Abstract
A recent paper demonstrated that decellularized extracellular matrix (DECM) deposited by synovium-derived stem cells (SDSCs), especially from fetal donors, could rejuvenate human adult SDSCs in both proliferation and chondrogenic potential, in which expanded cells and corresponding culture substrate (such as DECM) were found to share a mutual reaction in both elasticity and protein profiles (see ref. 1). It seems that young DECM may assist in the development of culture strategies that optimize proliferation and maintain “stemness” of mesenchymal stem cells (MSCs), helping to overcome one of the primary difficulties in MSC-based regenerative therapies. In this paper, the effects of age on the proliferative capacity and differentiation potential of MSCs are reviewed, along with the ability of DECM from young cells to rejuvenate old cells. In an effort to highlight some of the potential molecular mechanisms responsible for this phenomenon, we discuss age-related changes to extracellular matrix (ECM)'s physical properties and chemical composition.
Keywords: mesenchymal stem cells, aging, extracellular matrix, tissue engineering, proliferation, differentiation, microenvironment
Abbreviations and Acronyms
- ACAN
aggrecan
- ALP
alkaline phosphatase
- ADSC
adipose derived mesenchymal stem cell
- BMSC
bone marrow derived mesenchymal stem cell
- CBFA1
core binding factor α 1
- CFU-OB
colony forming unit of osteoblasts
- COL2A1
collagen type 2 alpha1
- DECM
decellularized extracellular matrix
- ECM
extracellular matrix
- ESC
embryonic stem cell
- FGF2
fibroblast growth factor basic
- GAG
glycosaminoglycan
- HGF
hepatocyte growth factor
- HSC
haematopoietic stem cell
- IGF-I
insulin-like growth factor I
- LOXL1
lysyl oxidase-like 1
- LPL
lipopolysaccharide
- LV
left ventricle
- miRNA
micro-RNA
- MMP
matrix metalloproteinase
- mRNA
mRNA
- MSC
mesenchymal stem cell
- ON
osteonectin
- PPARG
peroxisome proliferator active receptor gamma
- ROS
reactive oxygen species
- RUNX2
runt-related transcription factor 2
- SD
Sprague-Dawley
- SDSC
synovium derived stem cell
- SIS-ECM
small intestinal submucosa extracellular matrix
- SOX9
SRY (sex determining region-Y)-box 9
- SPARC
secreted protein, acidic and rich in cysteine
- TGFβ
transforming growth factor β
- TIMP
tissue inhibitor of metalloproteinases
- UDSC
umbilical cord derived mesenchymal stem cell
- VEGF
vascular endothelial growth factor
Introduction
Multipotent and present in many tissues, mesenchymal stem cells (MSCs) can be extracted, grown in vitro, differentiated into a variety of cell types, and subsequently implanted in the original donor without risk of immunological rejection. These properties give MSCs great potential for the treatment of degenerative diseases. Significant resources have been devoted to developing effective MSC-based therapies. Since degenerative diseases primarily affect the aged, it is important to appreciate the changes that the MSC population undergoes with aging, and to develop techniques to reverse age-associated handicaps to the MSC population.2
Figure 1.
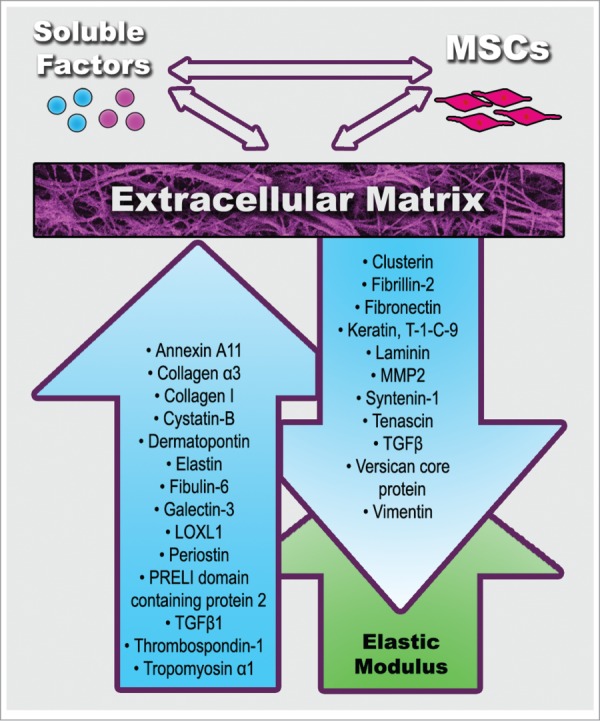
Physical (green color) and compositional (blue color) age-related changes in extracellular matrix (ECM) formed by mesenchymal stem cells (MSCs). Up/down arrows indicate increase or decrease. See Tables 1 and 2 for references.
A comprehensive discussion of MSC aging ought to address intrinsic aging, defined by Sharpless as changes located within the stem cell and its progeny, as well as extrinsic differences between young and old MSCs.3,4,5 For brevity's sake, this review will focus on extrinsic aging. Important intrinsic changes, including but not limited to micro RNA (miRNA) expression,6,7 telomere length,8,9 expression of apoptotic proteins and cell cycle regulators,7,10 cellular secretome (reviewed in refs.11,12), transmembrane receptors,13 and general differences in gene expression14 have been reviewed elsewhere.15,16
Relative to intrinsic changes, the extrinsic changes that accompany cellular aging have been sparsely studied, which is unfortunate given the close relationship between the extracellular matrix (ECM) that makes up the MSC microenvironment and cellular behavior.17,18 It has been suggested that MSCs from different mouse strains (SAMP6, SAMR1, C57BL/6) with different life expectancies may be intrinsically similar in their in vitro proliferation and differentiation capacity, despite exhibiting dramatically different in vivo differentiation and proliferation capacity due to the influence of heterogeneous microenvironments.19 Furthermore, age is associated with changes in the ECM that have been linked to multiple pathologies (reviewed in ref.20), including cancer.17 Consequently, it is vital that the impact of ECM aging on MSC behavior needs to be addressed in order to better understand age-associated diseases and MSC-based regenerative therapy. This review aims to succinctly discuss the current understanding of how ECM ages and to highlight the impact this process has on MSC proliferation and differentiation (Fig. 1).
