Abstract
Congenital defects are those abnormalities present at birth. During embryogenesis, many anomalies can occur. The primitive gut tube lengthens quickly and rotates, allowing the gastrointestinal tract acquire its final position and orientation. Because the colon of large animals is complex, most changes occur in this segment. Thus, in ruminants, colon atresia is the most frequent malformation, affecting mainly ascending colon, at the level of the spiral loop. There are no previous references about a very atypical colon atresia at the junction of distal loop and transverse colon, such we have described in a 5-day-old calf, after a history of abdominal distention and absence of feces at birth, even with a patent anal opening. Atresia coli was detected at distal position of the typical colon atresia, at the junction of distal loop and transverse colon. In addition, the distal blind end was bent into a U-shape supported by the mesocolon. Besides the anatomical findings of this worthwhile atresia coli we discuss its possible etiology, in which local factors, such as a compromised blood supply during embryogenesis, are more consistent than genetic factors. Finding out the causes of atresia coli would help to reduce its incidence, lessen animal suffering and economic loss.
Keywords: atresia coli, congenital defect, digestive system, embryology, malformation
Introduction
The anomalies existing at birth derived from mistakes originated during gestation are called congenital defects.1 These anomalies of structure or function may be produced by genetic causes or habitat influence, or both. Developmental defects may be lethal, semi-lethal, or compatible with life.2 In many cases, the causes are unknown.
Gastrulation is the first crucial step that takes place during embryogenesis in pluricellular organisms. Early embryonic cells arrange into 3 primary cell layers: ectoderm, mesoderm, and endoderm. In mammals, the gastrointestinal tract stems from each of these cell layers. The endoderm gives rise to the epithelium that lines the digestive system, while the mesoderm forms the connective tissue and smooth muscle component of the digestive tract. The ectdoderm is the transitional epithelium in the initial (mouth) and final (anal canal) portions of the alimentary canal.
During embryonic development, the gut and digestive organs derive from the primitive yolk sac as a result of lateral folds that previously divided it into an intra-embryonic tube and an extra-embryonic sac. These compartments are communicated through an opening, quite wide at the beginning and narrow later, that will form the vitelline duct. In mammals, after the primordium of the gut tube has closed, the intestine is suspended by a relatively straight dorsal mesentery, except at the site of the yolk sac attachment. Here, the gut loops ventrally toward and, later, through the umbilicus. At this site, the dorsal mesentery is expanded and a large blood vessel courses through it. This is the right vitelline artery, which becomes the cranial mesenteric artery in the fetus and adult, and the vitelline veins constitute the basis for the hepatic sinusoids.1,3,4
The primitive gut tube possesses anterior-posterior polarity and is divided into 3 parts, named foregut, midgut, and hindgut. The midgut develops into the caudal part of the duodenum, jejunum, ileum, cecum and cranial part of the colon. The cranial limb of the primitive intestinal loop of the midgut develops into the distal part of the duodenum, the jejunum, and the proximal part of the ileum. Meanwhile, the caudal limb of the primitive intestinal loop develops into the distal part of the ileum, the cecum, the ascending colon, and the proximal part of transverse colon.1,4
During embryogenesis, the gastrointestinal system lengthens quickly and disproportionally, compared to other parts of the embryo. As the gut tube lengthens, it protrudes through the umbilicus, with its vasculature, causing a physiological hernia. Then, it gyrates and re-enters into the abdominal cavity acquiring its definitive place and orientation. Changes in gastrointestinal rotation and positioning are pathologically important since mal-rotation and non-rotation may obstruct the gastrointestinal tract and its blood vessels.5 The intestinal tract and esophagus normally undergo temporary atresia (occluded lumen) during development, as a result of epithelial proliferation. Recanalization occurs by the formation of vacuoles that coalesce to form the ultimate lumen.5,6
