer EB, Flinsenberg TW, Boon L, Hoekstra ME, Boes M. Tubulation of endosomal structures in human dendritic cells by Toll-like receptor ligation and lymphocyte contact accompanies antigen cross-presentation. J Biol Chem 2014; 289(1):520-8; PMID:24235148; http://dx.doi.org/ 10.1074/jbc.M113.511147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boes M, Cerny J, Massol R, Op den BM, Kirchhausen T, Chen J, Ploegh HL. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 2002; 418(6901):983-8; PMID:12198548; http://dx.doi.org/ 10.1038/nature01004 [DOI] [PubMed] [Google Scholar]
- 7. Boes M, Bertho N, Cerny J, Op den BM, Kirchhausen T, Ploegh H. T cells induce extended class II MHC compartments in dendritic cells in a Toll-like receptor-dependent manner. J Immunol 2003; 171(8):4081-8; PMID:14530329; http://dx.doi.org/ 10.4049/jimmunol.171.8.4081 [DOI] [PubMed] [Google Scholar]
- 8. Bertho N, Cerny J, Kim YM, Fiebiger E, Ploegh H, Boes M. Requirements for T cell-polarized tubulation of class II+ compartments in dendritic cells. J Immunol 2003; 171(11):5689-96; PMID:14634076; http://dx.doi.org/ 10.4049/jimmunol.171.11.5689 [DOI] [PubMed] [Google Scholar]
- 9. van Nispen tot Pannerden HE, Geerts WJ, Kleijmeer MJ, Heijnen HF. Spatial organization of the transforming MHC class II compartment. Biol Cell 2010; 102(11):581-91; PMID:20712599; http://dx.doi.org/ 10.1042/BC20100046 [DOI] [PubMed] [Google Scholar]
- 10. Traer CJ, Rutherford AC, Palmer KJ, Wassmer T, Oakley J, Attar N, Carlton JG, Kremerskothen J, Stephens DJ, Cullen PJ. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat Cell Biol 2007; 9(12):1370-80; PMID:17994011; http://dx.doi.org/ 10.1038/ncb1656 [DOI] [PubMed] [Google Scholar]
- 11. Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol 2004; 164(7):1065-76; PMID:15051738; http://dx.doi.org/ 10.1083/jcb.200311064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marsh M, Griffiths G, Dean GE, Mellman I, Helenius A. Three-dimensional structure of endosomes in BHK-21 cells. Proc Natl Acad Sci U S A 1986; 83(9):2899-903; PMID:3458249; http://dx.doi.org/ 10.1073/pnas.83.9.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rahajeng J, Giridharan SS, Cai B, Naslavsky N, Caplan S. MICAL-L1 is a tubular endosomal membrane hub that connects Rab35 and Arf6 with Rab8a. Traffic 2012; 13(1):82-93; PMID:21951725; http://dx.doi.org/ 10.1111/j.1600-0854.2011.01294.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ali MY, Lu H, Bookwalter CS, Warshaw DM, Trybus KM. Myosin V and Kinesin act as tethers to enhance each others' processivity. Proc Natl Acad Sci U S A 2008; 105(12):4691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J 2005; 24(8):1537-45; PMID:15791208; http://dx.doi.org/ 10.1038/sj.emboj.7600631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 2004; 5(2):121-32; PMID:15040445; http://dx.doi.org/ 10.1038/nrm1315 [DOI] [PubMed] [Google Scholar]
- 17. Zhu C, Das SL, Baumgart T. Nonlinear sorting, curvature generation, and crowding of endophilin N-BAR on tubular membranes. Biophys J 2012; 102(8):1837-45; PMID:22768939; http://dx.doi.org/ 10.1016/j.bpj.2012.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura N, Lill JR, Phung Q, Jiang Z, Bakalarski C, de Mazière A, Klumperman J, Schlatter M, Delamarre L, Mellman I. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 2014; 509(7499):240-4; PMID:24695226; http://dx.doi.org/ 10.1038/nature13133 [DOI] [PubMed] [Google Scholar]