Donor Age Dependent Cell Senescence
Aging affects MSC proliferative capacity
Like many of the body's cells, MSCs change with age (reviewed in ref.15). Aging is associated with depressed proliferation and elevated apoptosis of MSCs. A recent report compared the self-renewal ability in murine (female C57BL/6 mice) bone marrow derived MSCs (BMSCs) from 3-month-old and 18-month-old mice. Three-month-old BMSCs generated 5 times the number of colony forming unit of osteoblasts (CFU-OB) after expansion, divided by a fraction of cells used for expansion, on plastic culture.21 Kretlow et al. found that murine BMSCs from younger animals had significantly elevated proliferation rates.22 It was further found that BMSCs from Wistar rats aged < 1 month old had a doubling time of 26.07 ± 1.81 hours and a doubling number of 3.64 ± 0.19 while rats aged > 12 months old had a doubling time of 32.20 ± 3.89 hours and a doubling number of 3.07 ± 0.18, suggesting that the young BMSCs replicated more quickly and to a greater degree than did the old BMSCs.23 This phenomenon was also observed in rhesus macaques where BMSCs from young monkeys had more rapid proliferation rates than those from older monkeys.6
The above animal studies have counterparts in human tissue research. Zhang and coworkers showed that human fetal BMSCs had a higher proliferative rate than adult adipose derived MSCs (ADSCs) and umbilical cord derived MSCs (UDSCs).24 It was observed by Stenderup and colleagues that BMSCs from young donors (18–29 y old) had greater proliferative capacity (41 ± 10 versus 24 ± 11 population doublings), slower progression to senescence, and greater proliferative rate (0.09 ± 0.02 vs. 0.05 ± 0.02 population doublings/day) than BMSCs from old donors (68–81 y old).25 Mareschi and coworkers contrasted BMSCs from pediatric donors with young adult donors and reported that, after 112 d of culture, BMSCs from pediatric donors had a cumulative population density almost double that of BMSCs from young adult donors (10.2 ± 1.9 versus 5.5 ± 3.7),26 suggesting that pediatric BMSCs have increased proliferative capacity in vitro. Similarly, Zaim et al. compared the proliferation of human BMSCs from young (0–12 y old), adult (25–60 y old), and elderly (over 60 y old) donors and reported that young BMSCs had a greater proliferative lifespan than cells from the other donor groups (38 ± 8 versus 30 ± 6 versus 10 ± 6 population doublings, respectively).27 Fickert observed that human BMSCs from donors younger than 50 y old or older than 65 y old had increased proliferation rates relative to donors between 50–65 y old (but donors older than 50 had a wider range in doubling times).28 Another publication reported that expression of apoptosis markers was higher in aged (older than 40 y old) human BMSCs than in young (younger than 19 y old) and adult (19–40 y old) human BMSCs and that, after 5 weeks of culture, proliferation in the aged BMSC cultures declined relative to BMSCs from adult donors.29
Aging may affect MSC differentiation, but reports conflict
There have also been many conflicting reports on whether age causes changes in MSC population size and differentiation capacity.30 For example, Asumda et al. reported that BMSCs from young Sprague-Dawley (SD) rats (4 months old) had greater adipogenic, chondrogenic, and osteogenic differentiation potential than BMSCs from old rats (15 months old).31 Similarly, Kretlow et al. reported that murine BMSCs had decreased chondrogenic and osteogenic differentiation capacity with age across all test groups (6 day, 6 week, 1 year), but that adipogenic differentiation ability declined only in cells from the oldest animals.22 Wilson and colleagues observed a progressive decline in osteogenic capacity with age in BMSCs taken from C57BL/6WT mice.32 In humans, bone marrow cells isolated from males between 37–80 y old exhibited an age dependent decline in alkaline phosphatase (ALP) activity (a marker of osteogenesis) and transcript number in osteogenic culture conditions, suggesting a diminished osteogenic capacity of BMSCs with age.33 This finding differs from results reported by Stenderup and coworkers, who found that human BMSCs from young donors (18–29 y old) and old donors (68–81 y old) had similar osteogenic and adipogenic capacity,25 as well as the results of Fickert et al., which did not reveal any difference in osteogenic differentiation between BMSCs harvested from humans younger than 50 y old or between 50–65 y old.28 However, the work of Zaim et al. illustrated that adipogenic, osteogenic, and neurogenic differentiation potential of human BMSCs declined with age, but that chondrogenic potential did not.27 Zhang reported that, following exposure to osteogenic induction medium, human fetal BMSCs exhibited greater osteogenic differentiation than adult ADSCs and UDSCs. Interestingly, they further found that the scaffold constructed by fetal BMSCs demonstrated elevated expression of osteogenic genes [runt-related transcription factor 2 (RUNX2), collagen type I (COL1A1), osteonectin (ON), and ALP], relative to the scaffolds formed by UDSCs or ADSCs.24 Kanawa and colleagues, however, reported that the adipogenic and osteogenic differentiation capacities of human BMSCs were unchanged by age, but that BMSCs' chondrogenic capacity declined.34
As has been reviewed elsewhere,35 molecular differences between young and aged MSCs likely partially account for the phenotypic differences between youthful and aged stem cells. Approximately 8000 genes were differentially expressed between murine (C57BL/6 WT) BMSCs from 2-, 8-, and 26-month-old sources. However, only 86 genes were downregulated across the entire 2–26 month time period studied; among these genes were osteogenic markers (ALP) and growth factors [vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and insulin-like growth factor I (IGFI)].32 Interestingly, Wilson and coworkers further reported that, in BMSCs of mice, adipogenic markers increased between 2–8 months old but declined between 8–26 months old.32 It was reported that expression of Nanog, an embryonic stem cell (ESC) marker, gradually declined as source animal age increased in BALB/c mice BMSCs,36 and that, in human BMSCs from donors aged 15–79 y old, expression of core binding factor α 1 (CBFA1), an osteogenic transcriptional factor, decreased with age (though the difference was not statistically significant). Meanwhile, expression of peroxisome proliferator active receptor gamma (PPARG), an adipogenic transcription factor, increased nearly fourfold and SRY (sex determining region Y)-box 9(SOX9, the master regulator of chondrogenesis) was unchanged.37 Kasper et al. studied the changes in SD rat BMSC proteomes associated with aging, and attributed the inverse correlation between age and replicative potential in part to a decline in cellular responsiveness to mechanical stimuli resulting from a less dynamic actin cytoskeleton.38
Veronesi and coworkers reviewed studies on aging's effects on ADSCs and BMSCs, and concluded that, although some authors found no differences in the proliferative capacity of MSCs, the majority of studies agreed that decreases in MSC proliferation rate and osteogenic capacity were observed with age.16 Some have postulated that inter-laboratory differences in MSC isolation and culture may partially account for conflicting reports on MSC proliferation rate,39 and others have raised the possibility that differences in murine strain might also be responsible, since inter-strain differences exist in murine haematopoietic stem cells (HSCs).30 The differentiation capacity and proliferative ability of MSCs in vitro is likely to correlate with their regenerative capacity in vivo,21,31 thus the diminished proliferative capacity of aged MSCs must be addressed before MSC-based therapies can be optimized for the treatment of degenerative diseases.
Rejuvenation of Elderly Cells by Young ECM
It has been proven that culture on decellularized ECM (DECM) substantially elevates MSC proliferative capacity relative to culture on plastic. Our laboratory has repeatedly demonstrated the superior ability of DECM, relative to plastic, to enhance MSC proliferation and chondrogenic potential (reviewed in ref.40) and other researchers have reported similar findings. For example, it was found that the proliferative ability of MSCs on plastic could be elevated up to 250-fold by culture on basement membrane ECM proteins.41 Enhanced proliferation and differentiation capacities were observed in both murine BMSCs42 and human BMSCs41 following culture on marrow cell produced DECM relative to plastic. For a more thorough review of the role of DECM in preventing senescence of cultured MSCs, see refs.2,40
While DECM culture in general is superior to culture on plastic, there is evidence to suggest that the properties of DECM and its efficacy in in vitro culture systems is highly influenced by the chronological age of the cells that formed it. Work by Conboy and colleagues showed that joining the circulatory systems of old (C57B1/6) and young (2–3 months old) mice (C57Bi/Ka-Ly5.2) elevated in vivo hepatocyte proliferation and enhanced in vivo repair of muscle damage in old (19–26 months old) mice, while also stimulating both in vitro and in vivo proliferation of aged satellite cells (myocyte precursors).42 Interestingly, Yu and colleagues reported that, in rhesus macaque BMSCs, conditioned medium obtained from young (1–5 y old) BMSCs was unable to elevate the proliferation rate of old (12–20 y old) BMSCs.6 This finding suggests that the factors secreted by young stem cells alone are unable to elevate the proliferation rates of old stem cells which, as will be discussed below, is not true of DECM formed by young stem cells.1 The combination of these reports highlights both the ability of the stem cell niche to regulate stem cell behavior and the importance of ECM as a component of that niche.
The ECM appears to convey to cells signals that regulate their proliferation and maintain “stemness.”40,43 Whether directly, through its own physical properties, or indirectly, though sequestration or concentration of soluble factors, the ECM plays a major role in regulating the activities of nearby cells.17,44 A comparison of decellularized organ scaffolds found that fetal and juvenile (3 months old to 1.5 y old) rhesus monkey kidney DECM allowed greater organ repopulation and tubular structure formation than adult (5–13 y old) DECM.45 Choi and colleagues found that DECM produced by young cells restored a youthful phenotype to senescent human fibroblasts, resulting in an additional 25 population doublings (a 39% increase in cellular lifespan).46 This finding was applied to female mouse (C57BL/6) BMSCs by Sun and colleagues, who reported that the defective replication of aged (18 months old) BMSCs was reversed by exposure to DECM from young (3 months old) animals. They found that cells from both young and old mice had a higher expression of telomerase when cultured on DECM from young donors than when cultured on DECM from aged donors or plastic, and that the young DECM diminished reactive oxygen species (ROS) levels in aged BMSCs by 50%.21
Our own laboratory has shown that expansion on DECM deposited by fetal synovium-derived stem cells (SDSCs) enhanced the proliferation of human adult SDSCs to a greater extent than plastic or DECM deposited by adult SDSCs. Further, fetal DECM diminished human adult SDSC ROS levels and promoted apoptotic resistance relative to plastic culture.1 Our findings are similar to the recent work of Ng and colleagues, who found that human adult BMSCs cultured on human fetal DECM had, after 10 d of culture, 1.6 times greater cell population size than BMSCs on plastic. Further, BMSCs that were cultured on plastic for 6 passages and then moved to fetal DECM displayed a 2.2-fold higher cell count after 3 additional passages relative to BMSCs cultured on plastic throughout, suggesting that fetal DECM was able to rescue the aged phenotype of adult BMSCs.47 Taken together, these studies show that DECM formed by young cells is able to independently enhance the proliferation of older cells. As mentioned above, this finding suggests a possible utility of fetal DECM in generating sufficiently large MSC populations derived from elderly donors for use in regenerative therapies.