The colon has a more complex configuration in larger domestic animals than in carnivores. The most extensive changes occur in the ascending colon and cecum. In ruminant species, the center of the ascending colon expands caudally around the root of the mesentery, then cranially on the left side and, finally, coils on the left side of the mesentery to produce the adult arrangement. The mesocolon fuses with the mesojejunum. The ascending colon of ruminants develops into an elongated loop which grows and coils to form the proximal, spiral and distal loops of the colon. Hence, the ascending colon becomes localized centrally in the same mesentery that carries the jejunum in its periphery. Finally, proximal and distal loops are formed orally and aborally to the spiral loop, respectively. Specifically in cattle, the spiral loops develop 2 centripetal turns, the central flexure, and 2 centrifugal turns. Due to the growth of the rumen, the intestine localizes in the supraomental recess on the right in the abdominal cavity. The cecum is straight and dilated, and situated on the right side of the dorsal abdomen with its blind end placed caudally.1,4
Among the congenital malformations of the intestinal tract, stenosis and atresia are the most common. The literature often does not differentiate between various types of atresia, and sometimes stenosis is also classified as atresia. Stenosis is defined as an incomplete occlusion of the intestinal lumen. This may be a localized narrowing of the bowel, or an incomplete membrane. In contrast, atresia denotes complete intrinsic occlusion of the intestinal lumen, as a result of the anomalous development of its wall.7 Atresias are categorized depending on increased importance of the anomaly in base of 3 facts: 1) amount of absent gut tissue, 2) interruption of the vasculature, and 3) the number of co-existing anomalies in the intestine.8 Accordingly, atresias are classified into 3 types: type 1: membrane atresia with a membrane occluding the lumen, type 2: cord atresia, produced by blind-ends connected by a tissue string (of muscular and/or fibrous origin) suspended or not by its mesentery, and type 3: blind-end atresia characterized by loss of intestinal tissue with separated blind-ends and a co-occurring cleft in the mesentery. They also described a fourth type, in fact a variant of type 3, exclusive to man, named “apple peel” or “Christmas tree” atresia.7,8
Results
No anomaly was detected in the exploration of the head, neck, and thoracic cavity. The thoracic organs (heart, lungs, thymus, and arterial and venous vessels) were completely normal and the diaphragm did not show any alterations either. In the abdominal and pelvic cavities, the liver had no deformity, with its lobes perfectly defined, and a well-developed biliar bladder. The spleen was normal, as was the peritoneum. The urinary system was normal, and no defects were seen in the genital tract. An apparent anomaly was detected in the gastrointestinal tract, at the level of the ascending colon (Fig. 1). A severe distention was identified in the small intestine and the proximal part of the crassum intestine: the cecum and ascending colon, whose distal loop finished in a blind end, were supported by the mesentery (Fig. 2). The spiral loop, although distended, did not show any morphological anomaly (Fig. 3). The proximal blind end was highly dilated with milk digested fluid; its diameter was around 10 cm. A severe vascular congestion was also detected in the distal loop of the colon (Fig. 4). The beginning of the transverse colon, with a very narrow diameter, was hidden among the intestinal loops. Both blind ends were completely detached, with no fibrous or muscular cord between them (Figs. 4 and 5). The transverse colon started with a 7 cm long U-shaped segment. The blind end was bent, coiled and attached to the distal part sharing the mesentery. Its lumen was very narrow (about 1 cm diameter) (Fig. 5). The descending colon, also with a small diameter, ran caudally close to the top of abdominal cavity, suspended by a normal mesocolon and filled with scarce yellowish mucous secretion (Fig. 6).
Figure 1.
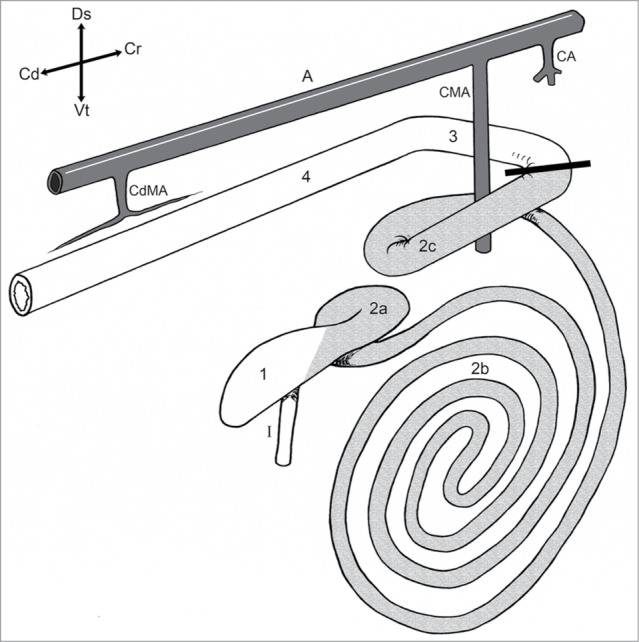
Schematic right lateral view of the bovine large intestinal tract. 1) Cecum; Ascending colon: 2a) Proximal loop, 2b) Spiral colon, 2c) Distal loop; 3) Transverse colon; 4) Descending colon; I) Ileum; A) Aorta; CA) Celiac artery; CMA) Cranial mesenteric artery; CdMA) Caudal mesenteric artery. The bold line shows where the atresia coli was placed. Modified from ref. 29.