- 19. Kozlov MM, Campelo F, Liska N, Chernomordik LV, Marrink SJ, McMahon HT. Mechanisms shaping cell membranes. Curr Opin Cell Biol 2014; 29C:53-60; http://dx.doi.org/ 10.1016/j.ceb.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farge E, Ojcius DM, Subtil A, utry-Varsat A. Enhancement of endocytosis due to aminophospholipid transport across the plasma membrane of living cells. Am J Physiol 1999; 276(3 Pt 1):C725-C733 [DOI] [PubMed] [Google Scholar]
- 21. Seifert U, Berndl K, Lipowsky R. Shape transformations of vesicles: Phase diagram for spontaneous- curvature and bilayer-coupling models. Phys Rev A 1991; 44(2):1182-202; PMID:9906067; http://dx.doi.org/ 10.1103/PhysRevA.44.1182 [DOI] [PubMed] [Google Scholar]
- 22. Ambroso MR, Hegde BG, Langen R. Endophilin A1 induces different membrane shapes using a conformational switch that is regulated by phosphorylation. Proc Natl Acad Sci U S A 2014; 111(19):6982-7; PMID:24778241; http://dx.doi.org/ 10.1073/pnas.1402233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garbett D, Sauvanet C, Viswanatha R, Bretscher A. The tails of apical scaffolding proteins EBP50 and E3KARP regulate their localization and dynamics. Mol Biol Cell 2013; 24(21):3381-92; PMID:23985317; http://dx.doi.org/ 10.1091/mbc.E13-06-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci U S A 2006; 103(40):14761-6; PMID:17001002; http://dx.doi.org/ 10.1073/pnas.0606881103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeniou-Meyer M, Zabari N, Ashery U, Chasserot-Golaz S, Haeberle AM, Demais V, Bailly Y, Gottfried I, Nakanishi H, Neiman AM, et al. . Phospholipase D1 production of phosphatidic acid at the plasma membrane promotes exocytosis of large dense-core granules at a late stage. J Biol Chem 2007; 282(30):21746-57; PMID:17540765; http://dx.doi.org/ 10.1074/jbc.M702968200 [DOI] [PubMed] [Google Scholar]
- 26. Rossy J, Ma Y, Gaus K. The organisation of the cell membrane: do proteins rule lipids? Curr Opin Chem Biol 2014; 20C:54-9; http://dx.doi.org/ 10.1016/j.cbpa.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 27. Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, Rapoport TA, Prinz WA. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 2008; 319(5867):1247-50; PMID:18309084; http://dx.doi.org/ 10.1126/science.1153634 [DOI] [PubMed] [Google Scholar]
- 28. Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, Geissler PL, Fletcher DA, Hayden CC. Membrane bending by protein-protein crowding. Nat Cell Biol 2012; 14(9):944-9; PMID:22902598; http://dx.doi.org/ 10.1038/ncb2561 [DOI] [PubMed] [Google Scholar]
- 29. Wang Q, Navarro MV, Peng G, Molinelli E, Goh SL, Judson BL, Rajashankar KR, Sondermann H. Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin Syndapin. Proc Natl Acad Sci U S A 2009; 106(31):12700-5; PMID:19549836; http://dx.doi.org/ 10.1073/pnas.0902974106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, von Zastrow M. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 2010; 143(5):761-73; PMID:21111236; http://dx.doi.org/ 10.1016/j.cell.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vyas JM, Kim YM, rtavanis-Tsakonas K, Love JC, Van d V, Ploegh HL. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol 2007; 178(11):7199-210; PMID:17513769; http://dx.doi.org/ 10.4049/jimmunol.178.11.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol 2011; 21(2):122-31; PMID:21067929; http://dx.doi.org/ 10.1016/j.tcb.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 2007; 449(7164):923-7; PMID:17914359; http://dx.doi.org/ 10.1038/nature06173 [DOI] [PubMed] [Google Scholar]