There is also evidence to suggest that a young ECM may not only rejuvenate the replicative capacity of old MSCs, but also their capacity to form tissues. Pre-culture of old murine BMSCs on DECM formed by young BMSCs resulted in greater in vivo bone formation by old BMSCs than by old BMSCs pre-cultured on old DECM or plastic.21 Kurtz et al. observed increased expression of pluripotency markers and differentiation potential in old MSCs following seeding on DECM formed by young ADSCs.48 Our work showed that, after incubation in chondrogenic or adipogenic medium, human adult SDSCs expanded on fetal DECM had greater production of chondrogenic marker genes [SOX9, aggrecan (ACAN), and collagen type II (COL2A1)], glycosaminoglycan (GAG) and collagen type II, or adipogenic markers lipopolysaccharide (LPL) and PPARG, respectively, though this pattern was not observed following treatment with osteogenic medium.1 These studies very strongly suggest that culture of old MSCs on young DECM rejuvenates the ability of old MSCs to differentiate.
Physical Changes of Matrix with Age and Potential Influence on MSC Commitment
General changes in matrix physical properties
Some researchers have noted that the stiffness of the ECM in muscle increases with age in animals49,50 and that an increase in ECM stiffness may enhance integrin signaling and cell proliferation.51,52 The effects of this change on aging satellite cells have been reviewed.53 It has long been established that collagen crosslinking increases as ECM ages as a result of the Maillard reaction which is believed to increase the stiffness of the matrix.54 Tottey and colleagues studied the small intestinal submucosa ECM (SIS-ECM) harvested from 3-, 12-, 26-, and >52 -week-old porcine. They reported that the ECM thickened with age and withstood less uniaxial stress. They also found that the elastic modulus of SIS-ECM from 3-week-old porcine was less than that of older animals, though the difference in SIS-ECM between 3 weeks old and >52 weeks old did not achieve statistical significance.55 In rat cardiac tissue, it was also observed that DECM stiffness of neonatal (P2–3) hearts was double that of fetal hearts, but this trend did not continue between neonatal and adult (2–3 months old) SD rats.56
Interestingly, age also correlated with the stretching and partial unfolding of fibronectin in ECM, resulting in gradually increasing ECM tension, which might augment the effects of elevated collagen crosslinking.57 The findings may partially account for Erickson et al.'s observation that the compressive modulus of adult (2–3 y old) and juvenile (3–6 months old) bovine cartilage was 50–75% greater than that of fetal (2nd or 3rd trimester) bovine cartilage.58 These changes in matrix elasticity are important because ECM elasticity has been shown to affect cell proliferation,59,60 although it is important to remember that the effects of matrix elasticity on MSC proliferation rates are sensitive to other factors, such as cell seeding density.61 In Table 1, age-associated changes in the physical properties of ECM are summarized.
Table 1.
Age-associated changes in ECM physical properties. Matrix proteins related to aging are upregulated (Direct)
| Correlation with age | Tissue source | Reference |
|---|---|---|
| GENERAL CHANGES | ||
| Elastic modulus, rigidity, stiffness | ||
| Direct | Porcine jejunum (3w, 12w, 26w, >52w) | 55 |
| Direct | Male Wistar rats, soleus muscle (4m, 24m) | 50 |
| Direct | Cardiac tissue of SD rats (fetal, neonatal, adult) | 56 |
| Direct (stiffness) | Epimysium of male Lewis rats (4 m, 28-30m) | 49 |
| Linear strength | ||
| Direct | Porcine jejunum (3w, 12w, 26w, >52 w) | 55 |
| Thickness | ||
| Direct | Porcine jejunum (3w, 12w, 26w, >52w) | 55 |
| MSC SPECIFIC CHANGES | ||
| Elastic modulus | ||
| Direct | Human synovium | 1 |
MSC specific changes in ECM physical properties
Cells on ECM interact with the matrix and sense the physical properties of the tissue that surrounds them,62,63 a process that affects cell proliferation and apoptosis.64,65 As discussed above, multiple studies have established that ECM changes with age, and therefore it stands to reason that age-related changes in the physical properties of ECM may affect MSC behavior. Matrix mechanical properties have been shown to affect stem cell proliferation rates66 and lineage commitment,67–70 likely by Rho GTPase dependent signaling.71 It may therefore be expected that age-induced changes in the mechanical properties of ECM can affect MSC proliferation and differentiation potential. Unfortunately, the physical properties of MSC deposited ECM are usually studied in the context of differentiation and proliferation rates only; the potential differences between old and young ECM are relatively unexplored.
Li et al. showed that DECM deposited by human adult SDSCs was more elastic than that deposited by fetal SDSCs,1 a finding in accord with the general findings of Tottey and coworkers.55 Interestingly, Gershlak and colleagues cultured rat BMSCs on DECM isolated from cardiac tissue of fetal, neonatal, and adult SD rats, and reported that rat BMSCs generated higher traction force when cultured on a fetal DECM hydrogel mixture with a stiffness of 48 kPa, than on adult or neonatal DECM mixtures of that same stiffness.56 Furthermore, the Badylak group, who grafted porcine SIS-ECM into the abdominal walls of adult female rats, observed that the SIS-ECM grafts taken from 3-week-old porcine withstood greater uniaxial tensile stress than those taken from 12-, 26-, and >52 -week-old porcine, as well as murine controls.72 Other investigators found that seeding on a collagen type I and fibronectin coated polyacrylamide gel with an elasticity of 250 Pa (similar to bone marrow and adipose tissue), caused BMSCs to halt progression through the cell cycle, but that seeding on stiffer substrates caused non-proliferative BMSCs to enter the cell cycle.66 These findings, viewed along with earlier reports that uniaxial tensile strength increases with age, suggests that the role of ECM in regulating MSCs is dependent not only on the physical properties of ECM, but on its chemical composition as well.56 Age-associated changes in the make-up of ECM are therefore reviewed below.
Compositional Changes with Age and Potential Influence on MSC Commitment
General changes in ECM composition
Like its mechanical properties, the protein composition of ECM changes with age; however, these changes are often tissue specific. Magnuson and colleagues noted that fibronectin, a key component of ECM, undergoes changes in alternative splicing with aging both in vitro and in vivo, but that these changes are tissue specific.73 Furthermore, it has been reported that the collagen content of human tissue increases with age in the left ventricle (LV) and uterine cervix,74 but decreases in spinal discs.75 Consequently, we divide our review of age-associated compositional modifications to the ECM by tissue type.
