Abstract
In the last 2 decades biomedical research has provided great insights into the molecular signatures underlying painful conditions. However, chronic pain still imposes substantial challenges to researchers, clinicians and patients alike. Under pathological conditions, pain therapeutics often lack efficacy and exhibit only minimal safety profiles, which can be largely attributed to the targeting of molecules with key physiological functions throughout the body. In light of these difficulties, the identification of molecules and associated protein complexes specifically involved in chronic pain states is of paramount importance for designing selective interventions. Ion channels and receptors represent primary targets, as they critically shape nociceptive signaling from the periphery to the brain. Moreover, their function requires tight control, which is usually implemented by protein-protein interactions (PPIs). Indeed, manipulation of such PPIs entails the modulation of ion channel activity with widespread implications for influencing nociceptive signaling in a more specific way. In this review, we highlight recent advances in modulating ion channels and receptors via their PPI networks in the pursuit of relieving chronic pain. Moreover, we critically discuss the potential of targeting PPIs for developing novel pain therapies exhibiting higher efficacy and improved safety profiles.
Keywords: inflammatory pain, ion channels, neuropathic pain, protein-protein interactions, protein complexes, pain, receptors
Introduction
The perception of harmful stimuli is critical for the maintenance of cellular homeostasis, avoidance of cell-damaging factors and consequently survival. In vertebrates, specialized somatic sensory neurons (so-called nociceptors) detect noxious chemical, mechanical and thermal stimuli and convey this information via the spinal cord to the brain where the sensory experience of pain is generated.1,2
Nociceptors exhibit a high threshold of activation, which is tightly controlled under physiological conditions to elicit withdrawal from potentially painful stimuli or removal of irritants (cough). In response to injury and inflammation, activation characteristics of such nociceptors can be modulated and sensitized to induce long-lasting plastic changes, which will activate secondary mechanisms in both the peripheral nervous system (PNS) and the central nervous system (CNS), leading to clinically relevant chronic pain syndromes.3 These are often manifested as hyperalgesia (increased pain sensitivity) and allodynia (pain in response to an innocuous stimulus).3
Chronic pain imposes major challenges as existing treatments are inadequate for the majority of patients and accompanied by adverse side effects.1,2 A reduction in pain (analgesia) can, in principle, be achieved by decreasing neuronal excitation, increasing inhibition or a combination of both. However, the targets of most interventions are widely expressed molecules involved in critical functions throughout the whole body, thereby accounting for the observed side effects and minimal safety profiles of current pain therapies.4-6
The development of new analgesic strategies has therefore focused on molecules that are highly enriched along pain transduction pathways among which are various ion channels and receptors.7 In this review, we discuss the significance of a number of ion channels and receptors (ion channels/receptors) as well as their modulation via protein-protein interactions (PPIs) in the context of pain. Moreover, we provide a critical appraisal of reported findings with implications for the design of novel pain therapies. Rather than providing a comprehensive literature review, we attempt to highlight studies that are representative for arising new developments in the pursuit of targeting ion channel/receptor-PPIs to relieve a variety of pain conditions in rodents. To this end, we focus on PPIs of selected ion channels/receptors (summarized in Table 1) with pivotal roles in detection and transmission of noxious stimuli along the pain axis, i.e. (i) several existing drug targets for human pain conditions,8 e.g. transient receptor potential vanilloid 1 (TRPV1) and voltage-gated calcium channels, (ii) proteins underlying human monogenic channelopathy-associated pain syndromes,9 e.g., voltage-gated sodium channels and transient receptor potential ankyrin 1 (TRPA1), and (iii) the large family of glutamate receptors crucially involved in central processes of nociceptive plasticity.
Table 1.
Ion channel/receptor-associated PPIs with in vivo relevance for pain sensation in rodents. This list does not represent a complete literature overview, but summarizes PPIs with proven functional significance for rodent pain behaviors as described in this review
| Channel/Receptor | Interacting protein | Modifying action | Reference |
|---|---|---|---|
| AMPAR | Stargazin | Knockdown attenuates AMPAR-dependent inflammatory pain | 117 |
| Knockdown attenuates post-operative pain | 118 | ||
| GRIP | Stabilizing interaction attenuates inflammatory pain | 113,115 | |
| Blocking interaction attenuates neuropathic pain | 110 | ||
| PICK1 | Blocking interaction attenuates neuropathic pain | 110 | |
| NSF | Blocking interaction attenuates neuropathic pain | 110 | |
| Cav2.2 | CRMP-2 | Disrupting interaction attenuates acute, inflammatory and neuropathic pain | 80 |
| Cav3.2 | USP5 | Disrupting interaction attenuates acute, inflammatory and neuropathic pain | 71,72 |
| mGluR1/5 | Homer proteins | Splice variant-dependent interaction affects inflammatory pain | 102 |
| Nav1.8 | Aquaporin-1 | Knockout exhibits reduced inflammatory pain | 65 |
| P11 | Knockout attenuates acute and neuropathic pain | 64 | |
| NMDAR | PSD-95 | Disrupting interaction attenuates inflammatory and neuropathic pain | 99 |
| Disrupting PSD-95-nNOS interaction disrupts NMDAR-PSD-95 interaction and reverses neuropathic pain | 100 | ||
| Src | Disrupting interaction attenuates inflammatory and neuropathic pain | 93 | |
| TRPA1 | AnxA2 | Knockout exhibits increased TRPA1-mediated pain | 55 |
| TRPV1 | AKAP79 | Preventing interaction attenuates inflammatory pain | 43 |
| GABAB1 | Activation blocks TRPV1-mediated pain hypersensitivity | 44 | |
| PIRT | Knockout exhibits reduced TRPV1-mediated pain | 45 | |
| TRPA1/Tmem100 | Tmem100 mutant enhances TRPV1-TRPA1 association and inhibits TRPA1-mediated pathological pain; Tmem100 knockout exhibits reduced TRPA1-mediated pain |
54 |
Rationale for targeting ion channels and receptors in pain
The identification of certain ion channels/receptors as noxious stimulus detectors in peripheral sensory neurons undoubtedly represents one of the great achievements of modern pain research. Besides stimulus detection, ion channels/receptors encode painful stimuli into a discharge of nerve impulses, determine nociceptor excitability and the activity of nociceptive pathways in the CNS. Hence, abnormalities in their function influence the perception of pain. Evidence for their crucial role is provided by targeted ablation in animals, as well as genetic studies identifying variants of ion channels that modulate the risk, severity and persistence of pain in humans.9-11
Functional alterations of ion channels/receptors reflect events that involve PPIs, such as alteration of intrinsic channel properties (activation threshold, open-probability, (in)activation kinetics), altered expression, trafficking, posttranslational modifications and turnover.7,10 The consequences of these processes include ectopic activity of nociceptors as well as facilitation and disinhibition of synaptic transmission in the CNS,8 all of which contribute to pain and increased tissue sensitivity.12 Therefore, a detailed understanding of the ion channels/receptors involved in the pathophysiology of pain conditions would provide far-reaching opportunities for the development of novel therapeutic strategies aimed specifically at the pain transduction pathway.