Figure 2.
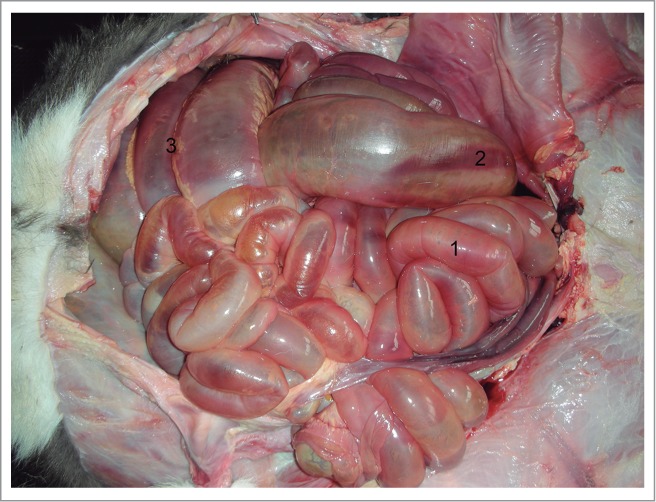
Dilated intestine: 1) Jejunum; 2) Cecum; 3) Spiral loop of ascending colon.
Figure 3.
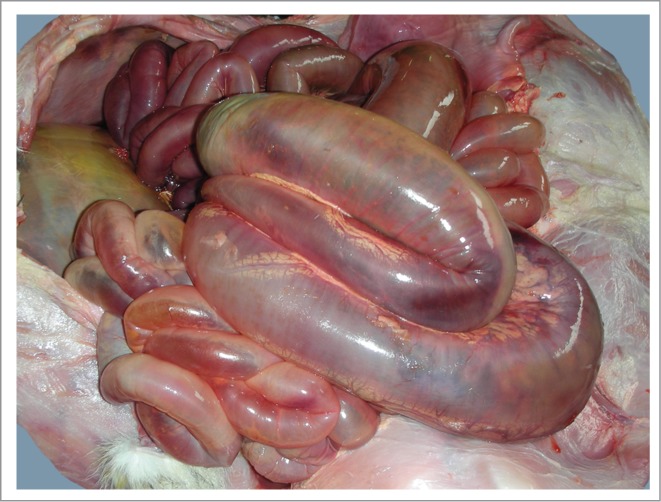
Distended spiral loop of the ascending colon.
Figure 4.
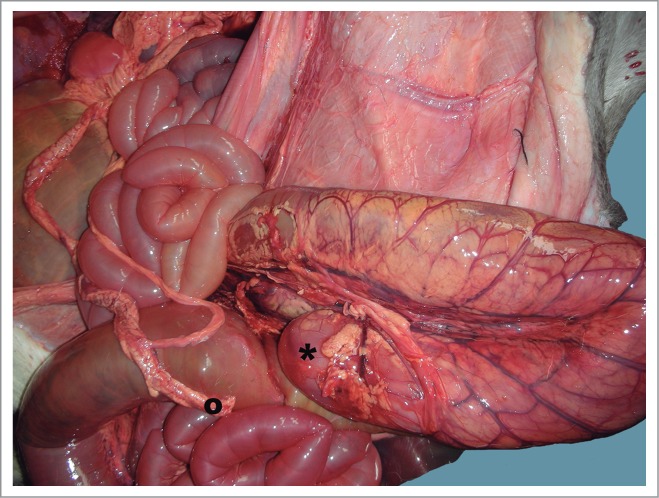
Distal loop of ascending colon showing congestion. This picture shows both blind ends of the colon: (*) proximal and (˚) distal ends.