In muscle, the collagen content of the LV increased with age in wild-type mice,76 sheep,77 and humans (independent of pathology),78–80 This change in LV collagen concentration may result from a variety of molecular factors recently reviewed.81 Additionally, older (20 months old) BALB/c mice had more LV hydroxyproline, collagen, fibronectin, α-1 integrin, and α-5 integrin, but less β-1 integrin, than young (2 months old) or middle-aged (12 months old) mice.82 Secreted protein, acidic and rich in cysteine (SPARC) increased with age in the LV of sheep77 and wild-type mice,76 an interesting finding in light of SPARC's proposed role in sequestering procollagen from the cell surface and processing it into mature collagen fibrils.76 As rat hearts matured from fetal to adult, there were significant increases in collagen types I and III and laminin.56 Lindsey et al. showed that matrix metalloproteinase 3 (MMP3), MMP8, MMP9, MMP12, and MMP14 increased with increasing age in murine hearts,83 though others observed a 40–45% decline in MMP2 activity in aged rat hearts.84 Kostrominova and Brooks observed an age-associated decrease in mRNA (mRNA) coding for collagen types I, III, and V, elastin, and proteoglycan 4 in murine tendons.85 It was found that age correlated directly with increased MMP2, MMP7, tissue inhibitor of metalloproteinase 1 (TIMP-1), TIMP-2, and TIMP-4 while MMP9 concentration decreased with age.86 mRNA levels of MMP2 and MMP9, as well as MMP2 and MMP9 activity, increased with age in tendons.87
Cartilage undergoes extensive modifications with age.88 In brief, as discussed by Gentilli and Cancedda in their review of cartilage and ECM, fetal chondrogenesis entails a net synthesis of cartilage matrix, while normal adult cartilage requires a balance between growth and anabolism.89 The total proteoglycan and collagen contents of the annulus fibrosus and nucleus pulposus decreased with age in humans, though some ECM proteins, such as fibromodulin and biglycan, increased in specific sections of the annulus fibrosus.75 It has also been reported that aged bovine chondrocytes secreted approximately 60% less collagen than fetal bovine chondrocytes.90 Erickson and colleagues showed that, without transforming growth factor β 3 (TGFβ3) treatment, the fetal bovine chondrocyte pellets had more collagen than juvenile or adult pellets.58 In humans, the cartilage-like ECM produced by immature human chondrocytes was superior to that formed by adult chondrocytes.91 Other researchers measured the ratio of collagen type III to collagen type I, and reported that age-related changes to this ratio were highly tissue specific in male Lewis rats; in cardiac tissues, the proportion of collagen type III increased from 1 day old to 6 month old rats, but fell between 1 y and 2 y. In the lungs, the proportion of collagen type III increased relatively steadily from birth until 2 years; in skin, the proportion of collagen type III decreased between 2 weeks and 1 month, but was constant after 2 months of age.92
Robert and colleagues thoroughly reviewed changes to the ECM with age and wrote that, in fibroblasts, fibronectin synthesis increased and collagen type III increased relative to collagen type I, while hyaluronan and GAG secretion decreased.20 In bovine cartilage, GAG content was unaffected by age.58 Proteoglycan and TGFβ1 decreased with age in the synovial fluid of New Zealand white rabbit knee joints.93 Takubo et al. found that the hydroxyproline content of whole murine lung was higher at 24 months of age than at 3 or 6 months of age in BALB/c mice.94 Senescent cells were shown to increase production of collagenases and to downregulate fibronectin and collagen types I, III, and IV,95 which can be expected to weaken the ECM. The overall ECM biosynthesis seems to decrease with age, though this is not true of all ECM components.20
MSC specific changes in ECM composition
Stem cells are not exempt from compositional modifications to the ECM with age and these modifications likely impact their behavior.96 Uncertainty exists regarding age-related changes in collagen synthesis by MSCs. Sun and colleagues found no difference in total protein content of ECM laid by young or old murine BMSCs, but did report that proteins weighing approximately 140 and 40 kDa were significantly less abundant in old ECM than in young.21 Ng et al., however, found that the amount of ECM produced per cell by human fetal BMSCs and adult BMSCs was 1.4 ± 0.6 and 0.5 ± 0.2 μg, respectively, indicating that ECM production by fetal BMSCs is significantly greater than that by adult BMSCs.47 BMSCs from elderly (1 y old) SD rats exhibited a diminished ability to generate a chondrogenic matrix in vitro relative to immature (1 week old) and young adult (12 weeks old) rats.97
Interestingly, Erickson and colleagues reported that bovine fetal BMSCs produced 2–15 times more GAG (in response to TGFβ3) and collagen than adult or juvenile BMSCs,58 and it was noted by our laboratory that human adult SDSC expansion on fetal DECM yielded a greater GAG content per pellet, as well as a higher GAG/DNA ratio, than did expansion on adult DECM or plastic.1 Sicari and colleagues found that the ECM produced by fetal porcine jejunum was enriched in GAG72 and other researchers reported diminished GAG concentration in ECM with aging of murine lungs,98 glomerular basement membranes, and cultured fibroblasts.20 Tottey et al. found that 3-week-old porcine jejunum ECM had less fibroblast growth factor basic (FGF2)/mg dry weight than 12-, 26-, and >52 -week-old sources and less VEGF than 12- and 26-week-old sources; ECM from 3- and 12-week-old sources had greater sulfated GAG/mg dry weight than 26- or >52 -week-old sources.55
Changes of GAG concentration in ECM could have important implications for stem cell proliferation, as GAG levels influenced male adult Wister rat MSC lineage commitment99 and proliferation.100 Investigators have also found that aggrecan and collagen type II were highly expressed by one-week-old rat BMSCs, but diminished with age, as did collagen type IV, which helps assemble collagen type II fibrils. LINK PROTEIN and SOX9 had increased expression in 12-week-old BMSCs compared to one-week-old or one-year-old BMSCs.97 Our laboratory identified several proteins unique to fetal DECM, such as fibrillin-2, tenascin, versican core proteins, and clusterin, while adult DECM, by contrast, had more abundant dermatopontin, elastin, fibulin-6, periostin, thrombospondin-1, and TGFβ1.1 In addition to its ability to interact with cells directly, ECM regulates the spatial distribution and availability of soluble factors that influence cell behavior.17,43,101 For instance, previous work demonstrated that a deficiency in the proteoglycans biglycan and decorin resulted in elevated TGFβ and apoptosis rates in murine BMSCs102 and GAGs were able to regulate murine bone formation by binding growth factors.103
Seck and colleagues showed that age diminished the bone matrix content of IGF-I and IGF-II in men and IGF-I in women. As IGF-I and IGF-II are both important regulators of osteoblastic differentiation and proliferation, it is possible that these changes may affect BMSCs, despite the fact that no correlation was observed between these growth factors and bone remodeling.104 Several other investigators have investigated the effect of age on the amount of growth factors sequestered within the matrix (reviewed in ref.30). It therefore seems clear that the compositional make-up of ECM changes with age and it is possible that these changes affect MSC proliferation both directly and indirectly by regulating the availability of growth factors. Table 2 summarizes age-related compositional changes in ECM.
Table 2.
Age-associated changes in ECM composition. Matrix proteins related to aging are either up- (Direct) or downregulated (Indirect)
| Protein | Correlation with age | Description | Tissue source | Reference |
|---|---|---|---|---|
| GENERAL CHANGES | ||||
| Collagens I and III | Direct (lung, heart) Indirect (skin) | Fraction of collagen III/(I+III), increased with age in lung and heart but decreased in skin | Male Lewis rat | 92 |
| Collagens I and III | Direct | Ratio of collagen III/I increased in fibroblast cultures | Human skin | 108 |
| Fibronectin | Direct | Plasma | Human | 109 |
| GAG | Indirect | Fibroblast culture | Human | 111 |
| Hyaluronic acid | Indirect | Skin | Human | 110 |
| Laminin | Direct | Glomerular Basement Membrane | Rat | 20 |
| Proteoglycans | Indirect | Glomerular Basement Membrane | Rat | 20 |
| MSC SPECIFIC CHANGES | ||||
| Annexin A11 | Direct | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Clusterin | Indirect | Adult versus Fetal DECM from SDSCs | Human | 1 |
| Collagen alpha-3(V) | Direct | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Collagen I | Direct | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Cystatin-B | Direct | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Dermatopontin | Direct | Adult versus Fetal DECM from SDSCs | Human | 1 |
| Elastin | Direct | Adult versus Fetal DECM from SDSCs | Human | 1 |
| Fibrillin-2 | Indirect | Adult versus Fetal DECM from SDSCs | Human | 1 |
| Fibulin-6 | Direct | Adult versus Fetal DECM from SDSCs | Human | 1 |
| Fibronectin | Indirect | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Galectin-3 | Direct | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Keratin, type 1 cytoskeletal 9 | Indirect | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Laminin | Indirect | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| LOXL1 | Direct | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Periostin | Direct | Adult versus Fetal DECM from SDSCs | Human | 1 |
| PRELI domain containing protein 2 | Direct | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| MMP2 | Indirect | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Syntenin-1 | Indirect | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Tenascin | Indirect | Adult versus Fetal DECM from SDSCs | Human | 1 |
| TGFβ | Indirect | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| TGFβ1 | Direct | Adult versus Fetal DECM from SDSCs | Human | 1 |
| Thrombospondin-1 | Direct | Adult versus Fetal DECM from SDSCs | Human | 1 |
| Tropomyosin alpha 1 chain | Direct | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
| Versican core protein | Indirect | Adult versus Fetal DECM from SDSCs | Human | 1 |
| Vimentin | Indirect | Young (P3) versus Senescent (P12) ADSC | Human | 48 |
Conclusions and Future Directions
We have reviewed age-associated changes in MSC proliferative capacity and differentiation ability, as well as the use of DECM from young cells to rejuvenate old cells. Further, we have briefly explored some of the reported differences between the mechanical and chemical properties of old and young ECM. In general, it seems that the stiffness of ECM increases with age, and collagen and GAG concentrations, as well as the concentrations of several other protein components of ECM, may change with age in a highly source-specific fashion.