Such ion channel/receptor-based analgesia may include agonists to activate inhibitory pathways, desensitize and ablate nociceptors (e.g. high-dose capsaicin patches), as well as antagonists to reduce their activity in the periphery and the CNS.7,13 However, even specifically blocking the activity of ion channels enriched in nociceptors has proven to be difficult and prone to adverse side-effects. Prime example is TRPV1, a noxious heat sensor. Several TRPV1 antagonists were developed, but failed in clinical trials due to the occurrence of hyperthermia and impaired noxious heat sensation, rendering common daily activities (e.g., taking a shower or consuming hot beverages) potentially dangerous.14 While these adverse effects might be circumvented by state-dependent blockers (i.e., antagonists specifically blocking sensitized or excessive ion channel activity), ion channel/receptor-based analgesia is additionally complicated by the inability to direct the drug-action to a specific tissue. For example, µ-opioid receptors are widely expressed throughout the body, hence application of their agonists (e.g. morphine) is accompanied with severe side-effects in the CNS (sedation, drowsiness) and the intestine (constipation).15 In the light of these difficulties, alternative strategies are in high demand and need to be considered in parallel.
Modulation of ion channel and receptor function via protein-protein interactions
A promising approach aims at affecting PPIs.16 Assembly into dynamic multi-protein complexes determines and modulates the multiple functions of a single protein and signaling pathways within a cell – a fact that became known as the concept of cellular “molecular machines.”17-19 Consequently, identifying the composition of such complexes and deciphering PPI networks can greatly facilitate insights into distinct signaling pathways. Targeting PPI networks therefore holds the opportunity to modulate specific pathways downstream of ion channel/receptor activation, which may provide 2 major benefits: (i) increase of tissue specificity and (ii) possibilities for subtle tuning of cellular functions.15 Taking into account these potential advantages of PPI-directed therapeutics, it is not surprising that they are considered a “gold mine” for drug development.20 Because cellular PPI networks are vast and essential, they theoretically bear many potential sites for targeted interference. Over the past years, considerable research efforts have been placed to identify specific PPI inhibitors, modulators and stabilizers.17 The results are promising and currently several PPI-modulating drugs are available on the market for various diseases. For example, the anti-HIV drug Maraviroc (Celsentri® from Pfizer), which functions as an entry-inhibitor by preventing the binding of viral gp120 with the chemokine receptor CCR5. In contrast, the immunosuppressive actions of Sirolimus (Rapamune® from Pfizer) depend on its ability to stabilize the binding of the receptor protein FKBP12 and the FKBP12-rapamycin binding domain of mTOR.21
Several other PPI-modulating agents have been identified and are of great clinical interest for cancer therapy and other diseases.17,22,23 In regard to PPI modulation in the nervous system, a prominent study by William Catterall and colleagues described the efficacy of peptides containing the synaptic protein interaction (synprint) site to dissociate N-type calcium channels from the synaptic core complex, thereby reducing synaptic transmission.24 These findings were later extended by the identification of several inhibitory peptides that prevent G-protein-mediated inhibition of calcium channels.25-27
Meanwhile, our knowledge about ion channel/receptor-associated PPIs in the nervous system and their impact on synaptic transmission has progressed rapidly, which can be attributed to increasingly sensitive techniques for their identification, including mass spectrometry-based interactomics.18,28,29 Although these studies are of outstanding interest, their detailed description is beyond the scope of this review. Instead, we will specifically focus on the modulation of selected ion channel/receptor-associated PPIs that are relevant for nociception, as shown by their potency to alter pain perception in rodents. Mere in vitro studies of PPIs lacking in vivo data on rodent pain behaviors are therefore excluded from our review.