Figure 5.
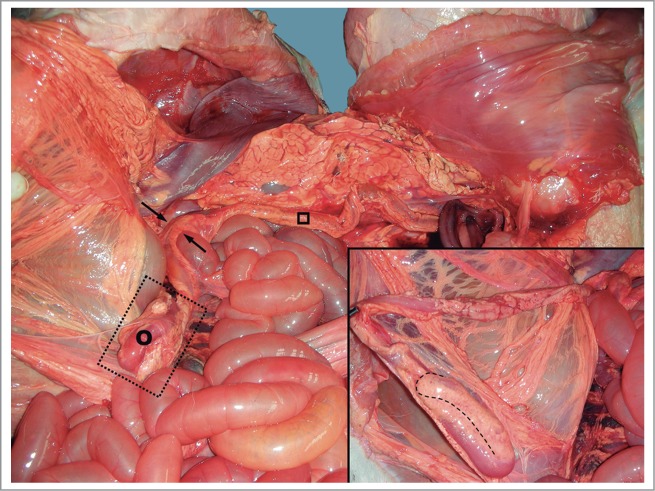
The distal blind end (transverse colon) was bent into a U-shape. (˚) Transverse colon; (□) descending colon; (→) Left colic flexure. Inset: major detail of the distal blind end.
Figure 6.
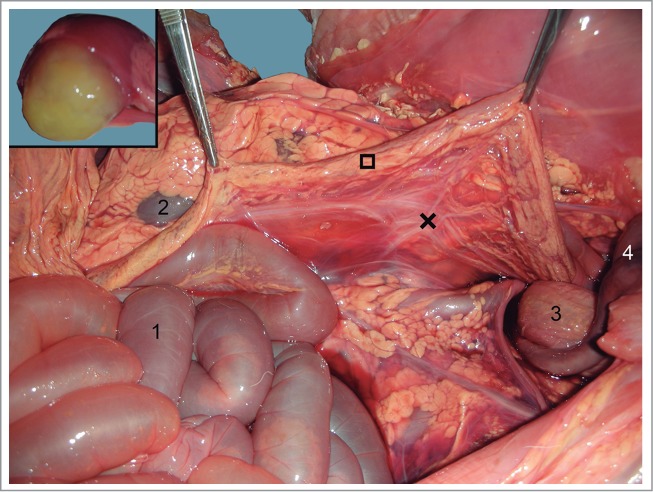
Descending colon (□) suspended by the mesocolon (×). 1) Jejunum; 2) Left kidney; 3) urine bladder; 4) Umbilical arteries. Inset: yellowish mucous secretion filling the descending colon lumen.
Discussion
Intestinal obstructions prevent the normal movement of gut content and meconium in the fetus and neonate. Therefore, they lead to dilation of the proximal segment, with progressive abdominal distention, which may become extensive. The bowel beyond the discontinuity is small in diameter, and filled with scarse mucus and desquamated cells. After birth, fecal content can not reach the anus.9
Intestinal atresia occurs with greater frequency at particular locations in different domestic animals. Variations in the digestive system anatomy among species may explain the diversity in the categorization of intestinal atresia, in addition to its incidence affecting different areas.10 According to the literature, stenosis in the large intestine is more frequent than in the small bowel.7,10 In domestic mammals, the most frequent abnormality regarding intestinal segments is atresia coli. It is seen particularly in the spiral colon of calves,10,11 and in the large and small colon of foals;10 it also occurs in cats.7,10 Atresia ilei, although much less frequent, is more prevalent in calves, but rare in foals, lambs, piglets, and pups.9
The exact etiological status of the intestinal atresia has not been elucidated yet. It is unclear whether it arises from some organ development disturbances or from hereditary defects. As far as the etiology is concerned, there are at least 3 hypotheses to date: heritable origin, a vascular incident, and failing recanalization of the embryonic gut. However, the principal feature(s) leading to intestinal atresia may differ within and between species.12
The causes of atresia coli in calves are not well understood and represent a matter of scientific controversy. As this atresia is more frequent in Holstein-Friesian calves than would be expected, a genetic origin has been proposed. It may be an autosomal recessive trait in Holsteins11 but, as its heritability was estimated to be 0.0875,13 other etiologies must be implied.14 In addition, this atresia was found in one of identical twin calves but not in the other.15
A predisposition to suffer from atresia coli has been described in male calves.16 In the same way, other authors17 have stated that all affected calves in their study were Holstein-Friesian males. In contrast, Durmus18 found a higher incidence of intestinal atresia in female than in male calves (23 females/11 males), as did Kiliç and Sarierler19 (15 females/7 males). Thus, in accordance with Jubb,9 the proportion of females/males affected is not consistent among studies. At present, most of the authors do not consider colonic atresia a genetic disease.11 In contrast, Puri and Fujimoto20 described 7 reports of hereditary multiple atresias affecting human babies. Their pathological findings suggested that all cases of hereditary multiple intestinal atresias were due to malformations in the digestive system instead of an ischemic accident. In calves, colonic atresia has been seen with coexisting anomalies14 such as: cleft palate, kidney agenesis, umbilical hernia, and cryptorchism; however, our case did not show any of them, suggesting that atresia coli might not be a genetic trait in this case.