The seminal study of Engler et al. demonstrated that artificial matrices influence MSC differentiation toward the tissue type whose mechanical properties most closely mimic the synthetic matrix,69 a finding that has been borne out repeatedly.66,99,105 Studies by our laboratory have found that SDSCs became less susceptible to osteogenic or adipogenic differentiation following culture on DECM deposited by SDSCs.106 Although this effect may be influenced by cell seeding density,61 it seems plausible to posit, alongside others,101 that in vivo ECM is optimized to encourage MSC differentiation toward their tissue of origin.
Our laboratory demonstrated that young ECM promotes MSC proliferation to a greater extent than plastic or even adult ECM. It is therefore tempting to speculate that fetal ECM may be optimized to promote the proliferation of stem cells in a manner similar to how adult ECM can direct MSC lineage choice. This hypothesis may explain, in part, the many reports (discussed above) that younger MSCs exhibit greater in vitro proliferative capacity than aged MSCs. It seems intuitively self-evident that fetal stem cells possess a greater proliferative capacity than their aged counterparts, and indeed, UDSCs have elevated proliferation capacity relative to human adult BMSCs.107 The many differences between fetal and adult ECM, therefore, might hold clues that will help researchers design culture techniques that optimize MSC proliferation and “stemness” maintenance in vitro, thereby removing one of the great obstacles to MSC-based regenerative medicine.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Suzanne Danley for her help in editing the manuscript and Tyler Pizzute for his assistance in artwork. This project was partially supported by Research Grants from the AO Foundation (S-12–19P) and NIH R03 (no. R03 AR062763–01A1).
Author Contributions
K.L.: collection and assembly of data, data analysis and interpretation, and manuscript writing; M.P.: conception and design, administrative support, manuscript writing, and final approval of the manuscript.
References
- 1. Li J, Hansen K, Zhang Y, Dong C, Dinu C, Dzieciatkowska M, Pei M. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials 2014; 35:642-53; PMID: 24148243; http://dx.doi.org/ 10.1016/j.biomaterials.2013.09.099 [DOI] [PubMed] [Google Scholar]
- 2. Li JT, Pei M. Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Eng Part B 2012; 18:270-87; PMID: 22273114; http://dx.doi.org/ 10.1089/ten.TEB.2011.0583 [DOI] [PubMed] [Google Scholar]
- 3. Sharpless N. Hot topics in stem cells and self renewal: 2010. Aging Cell 2010; 9:457-61; PMID: 20579010; http://dx.doi.org/ 10.1111/j.1474-9726.2010.00592.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bajek A, Czerwinski M, Olkowska J, Gurtowska N, Kloskowski T, Drewa T. Does aging of mesenchymal stem cells limit their potential application in clinical practice? Aging Clin Exp Res 2012; 24:404-11; PMID: 22595834; http://dx.doi.org/ 10.3275/8424 [DOI] [PubMed] [Google Scholar]
- 5. Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev 2006; 5:91-116; PMID: 16310414; http://dx.doi.org/ 10.1016/j.arr.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 6. Yu J, Wu X, Gimble J, Guan X, Freitas M, Bunnell B. Age related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell 2011; 10:66-79; PMID: 20969724; http://dx.doi.org/ 10.1111/j.1474-9726.2010.00646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alt E, Senst C, Murty S, Slakey D, Dupin C, Chaffin A, Kadowitz P, Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res 2012; 8:215-25; PMID: 22265741; http://dx.doi.org/ 10.1016/j.scr.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 8. Efimenko A, Dzhoyashviu N, Kalinina N, Kochegura T, Akchurin R, Tkachuk V, Parfyonova Y. Adipose derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl Med 2014; 3:32-41; PMID: 24353175; http://dx.doi.org/ 10.5966/sctm.2013-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choumerianou DM, Martimianaki G, Stiakaki E, Kalmanti L, Kalmanti M, Dimitriou H. Comparative study of stemness characteristics of mesenchymal cells from bone marrow of children and adults. Cytotherapy 2010; 12:881-7; PMID: 20662612; http://dx.doi.org/ 10.3109/14653249.2010.501790 [DOI] [PubMed] [Google Scholar]
- 10. Choudhery M, Kan M, Mahmood R, Mehmood A, Khan S, Riazuddin S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol Int 2012; 36:747-53; PMID: 22352320; http://dx.doi.org/ 10.1042/CBI20110183 [DOI] [PubMed] [Google Scholar]
- 11. Kuilman T, Peeper D. Senescence messaging secretome: SMSing cellular stress. Nat Rev Cancer 2009; 9:81-94; PMID: 19132009; http://dx.doi.org/ 10.1038/nrc2560 [DOI] [PubMed] [Google Scholar]
- 12. Campisi J, Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007; 8:729-40; PMID: 17667954; http://dx.doi.org/ 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 13. Guang L, Boskey A, Zhu W. Age-related CXC chemokine receptor-4-deficiency impairs osteogenic differentiation potency of mouse bone marrow mesenchymal stromal stem cells. Int J Biochem Cell Biol 2013; 45:1813-20; PMID: 23742988; http://dx.doi.org/ 10.1016/j.biocel.2013.05.034 [DOI] [PubMed] [Google Scholar]
- 14. Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber F, Eckstein V, et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One 2009; 4:e5846; PMID: 19513108; http://dx.doi.org/ 10.1371/journal.pone.0005846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mimeault M, Batra S. Recent insights into the molecular mechanisms involved in aging and the malignant transformation of adult stem/progenitor cells and their therapeutic implications. Ageing Res Rev 2009; 8:94-112; PMID: 19114129; http://dx.doi.org/ 10.1016/j.arr.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veronesi F, Torricelli P, Borsari V, Tschon M, Rimondini L, Fini M. Mesenchymal stem cells in the aging and osteoporotic population. Crit Rev Eukaryot Gene Expr 2011; 21:363-77; PMID: 22181705; http://dx.doi.org/ 10.1615/CritRevEukarGeneExpr.v21.i4.60 [DOI] [PubMed] [Google Scholar]
- 17. Lu P, Weaver V, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012; 196:395-406; PMID: 22351925; http://dx.doi.org/ 10.1083/jcb.201102147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hynes R. Extracellular matrix: not just pretty fibrils. Science 2009; 326:1216-9; PMID: 19965464; http://dx.doi.org/ 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fehrer C, Laschober G, Lepperdinger G. Aging of murine mesenchymal stem cells. Ann NY Acad Sci 2006; 1067:235-42; PMID: 16803992; http://dx.doi.org/ 10.1196/annals.1354.030 [DOI] [PubMed] [Google Scholar]
- 20. Labat-Robert J, Robert AM, Robert L. Aging of the extracellular matrix. Med Longevite 2012; 4:3-32; http://dx.doi.org/ 10.1016/j.mlong.2012.02.003 [DOI] [Google Scholar]
- 21. Sun Y, Li W, Lu Z, Chen R, Ling J, Ran Q, Jilka RL, Chen XD. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J 2011; 25:1474-85; PMID: 21248241; http://dx.doi.org/ 10.1096/fj.10-161497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kretlow J, Jin Y, Liu W, Zhang W, Hong T, Zhou G, Baggett L, Mikos A, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow derived stem cells. BMC Cell Biol 2008; 9:60; PMID: 18957087; http://dx.doi.org/ 10.1186/1471-2121-9-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valyushina MP, Buravkova LB. Age related differences in rat multipotent mesenchymal stromal bone marrow cells. Bull Exp Biol Med 2013; 155:129-33; PMID: 23667890; http://dx.doi.org/ 10.1007/s10517-013-2097-1 [DOI] [PubMed] [Google Scholar]