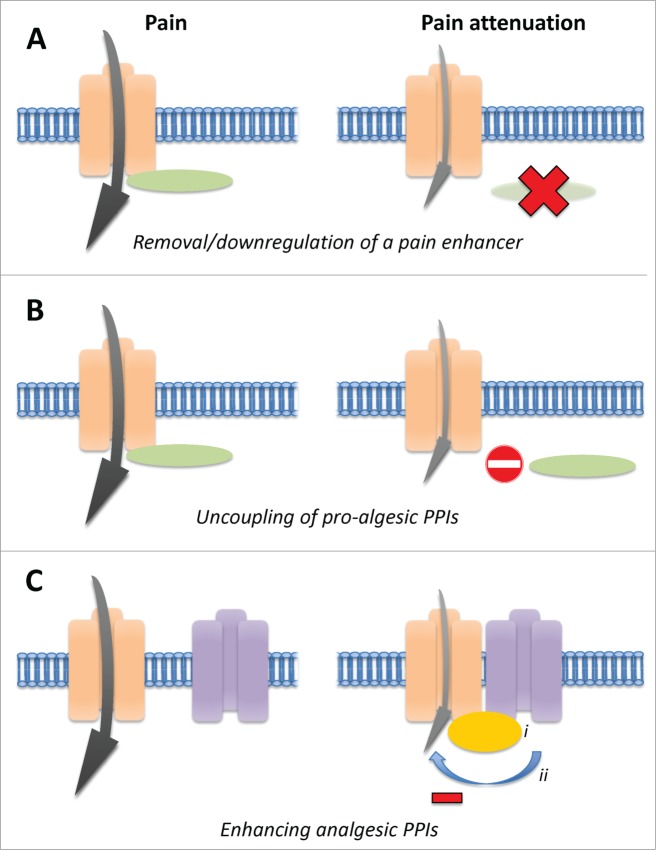
Technically, the modulation of PPIs to alter ion channel/receptor function with the aim to attenuate pain behaviors in vivo might be achieved by several means (Fig. 1). On the one hand, researchers identified proteins that enhance the activity of a pro-nociceptive ion channel, hence they are considered as pain enhancers. Consequently, either their removal or dissociation from the ion channel (referred to as uncoupling of the PPI) would lead to decreased pain behaviors. To achieve the first, gene deletion and siRNA-mediated knockdown may be employed. For the latter, direct injection of interfering peptides (often fused to the TAT domain of HIV to confer cell permeability) or virus-mediated delivery of peptide-encoding DNA has proven to be effective in many studies. On the other hand, a growing body of evidence describes the existence of PPIs with analgesic potential. Cellular mechanisms may involve direct inhibition of ion channel/receptor activity by an associated protein or the stabilization of such an interaction via additional interaction partners (Fig. 1C). Exploiting these analgesic PPIs to shut down excessive activity of pro-nociceptive ion channels/receptors during chronic pain states holds considerable promise for powerful therapeutic opportunities.
Figure 1.
Strategies for pain relief targeting protein-protein interactions of ion channels/receptors in vivo. The gray arrow symbolizes the activity of the pro-algesic ion channel/receptor. (A) Knockout or knockdown of a pain-enhancing interactor. (B) Uncoupling of the physical interaction between a pro-algesic ion channel/receptor and its activating interactor. (C) Enhancing analgesic PPIs by i) introducing a stabilizing protein or ii) activating inhibitory signaling pathways.
In the following sections, we will introduce and discuss representative examples of recent research efforts targeting a number of promising ion channel/receptor-PPIs to relieve a variety of pain conditions in rodents (summarized in Table 1).
Protein complexes associated with TRPA1 and channels
TRPA1 and TRPV1 channels belong to the large family of mammalian TRP ion channels and are highly expressed in nociceptive neurons.7 They are activated by a plethora of plant-derived compounds, natural irritants (e.g., TRPV1 by capsaicin, an active ingredient of chili peppers, and TRPA1 by mustard oil, an active ingredient in mustard), chemicals, and toxins, which endows them with the capacity to function as primary cellular sensors.7,30-32 In fact, Trpv1 and Trpa1 knockout mice exhibit impaired pain behavior to distinct noxious stimuli and profound deficiencies in models of inflammatory pain.31,33-35 Moreover, there is strong evidence for their involvement in human pain syndromes: gain-of-function mutations in human TRPA1 channels were found to be associated with familial episodic pain syndrome (FEPS), a rare but highly debilitating disorder characterized by sudden attacks of intense pain.36 TRPV1 channels, on the other hand, represent a major existing drug target for the treatment of several human pain conditions with capsaicin and resiniferatoxin patches.7,8 Therefore, the channel attracted attention to design novel analgesics and several TRPV1-antagonist have already reached preclinical trials.7 As mentioned earlier in this review, their safety profile has to be closely monitored due to complications resulting in hyperthermia.
Similar to TRPA1, TRPV1 channels have been shown to be sensitized by several inflammatory mediators, such as Bradykinin (Bk) and Prostaglandin-E2 (PGE2). Mechanistically, TRPV1 phosphorylation by downstream effectors like protein kinase C (PKC) and protein kinase A (PKA) seems to be involved.37-42 Interestingly, A-kinase anchor protein 79/150 (AKAP79/150) binds to TRPV1 and seems to be required for its sensitization via PGE2/PKA and Bk/PKC in vitro.39-42 Uncoupling the AKAP79/150-TRPV1 interaction has recently been shown by Fischer et al. to be a promising strategy to prevent TRPV1-mediated pain hypersensitivity. The authors defined the key residues in the AKAP-binding site of TRPV1 and designed a peptide (fused to the TAT sequence of HIV to confer cell permeability) capable of disrupting this interaction. Pain-related behavior after injection of formalin and carrageenan-induced inflammatory thermal hyperalgesia were reduced in mice pre-injected with this peptide. Altogether, these results suggest that TRPV1 sensitization by inflammatory mediators can be attenuated by uncoupling of the AKAP79/150-TRPV1 interaction.43 To which extent also one of the other interactors of AKAP might be mechanistically involved needs to be elucidated.
A very recent study by Hanack et al. provides new insights, specifically into TRPV1 sensitization and its modulation by the GABAB1 receptor subunit.44 The GABAB receptor has long been known to provide inhibitory input to pain transduction. This new work mechanistically investigated its action, and demonstrated that activation of GABAB1 selectively reverts the sensitized state of TRPV1 channels implicated in pathological pain but leaves acute TRPV1 pain signaling intact. Interestingly, GABAB1 is activated by its endogenous agonist GABA, which itself is released from hyper-active nociceptive nerve terminals expressing TRPV1. Hence, this study revealed the existence of an autocrine feedback mechanism to counteract excessive TRPV1 activity based on physical and functional interaction of TRPV1 with GABAB1 (according to the principle described in Fig. 1C). The exact mechanism how GABAB1 regulates the sensitization status of TRPV1 remains to be investigated, but may involve PKC phosphorylation-dependent TRPV1 gating.