- 34. Cai B, Giridharan SS, Zhang J, Saxena S, Bahl K, Schmidt JA, Sorgen PL, Guo W, Naslavsky N, Caplan S. Differential roles of C-terminal Eps15 homology domain proteins as vesiculators and tubulators of recycling endosomes. J Biol Chem 2013; 288(42):30172-80; PMID:24019528; http://dx.doi.org/ 10.1074/jbc.M113.488627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lebedeva T, Anikeeva N, Kalams SA, Walker BD, Gaidarov I, Keen JH, Sykulev Y. Major histocompatibility complex class I-intercellular adhesion molecule-1 association on the surface of target cells: implications for antigen presentation to cytotoxic T lymphocytes. Immunology 2004; 113(4):460-71; PMID:15554924; http://dx.doi.org/ 10.1111/j.1365-2567.2004.01985.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaplan KB, Bibbins KB, Swedlow JR, Arnaud M, Morgan DO, Varmus HE. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine 527. EMBO J 1994; 13(20):4745-56; PMID:7525268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reinecke JB, Katafiasz D, Naslavsky N, Caplan S. Regulation of Src trafficking and activation by the endocytic regulatory proteins MICAL-L1 and EHD1. J Cell Sci 2014; 127(Pt 8):1684-98; PMID:24481818; http://dx.doi.org/ 10.1242/jcs.133892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, Bonifacino JS. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J 2002; 21(11):2557-67; PMID:12032069; http://dx.doi.org/ 10.1093/emboj/21.11.2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giridharan SS, Cai B, Vitale N, Naslavsky N, Caplan S. Cooperation of MICAL-L1, syndapin2, and phosphatidic acid in tubular recycling endosome biogenesis. Mol Biol Cell 2013; 24(11):1776-15; PMID:23596323; http://dx.doi.org/ 10.1091/mbc.E13-01-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharma M, Giridharan SS, Rahajeng J, Naslavsky N, Caplan S. MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling. Mol Biol Cell 2009; 20(24):5181-94; PMID:19864458; http://dx.doi.org/ 10.1091/mbc.E09-06-0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 2009; 10(9):597-608; PMID:19696797; http://dx.doi.org/ 10.1038/nrm2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bai X, Zheng X. Tip-to-tip interaction in the crystal packing of PACSIN 2 is important in regulating tubulation activity. Protein Cell 2013; [Epub ahead of print]; PMID:23888307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Da C, Sr., Sou E, Xie J, Yarber FA, Okamoto CT, Pidgeon M, Kessels MM, Mircheff AK, Schechter JE, Qualmann B, et al. . Impairing actin filament or syndapin functions promotes accumulation of clathrin-coated vesicles at the apical plasma membrane of acinar epithelial cells. Mol Biol Cell 2003; 14(11):4397-413; PMID:12937279; http://dx.doi.org/ 10.1091/mbc.E03-05-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hobo W, Novobrantseva TI, Fredrix H, Wong J, Milstein S, Epstein-Barash H, Liu J, Schaap N, van der Voort R, Dolstra H. Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol Immunother 2013; 62(2):285-97; PMID:22903385; http://dx.doi.org/ 10.1007/s00262-012-1334-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flinsenberg TW, Compeer EB, Koning D, Klein M, Amelung FJ, van BD, Boelens JJ, Boes M. Fcgamma receptor antigen targeting potentiates cross-presentation by human blood and lymphoid tissue BDCA-3+ dendritic cells. Blood 2012; 120(26):5163-72; PMID:23093620; http://dx.doi.org/ 10.1182/blood-2012-06-434498 [DOI] [PubMed] [Google Scholar]
- 46. Hunt SD, Townley AK, Danson CM, Cullen PJ, Stephens DJ. Microtubule motors mediate endosomal sorting by maintaining functional domain organization. J Cell Sci 2013; 126(Pt 11):2493-501; PMID:23549789; http://dx.doi.org/ 10.1242/jcs.122317 [DOI] [PMC free article] [PubMed] [Google Scholar]