- 24. Zhang Z, Teoh S, Chong M, Schantz J, Fisk N, Choolani M, Chan J. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells 2009; 27:126-37; PMID: 18832592; http://dx.doi.org/ 10.1634/stemcells.2008-0456 [DOI] [PubMed] [Google Scholar]
- 25. Stenderup K, Justensen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003; 22:919-26; PMID: 14678851; http://dx.doi.org/ 10.1016/j.bone.2003.07.005 [DOI] [PubMed] [Google Scholar]
- 26. Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, Madon E, Fagioli F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem 2006; 97:744-54; PMID: 16229018; http://dx.doi.org/ 10.1002/jcb.20681 [DOI] [PubMed] [Google Scholar]
- 27. Zaim M, Karaman S, Cetin G, Isik S. Donor age and long term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol 2012; 91:1175-86; PMID: 22395436; http://dx.doi.org/ 10.1007/s00277-012-1438-x [DOI] [PubMed] [Google Scholar]
- 28. Fickert S, Bobsin U, Grob A, Hempel U, Wojciechowski C, Rentsch C, Corbeil D, Gunther K. Human mesenchymal stem cell proliferation and osteogenic differentiation during long term ex vivo cultivation is not age dependent. J Bone Mineral Metab 2011; 29:224-35; PMID: 20811759; http://dx.doi.org/ 10.1007/s00774-010-0215-y [DOI] [PubMed] [Google Scholar]
- 29. Stolzing A, Jones E, McGonagle D, Scutt A. Age related changes in human bone marrow derived mesenchymal stem cells: consequences for cell therapy. Mech Ageing Dev 2008; 129:163-73; PMID: 18241911; http://dx.doi.org/ 10.1016/j.mad.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 30. Bellantuono I, Aldahmash A, Kassem M. Aging of stromal (skeletal) stem cells and their contribution to age related bone loss. Biochim Biophys Acta 2009; 1792:364-70; PMID: 19419706; http://dx.doi.org/ 10.1016/j.bbadis.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 31. Asumda FZ, Chase PB. Age related changes in rat bone marrow mesenchymal stem cell plasticity. BMC Cell Biol 2011; 12:44; PMID: 21992089; http://dx.doi.org/ 10.1186/1471-2121-12-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson A, Shehadeh L, Yu H, Webster K. Age related molecular genetic changes of murine bone marrow mesenchymal stem cells. BMC Genomics 2010; 11:229; PMID: 20374652; http://dx.doi.org/ 10.1186/1471-2164-11-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mueller S, Glowacki J. Age related decline in the osteogenic potential of human bone marrow cells cultured in three dimensional collagen sponges. J Cell Biochem 2001; 82:583-90; PMID: 11500936; http://dx.doi.org/ 10.1002/jcb.1174 [DOI] [PubMed] [Google Scholar]
- 34. Kanawa M, Igarashi A, Ronald V, Higashi Y, Kurihara H, Sugiyama M, Saskianti T, Pan H, Kato Y. Age dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor 2. Cytotherapy 2013; 15:1062-72; PMID: 23800732; http://dx.doi.org/ 10.1016/j.jcyt.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 35. Kim M, Kim C, Choi Y, Kim M, Park C, Suh Y. Age related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: implications to age associated bone diseases and defects. Mech Ageing Dev 2012; 133:215-25; PMID: 22738657; http://dx.doi.org/ 10.1016/j.mad.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 36. Katsara O, Mahaira L, Iliopoulou E, Moustaki A, Antsaklis A, Loutradis D, Stefanidis K, Baxevanis C, Papamichail M, Perez S. Effects of donor age, gender and in vitro cellular aging on the phenotypic, functional and molecular characteristics of mouse bone marrow derived mesenchymal stem cells. Stem cells Dev 2011; 20:1549-61; PMID: 21204633; http://dx.doi.org/ 10.1089/scd.2010.0280 [DOI] [PubMed] [Google Scholar]
- 37. Jiang Y, Mishima H, Sakai S, Liu Y, Ohyabu Y, Uemura T. Gene expression analysis of major lineage defining factors in human bone marrow cells: effect of aging, gender, and age related disorders. J Orthop Res 2008; 26:910-7; PMID: 18302252; http://dx.doi.org/ 10.1002/jor.20623 [DOI] [PubMed] [Google Scholar]
- 38. Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kühnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, et al. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells 2009; 27:1288-97; PMID: 19492299; http://dx.doi.org/ 10.1002/stem.49 [DOI] [PubMed] [Google Scholar]
- 39. Raveh-Amit H, Berzsenyi S, Vas V, Ye D, Dinnyes A. Tissue resident stem cells: till death do us part. Biogerontology 2013; 14:573-90; PMID: 24085521; http://dx.doi.org/ 10.1007/s10522-013-9469-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pei M, Li J, Shoukry M, Zhang Y. Decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cells Mater 2011; 22:333-43; PMID: 22116651 [DOI] [PubMed] [Google Scholar]
- 41. Lindner U, Kramer J, Behrends J, Driller B, Wendler N, Boehrnsen F, Rohwedel J, Schlenke P. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement membrane extracellular matrix proteins. Cytotherapy 2010; 12:992-1005; PMID: 20807021; http://dx.doi.org/ 10.3109/14653249.2010.510503 [DOI] [PubMed] [Google Scholar]
- 42. Conboy I, Conboy M, Wagers A, Girma E, Weissman I, Rando T. Rejuvenation of aged progenitor cells by exposure to a young system environment. Nature 2005; 433:760-4; PMID: 15716955; http://dx.doi.org/ 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- 43. Chen X. Extracellular matrix provides an optimal niche for the maintenance and propagation of mesenchymal stem cells. Birth Defects Res (Part C) 2010; 90:45-54; PMID: 20301219; http://dx.doi.org/ 10.1002/bdrc.20171 [DOI] [PubMed] [Google Scholar]
- 44. Wagers A. The stem cell niche in regenerative medicine. Cell Stem Cell 2012; 10:362-9; PMID: 22482502; http://dx.doi.org/ 10.1016/j.stem.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 45. Nakayama K, Batchelder C, Lee C, Tarantal A. Renal tissue engineering with decellularized rhesus monkeys kidneys: age related differences. Tissue Eng Part A 2011; 17:2891-901; PMID: 21902603; http://dx.doi.org/ 10.1089/ten.TEA.2010.0714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Choi H, Cho K, Kang H, Lee J, Kaeberlein M, Suh Y, Chung K, Park S. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix. Aging cell 2011; 10:148-57; PMID: 21108727; http://dx.doi.org/ 10.1111/j.1474-9726.2010.00654.x [DOI] [PubMed] [Google Scholar]
- 47. Ng CP, Sharif AR, Heath DE, Chow JW, Zhang CB, Chan-Park MB, Hammond PT, Chan JK, Griffith LG. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials 2014; 35:4046-57; PMID: 24560460; http://dx.doi.org/ 10.1016/j.biomaterials.2014.01.081 [DOI] [PubMed] [Google Scholar]
- 48. Kurtz A, Oh SJ. Age related changes of the extracellular matrix and stem cell maintenance. Prev Med 2012; 54 Suppl: S50-6; PMID: 22285947; http://dx.doi.org/ 10.1016/j.ypmed.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 49. Gao Y, Kostrominova T, Faulkner J, Wineman A. Age related changes in mechanical properties of the epimysium in skeletal muscles of rats. J Biomech 2008; 41:465-9; PMID: 18031752; http://doi.org/ 10.1016/j.jbiomech.2007.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosant C, Nagel M, Perot C. Aging affects passive stiffness and spindle function of the rat soleus muscle. Exp Gerontol 2007; 42:301-8; PMID: 17118602; http://dx.doi.org/ 10.1016/j.exger.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 51. Wozniak M, Desai R, Solski P, Der C, Keely P. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol 2003; 163:583-95; PMID: 14610060; http://doi.org/ 10.1083/jcb.200305010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paszek M, Zahir N, Johnson K, Lakins J, Rozenberg G, Gefen A, Reinhart-King C, Margulies S, Dembo M, Boettinger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005; 8:241-54; PMID: 16169468; http://dx.doi.org/ 10.1016/j.ccr.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 53. Cosgrove B, Sacco A, Gilbert P, Blau H. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation 2009; 78:185-94; PMID: 19751902; http://dx.doi.org/ 10.1016/j.diff.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robert L, Labat-Robert J. Aging of connective tissues: from genetic to epigenetic mechanisms. Biogerontology 2000; 1:123-31; PMID: 11707928 [DOI] [PubMed] [Google Scholar]