The above-mentioned examples fundamentally confirm the feasibility of attenuating pain either via uncoupling of pro-algesic PPIs or stabilization of analgesic PPIs (see also Fig. 1). It is noteworthy that pain thresholds in the absence of inflammation were unaffected by AKAP79/150 and GABAB1, which is a critical requirement for translating these findings into future analgesic strategies.
This is in contrast to the regulation of TRPV1 exerted by its binding partner Pirt (Phosphoinositide-interacting regulator of TRP).45 Pirt knockout mice display reduced TRPV1 activity in sensory neurons and impaired basal pain behaviors to TRPV1, capsaicin and noxious heat. These results lead to the notion of Pirt being a key component of the TRPV1 protein complexes. However, the functional relevance of Pirt might extend beyond TRPV1 channels, as (i) Pirt interacts with PIP2 known to be linked to ion channel function in many contexts, (ii) Pirt regulates TRPM846 and potentially other TRP channels and (iii) Pirt knockout mice exhibit deficits in TRPV1-independent models of itch.47 Despite its seemingly widespread functions, Pirt might still serve as a potential therapeutic target for pain and itch considering its limited and specific expression in somatosensory neurons.45 Many more putative interactors of TRPV1 are described; however, only few of them have been shown to have functional consequences in vivo. An overview is given by the TRIP database, a manually curated database containing interacting proteins for all mammalian TRP channels.48
In addition, a physical and functional interaction of TRPV1 and TRPA1 channels has been described. TRPV1 and TRPA1 are largely co-expressed in sensory neurons and several studies suggest that they form heteromers,49-51 which affects their functional properties and sensitization.49,51-53 Just recently, the membrane adaptor protein Tmem100 was found to bind both TRP channels, thereby weakening their association and selectively potentiating TRPA1 activity.54 A Tmem100 mutant, Tmem100–3Q, and its mimicking cell-permeable peptide show the opposite effect; i.e. it stabilizes the association of TRPA1 and TRPV1, attenuates TRPA1 responses in vitro and blocks TRPA1-mediated inflammatory pain as well as chemotherapy-induced neuropathy.54 This result adds to the growing body of evidence for the physiological relevance of stabilizing analgesic PPIs and their enormous potential to specifically interfere with the activity of one interaction partner (in this case TRPA1), while leaving the function of the other intact (in this case TRPV1).
Furthermore, we reported the physical association of TRPA1 with AnnexinA2 (AnxA2), a calcium-regulated protein implicated in membrane transport processes.55 AnxA2 seems to limit the availability of TRPA1 at the plasma membrane of sensory neurons, which could contribute to increased TRPA1-mediated pain behaviors we observed in AnxA2 knockout mice. Strikingly, TRPV1-dependent nociceptive signaling was unaffected by AnxA2, suggesting a certain degree of specificity – a fact that could be therapeutically exploited.55 However, analgesic strategies based on the AnxA2-TRPA1 interaction might presumably be limited to local applications in the periphery due to ubiquitous functions of AnxA2 as a calcium effector protein mediating various membrane transport processes.56
Voltage-gated sodium channels and their associated proteins
Throughout the nervous system, voltage-gated sodium channels (VGSCs) are indispensable for action potential propagation and nerve excitability.57 Thus, changes in VGSC function can have profound effects on pain signaling, a fact which is exploited by local anesthetics, such as lidocaine.13,58,59 In nociceptive neurons of the PNS the tetrodotoxin-sensitive (TTX-S) channel Nav1.7 and 2 tetrodotoxin-resistant (TTX-R) channels, Nav1.8 and Nav1.9, are abundantly expressed. Mutations of Nav1.7 have been linked to 3 human pain disorders including congenital insensitivity to pain.57 In general, sodium channel blockers effectively relieve pain, however, with narrow safety windows due to their widespread function (e.g. cardiac side effects).57-59
VGSCs form multi-protein complexes consisting of pore-forming α-subunits associated with regulatory β-subunits and a plethora of accessory proteins necessary for their dynamic regulation and trafficking in different cell types. These regulatory subunits are often shared among VGSC isoforms.57 In contrast, modulating accessory proteins may provide a mechanism for selective control over individual Nav isoforms. The latter is exemplified by poor expression of Nav1.8 in heterologous cells, which can be overcome by the co-transfection of P11.60,61 P11 (also known as S100A10) is a member of the S100 family of calcium-binding proteins62 and promotes Nav1.8 membrane translocation and function in cultured sensory neurons.61,63 This regulation is specific to Nav1.8, while other VGSCs are unaffected.61 Furthermore, P11 deletion in nociceptors in mice showed reduced TTX-R current density and attenuated pain behaviors mostly similar to those of the Nav1.8 null mutant.64
Zhang et al. uncovered the interaction of Nav1.8 with the water-transporting protein Aquaporin-1 (AQP1) in mouse sensory neurons.65 Behavioral studies in Aqp1 knockout mice showed greatly reduced thermal inflammatory pain perception evoked by Bk, PGE2 and capsaicin as well as reduced cold pain perception. Electrophysiological recordings revealed impaired Nav1.8 function in the absence of AQP1 indicating that the interaction between the channels accounts, at least in part, for the observed phenotype.65
Our knowledge on PPIs of VGSCs relevant for pain perception is still scarce. Nevertheless, continuous research efforts focused on determining PPIs specific for VGSC isoforms or their accessory subunits might yield fruitful insights and could become the source of novel VGSC-based therapeutics.