The inheritability of intestinal atresia is controversial and poorly understood. Stenosis and atresia affecting duodenum may be more influenced by genetic components than atresia in other intestinal segments (jejunum, ileum or colon) because a remarkable number of patients have other co-occurring malformations.12 In addition, a hereditary-recessive cause of intestinal atresia has been reported in the jejunum of Jersey cattle and in the in the ileum of Swedish Highland cattle.19
Regarding a vascular incident, Lejeune et al.21 stated that intestinal atresia may be caused by local ischemia during temporary herniation and rotation of the fetal gut into the extra-embryonic coelom. The most accepted explication is that vascularization becomes deficient in small areas of the intestine because it is not well established or it atrophies soon in development.1,9 Depending on the importance of the vascular failure, the affected intestinal portions develop hypoplasia or atrophy.1 Among farm animals, atresia coli has higher incidence in ruminants, mainly in the calf, probably because an ample spiralling of colon happens together with its blood supply. The sinuosity of the vasculature insinuates that, during intrauterine period, vessels may suffer from strangulation, inducing spiral colon atresia12. In fact, atresia has been induced by obstructing the gut blood vessels in experimental trials with ovine fetuses,22 dogs,23 rabbits,24 and chickens.25,26 In addition, the duration of the vascular occlusion seemed to be a significant factor in chicken.26 Unfortunately, no data are yet available for calves.
An association has been postulated between pressure on the amniotic vesicle during palpation of the embryo for pregnancy diagnosis prior to 41–42 d of gestation, and the development of atresia of the jejunum or colon in calves.11 Syed and Shanks16 stated that early pregnancy diagnosis in dams may contribute, but was not essential, to atresia coli in Holstein calves. Nevertheless, the mechanism of this effect is uncertain.9,12 The hypothesis of ischemia after early pregnancy diagnosis is an unlikely theory for explaining the pathogenesis of atresia coli in our case, as the gestation diagnosis was made by ultrasonography.
The third theory regarding the etiology of intestinal atresia refers to the lack of revacuolization of the slid cord stage during intestinal development. This fact might explain most cases of intestinal atresias, especially those that occur in the anterior portion of the duodenum, where the intestine failed to re-establish patency after the transient stage of luminal occlusion.27,28
In conclusion, this is the first record of a very uncommon case of atresia regarding the distal loop of the ascending colon of a calf, without any other anomalies. Unfortunately, the etiology is still unknown. However, as this calf has no other malformations concurrently, the etiopathogenesis might be due to local factors instead of genetic component. Therefore more studies are needed in order to elucidate the causes of atresia coli. Determining the causes would be decisive in reducing the occurrence of this congenital malformation, avoiding animal suffering and economic loss.