- 55. Tottey S, Johnson SA, Crapo PM, Reing JE, Zhang L, Jiang H, Medberry CJ, Reines B, Badylak SF. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 2011; 32:128-36; PMID: 20870285; http://dx.doi.org/ 10.1016/j.biomaterials.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gershlak JR, Resnikoff JI, Sullivan KE, Williams C, Wang RM, Black LD, 3rd. Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochem Biophys Res Commun 2013; 439:161-6; PMID: 23994333; http://dx.doi.org/ 10.1016/j.bbrc.2013.08.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Antia M, Baneyx G, Kubow K, Vogel V. Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Farady Discuss 2008; 139:229-49; PMID: 19048998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Erickson I, van Veen S, Sengupta S, Kestle S, Mauck R. Cartilage matrix formation by bovine mesenchymal stem cells in three dimensional culture is age dependent. Clin Orthop Relat Res 2011; 469:2744-53; PMID: 21424832; http://dx.doi.org/ 10.1007/s11999-011-1869-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guilak F, Cohen D, Estes B, Gimble J, Liedtke W, Chen C. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009; 5:17-26; PMID: 19570510; http://dx.doi.org/ 10.1016/j.stem.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lopez J, Mouw J, Weaver V. Biomechanical regulation of cell orientation and fate. Oncogene 2008; 27:6981-93; PMID: 19029939; http://dx.doi.org/ 10.1038/onc.2008.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xue R, Li J, Yeh Y, Yang L, Chien S. Effects of matrix elasticity and cell density on human mesenchymal stem cells differentiation. J Orthop Res 2013; 31:1360-5; PMID: 23606500; http://dx.doi.org/ 10.1002/jor.22374 [DOI] [PubMed] [Google Scholar]
- 62. Ogneva I. Cell mechanosensitivity: mechanical properites and interaction with gravitational field. BioMed Res Int 2013; 2013:598461; PMID: 23509748; http://dx.doi.org/ http://dx.doi.org/ 10.1155/2013/598461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ingber D. Mechanobiology and diseases of mechanotransduction. Ann Med 2003; 35:564-77; PMID: 14708967; http://dx.doi.org/ 10.1080/07853890310016333 [DOI] [PubMed] [Google Scholar]
- 64. Hadjipanayi E, Mudera V, Brown R. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Reg Med 2009; 3:77-84; PMID: 19051218; http://dx.doi.org/ 10.1002/term.136 [DOI] [PubMed] [Google Scholar]
- 65. Wang W, Passaniti A. Extracellular matrix inhibits apoptosis and enhances endothelial cell differentiation by a NFkappaB dependent mechanism. J Cell Biochem 1999; 73:321-31; PMID: 10321832; http://dx.doi.org/ 10.1002/(SICI)1097-4644(19990601)73:3 [DOI] [PubMed] [Google Scholar]
- 66. Winer J, Janmey P, McCormick M, Funaki M. Bone marrow derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A 2009; 15:147-54; PMID: 18673086; http://dx.doi.org/ 10.1089/ten.tea.2007.0388 [DOI] [PubMed] [Google Scholar]
- 67. Lee J, Abdeen A, Huang T, Kilian K. Controlling cell geometry on substrates of variable stiffness can tune the degree of osteogenesis in human mesenchymal stem cells. J Mech Behav Biomed Mater 2014; 38:209-18; pii: S1751-6161(14)00010-1; PMID: 24556045; http://dx.doi.org/ 10.1016/j.jmbbm.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 68. Kolf C, Cho E, Tuan R. Mesenchymal stromal Cells. Biology of adult mesenchymal stem cells: regulation of niche, self renewal and differentiation. Arth Res Ther 2007; 9:204; PMID: 17316462; http://dx.doi.org/ 10.1186/ar2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Engler A, Sen S, Sweeney L, Dischler D. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126:677-89; PMID: 16923388; http://dx.doi.org/ 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- 70. Trappmann B, Gautrot J, Connelly J, Strange D, Li Y, Oyen M, Stuart C, Boehm H, Li B, Vogel V, et al. Extracellular matrix tethering regulates stem cell fate. Nat Mater 2012; 11:642-9; PMID: 22635042; http://dx.doi.org/ 10.1038/nmat3339 [DOI] [PubMed] [Google Scholar]
- 71. Keung A, Juan-Pardo E, Schaffer D, Kumar S. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 2011; 29:1886-97; PMID: 21956892; http://dx.doi.org/ 10.1002/stem.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sicari B, Johnson S, Siu B, Crapo P, Daly K, Jiang H, Medberry C, Tottey S, Turner N, Badylak S. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 2012; 33:5524-33; PMID: 22575834; http://dx.doi.org/ 10.1016/j.biomaterials.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Magnuson V, Young M, Schattenberg D, Mancini M, Chen D, Steffensen B, Klebe R. The alternative splicing of fibronectin pre-mRNA is altered during aging and in response to growth factors. J Biol Chem 1991; 266:14654-62; PMID: 1713586 [PubMed] [Google Scholar]
- 74. Oxlund B, Ortoft G, Bruel A, Danielsen C, Bor P, Oxlund H, Uldbjerg N. Collagen concentration and biomechanical properties of samples from the lower uterine cervix in relation to age and parity in non-pregnant women. Reprod Biol Endocrinol 2010; 8:82; PMID: 20604933; http://dx.doi.org/ 10.1186/1477-7827-8-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Singh K, Masuda K, Thonar E, An H, Cs-Szabo G. Age related changes in the extracellular matrix of nucleus puposus and annulus fibrosus of human intervertebral disc. Spine 2009; 34:10-6; PMID: 19127156; http://dx.doi.org/ 10.1097/BRS.0b013e31818e5ddd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bradshaw A, Baicu C, Rentz T, Laer A, Bonnema D, Zile M. Age dependent alternation in fibrillar collagen content and myocardial diastolic function: role of SPARC in post synthetic procollagen processing. Am J Physiol Heart Circ Physiol 2010; 298:H614-22; PMID: 20604933; http://dx.doi.org/ 10.1186/1477-7827-8-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Horn M, Graham H, Richards M, Clarke J, Greensmith D, Briston S, Hall M, Dibb K, Trafford A. Age related divergent remodeling of the cardiac extracellular matrix in heart failure: collagen accumulation in the young and loss in the aged. J Mol Cell Cardiol 2012; 53:82-90; PMID: 22516365; http://dx.doi.org/ 10.1016/j.yjmcc.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 78. Lakatta E. Cardiovascular ageing in health sets the stage for cardiovascular disease. Heart Lung Circ 2002; 11:76-91; PMID: 16352074; http://dx.doi.org/ 10.1046/j.1444-2892.2002.00126.x [DOI] [PubMed] [Google Scholar]
- 79. Lakatta E. Cardiovascular aging research: the next horizons. J Am Geriatr Soc 1999; 47:613-25; PMID: 10323658 [DOI] [PubMed] [Google Scholar]
- 80. Debessa C, Maifrino L, de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev 2001; 122:1049-58; PMID: 11389923; http://dx.doi.org/ 10.1016/S0047-6374(01)00238-X [DOI] [PubMed] [Google Scholar]
- 81. Kwak H. Aging, exercise, and the extracellular matrix in the heart. J Exerc Rehabil 2013; 9:338-47; PMID: 24278882; http://dx.doi.org/ 10.12965/jer.130049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Burgess M, McCrea J, Hedrick H. Age associated changes in cardiac matrix and integrins. Mech ageing Dev 2001; 122:1739-56; PMID: 11557277; http://dx.doi.org/ 10.1016/S0047-6374(01)00296-2 [DOI] [PubMed] [Google Scholar]