Protein-protein interactions of voltage-gated calcium channels
Several types of voltage-gated calcium channels (VGCCs) play a crucial role in signal transmission in the pain pathway with regard to modulation of the general neuronal excitability and presynaptic neurotransmitter release. Moreover, changes in their expression and/or activity facilitate pain hypersensitivity in pathological conditions.66-68 However, the use of VGCC blockers is limited due to their narrow therapeutic window and systemic side effects such as hypotension and memory loss.69 Instead, the idea of targeting PPIs to selectively interfere with the – often aberrant – activity of VGCCs during chronic pain states yielded promising results in several studies.
For example, in mice Cav3.2 ion channel protein levels and function was found to be upregulated in diverse chronic pain conditions (originating from inflammation, diabetes or nerve injury).68 While various mechanisms might contribute to Cav3.2 hyperactivity, several reports hint toward the involvement of posttranslational modifications and proteasomal degradation of the channels.70-72 The latter appears to be counteracted by the deubiquitinating enzyme USP5. Garcia-Caballero and colleagues observed that knockdown of USP5 facilitates Cav3.2 ubiquitination and concomitantly decreased Cav3.2 protein levels as well as currents.71,72 These results correlated very well with analgesia in mouse models of inflammatory and neuropathic pain upon in vivo knockdown of USP5 or uncoupling the Cav3.2-USP5 interaction using TAT peptides.71
In a very recent follow-up study the same group confirmed the efficacy of this Cav3.2-USP5-TAT peptide in animal models of diabetic neuropathy and acute visceral pain.72 In addition, they screened a library of bioactive small molecules and found 2 compounds (suramin and gossypetin) capable of disrupting the Cav3.2-USP5 interaction. Both compounds turned out to protect mice from neuropathic and inflammatory pain upon intrathecal injection, and suramin was even effective in visceral and diabetic pain. Importantly, the implementation of careful controls, i.e., experiments in Cav3.2 null mice and electrophysiology to rule out direct inhibition of Cav3.2, established that the action of these compounds targeted the Cav3.2-USP5 interaction.72 Collectively these findings not only demonstrate that preventing pro-algesic PPIs relieves chronic pain (see also Fig. 1B) and may be achieved either by uncoupling peptides or by small molecules, but also highlight in an exemplary manner the necessity of meticulous controls to ensure on-target action of interventions.
Another prominent group of VGCCs, which are intimately linked to pain signaling are high voltage-activated (HVA) calcium channels. They form heteromultimeric protein complexes comprised of the pore-forming α1-subunit and auxiliary subunits (α2δ-, β- and γ-subunits).73 The latter are proposed to modulate cell surface expression, gating properties of HVA channels and potentially the susceptibility to second messengers, offering the possibility to control HVA channel function.74-76 This is exemplified by pregabalin, an anti-epileptic drug often used to treat several chronic pain syndromes in humans.5 The analgesic properties of pregabalin are not fully understood and several mechanisms have been proposed,66,77 e.g., inhibition of neuropathic pain-induced membrane trafficking of the α2δ1-subunit and concomitantly prevention of excessive HVA channel function.78,79
In contrast to the inhibitory action of pregabalin, collapsin response mediator protein 2 (CRMP-2) was reported to bind to HVA N-type calcium channels (Cav2.2) resulting in increased surface expression as well as calcium currents in hippocampal and sensory neurons.80-83 Further work established the notion that CRMP-2 expression levels allow the control of Cav2.2 function, nociceptor excitability and transmitter release.80,83 These findings prompted the mapping of the mutual binding sites of CRMP-2 and Cav2.2 with the goal to uncouple their physical interaction and thereby antagonize CRMP-2-mediated Cav2.2 hyperactivity.80,84 Several synthetic CRMP-2 as well as calcium channel peptides were fused to the TAT protein and significantly alleviated different pain conditions in mice upon their injection.80,84,85 For example, a seminal study by Brittain and colleagues showed that a CRMP-2 peptide was capable of effectively suppressing many of the reported functions of CRMP-2 within the nociceptive pathway, i.e. (i) the Cav2.2-CRMP-2 interaction, (ii) calcium currents in DRG and (iii) synaptic transmission as well as calcitonin gene related peptide (CGRP) release in the spinal cord. In addition, they observed a pronounced reduction of acute, inflammatory and neuropathic pain using various mouse models.80 Despite the fact that pharmacological blockade of N-type channels has been linked to anxiety and depression, CRMP-2 peptides were only mildly anxiolytic without detectable effects on depression, sensorimotor function or memory retrieval.80 Taken together, these studies provide unambiguous evidence for the impact of PPI on ion channels modulation specifically during pain states and simultaneously offer an elegant way to interfere with dysregulated ion channels for pain relief with minimized side effects.