Materials and Methods
A mixed breed male calf (Friesian x Galician Blond) from a local farm, with a history of absence of defecation and abdominal distention, was suspected of having a congenital defect in the gastrointestinal tract. External exploration was completely free of any morphological malformation, including anal atresia. Finger exploration through the rectum only revealed the presence of a mucous secretion but not meconium. The calf was fed orally for 4 d and died at 5 d of age. No hemato-biochemical analyses were performed. After its death, careful examination and dissection were performed.
The calf was born at 9 months of gestation from a Friesian cow. The pregnancy was confirmed by transrectal real-time ultrasonography at 30 d post-insemination. The gestation and delivery were without any remarkable events. The Galician Blond bull, whose semen was used in artificial insemination, had no previously reported malformations in its offspring.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author's Declarations
–This manuscript is not submitted or being considered for publication in any other journal.
–All research meets the ethical guidelines of the European Union (Directive 2010/63/UE).
–The authors declared that they had no conflicts of interests with respect to their authorship or the publication of this article, and they received no financial support for their research and/or authorship of this article.
–Both authors participated equally in the conception of this study, the drafting and revision, and approved the final version before being submitted.
References
- 1. Noden DM, De Lahunta A. Digestive system. In: The Embryology of Domestic Animals. Developmental Mechanisms and Malformations. Baltimore: Williams & Wilkins, 1985; 292-311. [Google Scholar]
- 2. Ghanem M, Yoshida C, Isobe N, Nakao T, Yamashiro H, Kubota H, Miyake Y, Nakada K. Atresia ani with diphallus and separate scrota in a calf: a case report. Theriogenology 2004; 61:1205-13; PMID:15036955; http://dx.doi.org/ 10.1016/j.theriogenology.2003.04.002 [DOI] [PubMed] [Google Scholar]
- 3. McGeady TA, Quinn PJ, Fitzpatrick ES, Ryan MT, Cahalan S. Veterinary Embryology. Oxford: Blackwell, 2006. [Google Scholar]
- 4. Hyttel P, Sinowatz F, Vejlsted M, Betteridge K. (Editor): Essentials of Domestic Animal Embryology. 1st ed Edinburgh: Elsevier, 2010. [Google Scholar]
- 5. Bates MD, Deutsch GH. Molecular insights into congenital disorders of the digestive system. Pediatr Dev Pathol 2003; 6:284-98; PMID:14692642; http://dx.doi.org/ 10.1007/s10024-002-2996-z [DOI] [PubMed] [Google Scholar]
- 6. Fletcher TF, Weber AF. Veterinary developmental anatomy. Veterinary Embryol-Cslass Notes 2013; Retrieved from http://vanat.cvm.umn.edu/WebSitesEmbryo.html (http://vanat.cvm.umn.edu/vanatpdf/EmbryoLectNotes.pdf) [Google Scholar]
- 7. Van der Gaag I, Tibboel D. Intestinal atresia and stenosis in animals: a report of 34 cases. Vet Pathol 1980; 17:565-74; PMID:7404967; http://dx.doi.org/ 10.1177/030098588001700505 [DOI] [PubMed] [Google Scholar]
- 8. Nichol PF, Reeder A, Botham R. Humans, mice, and mechanisms of intestinal atresias: a window into understanding early intestinal development. J Gastrointest Surg 2011; 15:694-700; PMID:21116726; http://dx.doi.org/ 10.1007/s11605-010-1400-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jubb K. Palmer's Pathology of Domestic Animals. 5th edn Edinburgh: Elsevier-Saunders, 2007. [Google Scholar]
- 10. Johnson R. Intestinal atresia and stenosis: a review comparing its morphology. Vet Res Commun 1986; 10:105-11; PMID:3962173; http://dx.doi.org/ 10.1007/BF02213973 [DOI] [PubMed] [Google Scholar]