- 83. Lindsey M, Gosorn D, Squires C, Escobar P, Hendrick J, Mingoia J, Sweterlitsch S, Spinale F. Aged dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res 2005; 66:410-9; PMID: 15820210; http://dx.doi.org/ 10.1016/j.cardiores.2004.11.029 [DOI] [PubMed] [Google Scholar]
- 84. Robert V, Besse S, Sabri A, Silvestre J, Asayag P, Thiem N, Swynghedauw B, Delcayre C. Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab Invest 1997; 76:729-38; PMID: 9166291 [PubMed] [Google Scholar]
- 85. Kostrominova T, Brooks S. Age related changes in structure and extracellular matrix protein expression levels in rat tendons. Age 2013; 35:2203-12; PMID: 23354684; http://dx.doi.org/ 10.1007/s11357-013-9514-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bonnema D, Webb C, Pennington W, Stroud R, Leonardi A, Clark L, McClure C, Finklea L, Spinale F, Zile M. Effects of age on plasma matrix metalloproteinases and tissue inhibitor of metalloproteinases. J Cardiac Failure 2007; 13:530-40; PMID: 17826643; http://dx.doi.org/ 10.1016/j.cardfail.2007.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yu T, Pang J, Wu K, Chen M, Chen C, Tsai W. Age is associated with increased activities of matrix metalloproteinase-2 and 9 in tenocytes. BMC Musculoskelet Disord 2013; 14:2; PMID: 23281803; http://dx.doi.org/ 10.1186/1471-2474-14-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Luria A, Chu C. Articular cartilage changes in maturing athletes: new targets for joint rejuvenation. Sports Health 2014; 6:18-30; PMID: 24427438; http://dx.doi.org/ 10.1177/1941738113514369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des 2009; 15:1334-48; PMID: 19355972 [DOI] [PubMed] [Google Scholar]
- 90. Tran-Khanh N, Hoemann C, McKee M, Henderson J, Buschmann M. Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J Orthop Res 2005; 23:1354-62; PMID: 16048738; http://dx.doi.org/ 10.1016/j.orthres.2005.05.009.1100230617 [DOI] [PubMed] [Google Scholar]
- 91. Adkisson H, Gillis M, Davis E, Maloney W, Hruska K. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res 2001; 391 Suppl: S280-94; PMID: 11603712; http://dx.doi.org/ 10.1097/00003086-200110001-00026 [DOI] [PubMed] [Google Scholar]
- 92. Mays P, Bishop J, Laurent G. Age related changes in the proportion of types 1 and 3 collagen. Mech Ageing Dev 1988; 45:203-12; PMID: 3266279; http://dx.doi.org/ 10.1016/0047-6374(88)90002-4 [DOI] [PubMed] [Google Scholar]
- 93. Wei X, Messner K. Age and injury dependent concnetrations of transforming growth factor b1 and proteoglycan fragments in rabbit knee joint fluid. Osteoarthritis Cartilage 1998; 6:10-8; PMID: 9616434; http://dx.doi.org/ 10.1053/joca.1997.0087 [DOI] [PubMed] [Google Scholar]
- 94. Takubo Y, Hirai T, Muro S, Kogishi K, Hosokawa M, Mishima M. Age associated changes in elastin and collagen content and the proportion of types I and III collagen in the lungs of mice. Exp Gerontol 1999; 34:353-64; PMID: 10433389; http://dx.doi.org/ 10.1016/S0531-5565(99)00017-0 [DOI] [PubMed] [Google Scholar]
- 95. Krizhanovsky V, Yon M, Dickins R, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe S. Senescence of activated stellate cells limits liver fibrosis. Cell 2008; 134:657-67; PMID: 18724938; http://dx.doi.org/ 10.1016/j.cell.2008.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huang N, Patlolla B, Abilez O, Sharma H, Rajadas J, Beygui R, Zarins C, Cooke J. A matrix micropatterning platform for cell localization and stem cell fate determination. Acta Biomater 2010; 6:4614-21; PMID: 20601236; http://dx.doi.org/ 10.1016/j.actbio.2010.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zheng H, Martin J, Duwayri Y, Falcon G, Buckwalter J. Impact of aging on rat bone marrow derived stem cell chondrogenesis. J Gerontol A Biol Sci Med Sci 2007; 62:136-48; PMID: 17339639 [DOI] [PubMed] [Google Scholar]
- 98. Sokocevic D, Bonenfant N, Wagner D, Borg Z, Lathrop M, Lam Y, Deng B, DeSarno M, Ashikaga T, Loi R, et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials 2013; 34:3256-69; PMID: 23384794; http://dx.doi.org/ 10.1016/j.biomaterials.2013.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Murphy C, Matsiko A, Haugh M, Gleeson J, O'Brien F. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen glycosaminoglycan scaffolds. J Mech Behav Biomed Mater 2012; 11:53-62; PMID: 22658154; http://dx.doi.org/ 10.1016/j.jmbbm.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 100. Frescaline G, Bouderlique T, Huynh M, Papy-Garcia D, Courty J, Albanese P. Glycosaminoglycans mimetic potentiate the clonogenicity, proliferation, migration and differentiation properties of rat mesenchymal stem cells. Stem Cell Res 2012; 8:180-92; PMID: 22265738; http://dx.doi.org/ 10.1016/j.scr.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 101. Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 2014; 1840:2506-19; PMID: 24418517; http://dx.doi.org/ 10.1016/j.bbagen.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bi Y, Stuelten C, Kilts T, Wadhwa S, Iozzo R, Robey P, Chen X, Young M. Glycobiology and extracellular matrices: extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem 2005; 280:30481-9; PMID: 15964849; http://dx.doi.org/ 10.1074/jbc.M500573200 [DOI] [PubMed] [Google Scholar]
- 103. Manton K, Leong D, Cool S, Nurcombe V. Disruption of heparin and chondroitin sulfate signaling enhances mesenchymal stem cell derived osteogenic differentiation via bone morphogenetic protein signaling pathways. Stem Cells 2007; 25:2845-54; PMID: 17702986; http://dx.doi.org/ 10.1634/stemcells.2007-0065 [DOI] [PubMed] [Google Scholar]
- 104. Seck T, Bretz A, Krempien R, Krempien B, Ziegler R, Pfeilschifter J. Age-related changes in insulin like growth factor I and II in human femoral cortical bone: lack of correlation with bone mass. Bone 1999; 24:387-93; PMID: 10221551; http:dx.doi/org/ 10.1016/S8756-3282(98)00186-0 [DOI] [PubMed] [Google Scholar]
- 105. Wang Y, Yu X, Cohen D, Wozniak M, Yang M, Gao L, Eyckmans J, Chen C. Bone morphogenetic protein 2 induced signaling and osteogenesis is regulated by cell shape, RhoA/Rock, and cytoskeletal tension. Stem Cells Dev 2012; 21:1176-8; PMID: 21967638; http://dx.doi.org/ 10.1089/scd.2011.0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. He F, Chen X, Pei M. Reconstruction of an in vitro tissue specific microenvironment to rejuvenate synovium derived stem cells for cartilage tissue engineering. Tissue Eng Part A 2009; 15:3809-21; PMID: 19545204; http://dx.doi.org/ 10.1089/ten.TEA.2009.0188 [DOI] [PubMed] [Google Scholar]
- 107. Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006; 24:1294-301; PMID: 16410387; http://dx.doi.org/ 10.1634/stemcells.2005-0342 [DOI] [PubMed] [Google Scholar]
- 108. Branchet MC, Boisnic S, Frances C, Lesty C, Robert L. Morphometric analysis of dermal collagen fibers in normal human skin as a function of age. Arch Gerontol Geriatr 1991; 13:1-14; PMID: 15374431 [DOI] [PubMed] [Google Scholar]
- 109. Labat-Robert J, Robert L. Modifications of fibronectin in age related diseases: diabetes and cancer. Arch Gerontol Geriatri 1984; 3:1-10; PMID: 6378113; http://dx.doi.org/ 10.1016/0167-4943(84)90011-6 [DOI] [PubMed] [Google Scholar]
- 110. Schachtschabel DO, Wever J. Age-related decline in the synthesis of glycosaminoglycans by cultured human fibroblasts (WI-38). Mech Age Develop 1978; 8:257-64; PMID: 703401 [DOI] [PubMed] [Google Scholar]
- 111. Robert L, Robert A-M, Renard G. Biological effects of hyaluronan in connective tissues, eye, skin, venous wall. Role in Aging. Pathol Biol 2010; 58:187-98; PMID: 19932571; http://dx.doi.org/ 10.1016/j.patbio.2009.09.010 [DOI] [PubMed] [Google Scholar]