Interestingly, CRMP-2 was initially identified in a screen for presynaptic Cav2.2-interacting proteins via a mass spectrometry-based proteomics approach.87 The significance of this discovery with respect to understanding the molecular underpinnings of chronic pain and its potential application in pain therapy highlights the enormous potential of mass spectrometry-based proteomics screens. Constant progress is made to enhance the sensitivity, accuracy and reproducibility of mass spectrometers, which is expected to provide detailed information about the composition of protein complexes associated with ion channels/receptors.86-88
Protein complexes regulating glutamate receptors
Glutamate is a major excitatory neurotransmitter and its receptors are ubiquitously expressed in the CNS and PNS.89 Glutamate receptors can be divided into 2 groups, ionotropic glutamate receptors (N-methyl-D-aspartate (NMDA) receptors, kainate receptors, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors) and metabotropic glutamate receptors (mGluRs). Owing to their crucial function in synaptic transmission and plasticity throughout the nervous system, their involvement in nociceptive signaling, central sensitization (i.e., activity-dependent and persistent increase of neuronal excitability in the dorsal horn), and pain hypersensitivity has been a matter of intensive research.66,90-92 While blockade of glutamate receptors to relieve acute and chronic pain states is effective, but therapeutically challenging,4 a growing body of evidence suggests that chronic pain-associated hypersensitivity can be attenuated with minimal side effects by uncoupling PPIs with glutamate receptors using TAT fusion peptides. Very promising strategies target the interaction with “regulatory hubs” through which other proteins co-assemble with NMDA receptors and facilitate receptor activity.93 Among them are postsynaptic density protein (PSD) 95 and the protein tyrosine kinase Src. PSD-95 plays a major role in spinal mechanisms governing central sensitization following noxious stimulation.94-98 The resulting synaptic plasticity is largely mediated by NMDA receptor subunits that bind to PSD-95 as manifested by experiments which aimed to selectively disrupt this interaction in the dorsal horn of rats. D'Mello and colleagues intrathecally injected a TAT fusion peptide harboring the PSD-95 binding domain of NMDA receptor subunit 2 (NR2B) and observed a reduction of NMDA receptor-dependent mechanical and cold hypersensitivity in rats with nerve injury-induced pain.99 Another signaling protein that is assembled together with NMDA receptors and PSD-95 is neuronal nitric oxide synthase (nNOS). Disruption of the nNOS-PSD-95 interaction by intrathecal injection of specific inhibitors reversed NMDA-induced thermal hyperalgesia in mice and mechanical allodynia following chronic constriction injury of the sciatic nerve.100 In addition, NR2B interacts with Src, which is physiologically relevant for inflammatory, i.e. formalin- and complete Freund´s adjuvant (CFA)-induced pain, as well as neuropathic pain upon peripheral nerve injury.93 Like in the studies mentioned above, these pain behaviors can be suppressed by intrathecal or intravenous injection of TAT-Src40–49, a peptide consisting of the amino acids 40–49 of Src representing the NMDA receptor binding domain. Mechanistically, these results may be explained by a reduction of Src-dependent NR2B phosphorylation, as repressing Src activity with a broad-spectrum protein tyrosine phosphatase inhibitor also alleviates thermal hypersensitivity elicited by CFA.93,101 It is remarkable that removal of Src from NMDA receptor complexes neither affected basal sensory thresholds and acute nociception nor locomotor and cognitive functions. This strongly suggests that specific alterations of NMDA receptor-harboring protein complexes in the dorsal horn may prove useful for relieving pain syndromes with minimized side effects.93,99,100
The extent and central role of multi-protein complexes surrounding NMDA receptors is highlighted by additional studies showing that the long-form Homer proteins, Homer1b and Homer1c, link metabotropic glutamate receptors (mGluR1 and mGluR5) to NMDA receptors at the cell surface.102,103 These interactions are antagonized by Homer1a, a short form of the Homer1 protein, which is upregulated in the spinal dorsal horn in rodent models of NMDA receptor–dependent peripheral inflammation and neuropathy.102 Consequently, Homer1a uncouples glutamatergic receptors from nociceptive signaling events in the dorsal horn and counteracts central sensitization, thereby reducing inflammatory hyperalgesia in mice. It is noteworthy, that transgenic mice overexpressing Homer1a in the forebrain also displayed diminished inflammatory pain behaviors, establishing Homer1a as an endogenous attenuator of nociceptive signaling along the pain transduction pathway.104
Besides NMDA and metabotropic glutamate receptors, AMPA receptors also display dynamic regulation during acute and chronic pain states.105,106 Mechanistically, several PPI-dependent processes have been reported to contribute to these events via AMPA receptor subunit-specific internalization and surface trafficking with concomitant changes in calcium permeability.107-113 Among them are interactions between the AMPA receptor subunit GluR2 and glutamate receptor-interacting protein (GRIP) as well as intracellular adaptor proteins such as protein interacting with C-Kinase (PICK1) and N-ethylmaleimide sensitive fusion protein (NSF) – all of which are linked to the maintenance of hyperalgesia following nerve injury.110,114
Many studies contributed to these findings and described the involvement of these interactions in other pain models; a complete listing of all is beyond the scope of this review. Taken together, the picture is emerging that the interplay of PPIs with AMPA receptors is rather complex with distinct contributions of certain interactions depending on the model used.110,111,113-116 For example, immunoprecipitation studies indicate that CFA-induced inflammation reduces the GluR2-GRIP interaction in the dorsal horn, whereas GluR2-PICK1 remains unaffected. As a consequence, GluR2 internalization is induced in a PKC phosphorylation-dependent manner and entails an increase in the calcium permeability of AMPA receptors resulting in CFA-induced hypersensitivity.113,115 The authors exploited this mechanism and mutated the GluR2 PKC phosphorylation site to prevent CFA-induced GluR2 internalization alongside with alleviation of CFA-evoked nociceptive hypersensitivity.113 Interestingly, PKC activation is triggered by spinal NMDA receptors, again exemplifying their principal position in nociceptive plasticity.113
Moreover, NMDA and AMPA receptors are functionally and physically linked via a PSD-95-stargazin bridge in the dorsal horn.113 Stargazin is a member of the AMPA receptor regulatory protein family and binds to the AMPA receptor subunit GluR1.117,118 In addition, stargazin represents a pain susceptibility gene in humans and its polymorphisms are associated with chronic pain after breast surgery in cancer patients.112,119 This function correlates well with studies in rodents implicating the stargazin-GluR1 interaction in formalin-induced inflammatory pain as well as in post-operative pain. In respect to the latter, plantar incision in rats enhanced stargazin binding to GluR1 in the dorsal horn as evidenced by co-immunoprecipitation studies, which could be counteracted by prior intrathecal administration of stargazin siRNA.117,118 Furthermore, downregulation of stargazin prior to incision alleviated post-operative pain in rats, opening the exciting possibility for its preventive use prior to surgical procedures.118
Conclusions
Numerous studies have established by now that ion channels/receptors are embedded into protein complexes, which critically influence their function. Importantly, these PPIs can be dynamic and specifically occur under certain (patho-)physiological states. A corollary of this concept is that the identification and characterization of PPIs can provide a stepping stone for further insights into ion channel/receptor function, specifically during disease states. The examples described in this review highlight the potential of such investigations to explore new molecular players relevant for nociceptive signaling. As a result of these promising studies in rodent models of pain, mounting interest has developed to translate PPI-targeting approaches to human pain syndromes, with the hope of gaining improved clinical efficacy and safety profiles. However, several questions remain unanswered and require careful considerations before the advantages of PPI modulators can be fully exploited for relieving human pain.