- 11. Fubini SL, Ducharme NG. Farm animal surgery. Saunders 2004. [Google Scholar]
- 12. Johnson R. Intestinal atresia and stenosis: a review comparing its etiopathogenesis. Vet Res Commun 1986; 10:95-104; PMID:3962177; http://dx.doi.org/ 10.1007/BF02213972 [DOI] [PubMed] [Google Scholar]
- 13. Willer Von S, Muller W, Schlegel F. Untersuchungen uber die genetisch bedingte variabilitat der angedorenen partiellen kolonaplase beim rind. Monatshefte Veterinarmed 1984; 39:473-6. [Google Scholar]
- 14. Ducharme NG, Arighi M, Horney FD, Barker IK, Livesey MA, Hurtig MH, Johnson RP. Colonic atresia in cattle: a prospective study of 43 cases. Can Vet J 1988; 29:818-824; PMID:17423141 [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffsis GF, Bruner RR. Atresia coli in a twin calf. J Am Vet Med Assoc 1977; 171:433-5; PMID:561771 [PubMed] [Google Scholar]
- 16. Syed M, Shanks RD. Incidence of atresia coli and relationships among the affected calves born in one herd of Holstein cattle. J Dairy Sci 1992; 75:1357-64; PMID:1597591; http://dx.doi.org/ 10.3168/jds.S0022-0302(92)77887-4 [DOI] [PubMed] [Google Scholar]
- 17. Modic T, Zadnik T. Atresia coli in newborn calves. In: Program of the XXVI Congresso Nazionale Società Italiana di Buiatria (SIB)-XXVIII Congresso Mondiale, Bologna, Italy 1994; 1423-5. [Google Scholar]
- 18. Durmus AS. Congenital intestinal atresia in calves. Indian Vet J 2009; 86:737-8. [Google Scholar]
- 19. Kiliç N, Sarierler M. Congenital intestinal atresia in calves: 61 cases (1999–2003). Rev Méd Vet 2004; 155:381-4. [Google Scholar]
- 20. Puri P, Fujimoto T. New observations on the pathogenesis of multiple intestinal atresias. J Pediatr Surg 1988; 23:221-5; PMID:3357137; http://dx.doi.org/ 10.1016/S0022-3468(88)80726-7 [DOI] [PubMed] [Google Scholar]
- 21. Lejeune B, Miclard J, Stoffel MH, Meylan M. Intestinal atresia and ectopia in a bovine fetus. Vet Pathol 2011; 48:830-3; PMID:20926733; http://dx.doi.org/ 10.1177/0300985810383872 [DOI] [PubMed] [Google Scholar]
- 22. Abrams JS. Experimental intestinal atresia. Surgery 1968; 64:185-91; PMID:5690620 [PubMed] [Google Scholar]
- 23. Koga Y, Hayashida Y, Ikeda K, Inokuchi K, Hashimoto N. Intestinal atresia in fetal dogs produced by localized ligation of mesenteric vessels. J Pediatr Surg 1975; 10:949-53; PMID:1202181; http://dx.doi.org/ 10.1016/S0022-3468(75)80101-1 [DOI] [PubMed] [Google Scholar]
- 24. Tsujimoto K, Sherman FE, Ravitch MM. Experimental intestinal atresia in the rabbit fetus. Sequential pathological studies. Johns Hopkins Med J 1972; 131:287-97; PMID:5081731. [PubMed] [Google Scholar]
- 25. Baglaj SM, Czernik J, Kuryszko J, Kuropka P. Natural history of experimental intestinal atresia: morphologic and ultrastructural study. J Pediatr Surg 2001; 36:1428-34; PMID:11528622; http://dx.doi.org/ 10.1053/jpsu.2001.26392. [DOI] [PubMed] [Google Scholar]
- 26. Tibboel D, van Nie CJ, Molenaar JC. The effects of temporary general hypoxia and local ischemia on the development of the intestines: an experimental study. J Pediatr Surg 1980; 15:57-62; PMID:7365660; http://dx.doi.org/ 10.1016/S0022-3468(80)80404-0 [DOI] [PubMed] [Google Scholar]
- 27. Dalla Vecchia LK, Grosfeld JL, West KW, Rescorla FJ, Scherer LR, Engum SA. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg 1998; 133:490-6; discussion 496-497; PMID:9605910 [DOI] [PubMed] [Google Scholar]
- 28. Sadler TW. Langman's Medical Embryology. 9th edn Baltimore: Lippincott & Wilkins Inc, 2004. [Google Scholar]
- 29. Dyce KM, Sack WO, Wensing CJG. Textbook of Veterinary Anatomy. 4th edn Philadelphia: Saunders-Elsevier, 2010. [Google Scholar]