Challenges in designing PPI modulators
Albeit many promising examples in animal models, clinical trials and approved drugs for several diseases targeting PPIs for therapeutic interventions is still in development and subject to major research and optimization efforts.17 Regarding their chemical composition, most PPI modulators considered to date are either peptides of peptidomimetic nature belonging to the group of small-molecule modulators, i.e., naturally occurring, or synthetic agents exhibiting a significant degree of structural complexity to allow selective and efficient binding.17 Regardless of their structure, several challenges in PPI modulator design have to be overcome, such as (i) large, hydrophobic and often undefined interaction interfaces, (ii) low stability and proteolytic lability of peptide-based modulators, as well as (iii) cell permeability.17,22,81
Based on the assumption that PPI interfaces are large and lack defined features, considerable concerns have been raised whether required selectivity and potency in targeting PPIs might be achievable.17 However, several studies support the notion that PPIs can be selectively and effectively targeted via so-called hot spots (a subset of a few amino acids mainly involved in protein binding) or post-translational modifications.17,22,120,121 Significant advances in determining PPI interfaces can also be expected from the ever increasing knowledge about the structure and, consequently, functional domains of ion channels/receptors. For example, the very recently published cryoelectron microscopy structure on TRPA1 channels reveals information about its stability, regulation and putatively “druggable” binding sites.122
While peptides are considered as excellent leads for the development of PPI modulators, the hurdles associated with their proteolytic lability, hydrophobicity and consequently limited cell permeability require novel approaches for rational chemical modifications.17,81 Among them are the development of smart linkers to increase stability, conjugation of the modulating peptide with cell-penetrating peptides (such as TAT domains mentioned throughout this review), as well as bioisosteric modifications. The latter involves directed replacement of chemical groups with the goal to maintain desired biological properties while simultaneously attenuating unwanted characteristics of a given compound.
Are PPI modulators effective, safe and tolerated in humans?
The existing treatment options for chronic pain syndromes are limited, mainly due to adverse side effects, tolerance and low efficacy among patients. The hopes are high that PPI modulators minimize these hurdles, as they are designed to selectively bind distinct sites of specific proteins, thereby enhancing efficacy, selectivity and specificity.81 This notion is reinforced by available therapeutics targeting PPIs in cancer, cardiovascular diseases and HIV infections.22 Peptide-based modulators represent valuable starting points for safe and tolerable drug design due to their specificity (ergo, low risk of triggering off-target effects), their low immunogenicity and toxicity.17,81
Nonetheless, the safety and tolerability of each new PPI modulator will have to be carefully investigated, especially when considering the heterogeneity of pain syndromes and the wealth of proteins involved. To this end, advances in several disciplines have to be bundled, such as rationale PPI modulator design, research efforts aimed at understanding the molecular signature underlying defined pathologies,11,16 screening technologies reflecting these pathological conditions, functional validation of PPI modulation in defined model systems, as well as stratification of chronic pain patients.17 The combination of these efforts will likely open new avenues to exploiting PPIs for pain therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Dr. Ben Cooper (MPI for Experimental Medicine, Göttingen) for critically reading the manuscript.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft through the Emmy Noether-Program of the DFG (SCHM 2533/2–1; to M.S.) and the Collaborative Research Center 889 (project A9 to M.S.). Julia Sondermann is the recipient of a PhD fellowship of the Max Planck Society.
References
- 1.Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron 2007; 55:353-64; PMID:17678850; http://dx.doi.org/ 10.1016/j.neuron.2007.07.016 [DOI] [PubMed] [Google Scholar]
- 2.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008; 70:1630-5; PMID:18003941; http://dx.doi.org/ 10.1212/01.wnl.0000282763.29778.59 [DOI] [PubMed] [Google Scholar]
- 3.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 2007; 55:365-76; PMID:17678851; http://dx.doi.org/ 10.1016/j.neuron.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 4.Lynch ME, Watson CP. The pharmacotherapy of chronic pain: a review. Pain Res Manag 2006; 11:11-38; PMID:16511612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, et al.. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007; 132:237-51; PMID:17920770; http://dx.doi.org/ 10.1016/j.pain.2007.08.033 [DOI] [PubMed] [Google Scholar]
- 6.Gilron I, Jensen TS, Dickenson AH. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol 2013; 12:1084-95; PMID:24074723; http://dx.doi.org/ 10.1016/S1474-4422(13)70193-5 [DOI] [PubMed] [Google Scholar]
- 7.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov 2009; 8:55-68; PMID:19116627; http://dx.doi.org/ 10.1038/nrd2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009; 32:1-32; PMID: 19400724; http://dx.doi.org/ 10.1146/annurev.neuro.051508.135531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raouf R, Quick K, Wood JN. Pain as a channelopathy. J Clin Invest 2010; 120:3745-52; PMID: 21041956; http://dx.doi.org/ 10.1172/JCI43158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett DL, Woods CG. Painful and painless channelopathies. Lancet Neurol 2014; 13:587-99; PMID:24813307; http://dx.doi.org/ 10.1016/S1474-4422(14)70024-9 [DOI] [PubMed] [Google Scholar]
- 11.Delmas P. SnapShot: ion channels and pain. Cell 2008; 134:366-e1; PMID:18662550; http://dx.doi.org/ 10.1016/j.cell.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 12.Gold MS. Ion channels: recent advances and clinical applications. In: Flor H, Kaslo E, Dostrovsky JO, eds. Proceedings of the 11th World Congress on Pain. Seattle, WA: IASP Press; 2006; pp. 73-92. [Google Scholar]
- 13.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010; 9:807-19; PMID:20650402; http://dx.doi.org/ 10.1016/S1474-4422(10)70143-5 [DOI] [PubMed] [Google Scholar]
- 14.Lee Y, Hong S, Cui M, Sharma PK, Lee J, Choi S. Transient receptor potential vanilloid type 1 antagonists: a patent review (2011 – 2014). Expert Opin Ther Pat 2015; 25:291-318; PMID:25666693; http://dx.doi.org/ 10.1517/13543776.2015.1008449 [DOI] [PubMed] [Google Scholar]
- 15.Blazer LL, Neubig RR. Small molecule protein-protein interaction inhibitors as CNS therapeutic agents: current progress and future hurdles. Neuropsychopharmacology 2009; 34:126-41; PMID:18800065; http://dx.doi.org/ 10.1038/npp.2008.151 [DOI] [PubMed] [Google Scholar]
- 16.Jamieson DG, Moss A, Kennedy M, Jones S, Nenadic G, Robertson DL, Sidders B. The pain interactome: connecting pain-specific protein interactions. Pain 2014; 155:2243-52; PMID:24978826; http://dx.doi.org/ 10.1016/j.pain.2014.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinzalla G, Thurston DE. Targeting protein-protein interactions for therapeutic intervention: a challenge for the future. Future Med Chem 2009; 1:65-93; PMID:21426071; http://dx.doi.org/ 10.4155/fmc.09.12 [DOI] [PubMed] [Google Scholar]
- 18.Schulte U, Muller CS, Fakler B. Ion channels and their molecular environments–glimpses and insights from functional proteomics. Semin Cell Dev Biol 2011; 22:132-44; PMID:20934526; http://dx.doi.org/ 10.1016/j.semcdb.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 19.Alberts B. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell 1998; 92:291-4; PMID:9476889; http://dx.doi.org/ 10.1016/S0092-8674(00)80922-8 [DOI] [PubMed] [Google Scholar]
- 20.Mullard A. Protein-protein interaction inhibitors get into the groove. Nat Rev Drug Discov 2012; 11:173-5; PMID:22378255; http://dx.doi.org/ 10.1038/nrd3680 [DOI] [PubMed] [Google Scholar]
- 21.Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science 1996; 273:239-42; PMID:8662507; http://dx.doi.org/ 10.1126/science.273.5272.239 [DOI] [PubMed] [Google Scholar]
- 22.Ivanov AA, Khuri FR, Fu H. Targeting protein-protein interactions as an anticancer strategy. Trends Pharmacol Sci 2013; 34:393-400; PMID:23725674; http://dx.doi.org/ 10.1016/j.tips.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurie NA, Shih CS, Dyer MA. Targeting MDM2 and MDMX in retinoblastoma. Curr Cancer Drug Targets 2007; 7:689-95; PMID:18045074; http://dx.doi.org/ 10.2174/156800907782418266 [DOI] [PubMed] [Google Scholar]
- 24.Mochida S, Sheng ZH, Baker C, Kobayashi H, Catterall WA. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron 1996; 17:781-8; PMID:8893034; http://dx.doi.org/ 10.1016/S0896-6273(00)80209-3 [DOI] [PubMed] [Google Scholar]
- 25.Li X, Hummer A, Han J, Xie M, Melnik-Martinez K, Moreno RL, Buck M, Mark MD, Herlitze S. G protein beta2 subunit-derived peptides for inhibition and induction of G protein pathways. Examination of voltage-gated Ca2+ and G protein inwardly rectifying K+ channels. J Biol Chem 2005; 280:23945-59; PMID:15824105; http://dx.doi.org/ 10.1074/jbc.M414078200 [DOI] [PubMed] [Google Scholar]
- 26.Bucci G, Mochida S, Stephens GJ. Inhibition of synaptic transmission and G protein modulation by synthetic CaV2.2 Ca(2)+ channel peptides. J Physiol 2011; 589:3085-101; PMID:21521766; http://dx.doi.org/ 10.1113/jphysiol.2010.204735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamponi GW, Snutch TP. Advances in voltage-gated calcium channel structure, function and physiology. Biochim Biophys Acta 2013; 1828:1521; PMID:23518035; http://dx.doi.org/ 10.1016/j.bbamem.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 28.Liao L, McClatchy DB, Yates JR. Shotgun proteomics in neuroscience. Neuron 2009; 63:12-26; PMID:19607789; http://dx.doi.org/ 10.1016/j.neuron.2009.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhary C, Mann M. Decoding signalling networks by mass spectrometry-based proteomics. Nat Rev Mol Cell Biol 2010; 11:427-39; PMID:20461098; http://dx.doi.org/ 10.1038/nrm2900 [DOI] [PubMed] [Google Scholar]
- 30.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol 2013; 75:181-200; PMID:23020579; http://dx.doi.org/ 10.1146/annurev-physiol-030212-183811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288:306-13; PMID:10764638; http://dx.doi.org/ 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- 32.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389:816-24; PMID:9349813; http://dx.doi.org/ 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 33.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006; 50:277-89; PMID:16630838; http://dx.doi.org/ 10.1016/j.neuron.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 34.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006; 124:1269-82; PMID:16564016; http://dx.doi.org/ 10.1016/j.cell.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 35.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007; 445:541-5; PMID:17237762; http://dx.doi.org/ 10.1038/nature05544 [DOI] [PubMed] [Google Scholar]
- 36.Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR, et al.. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010; 66:671-80; PMID:20547126; http://dx.doi.org/ 10.1016/j.neuron.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW 4th. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci U S A 2003; 100:12480-5; PMID:14523239; http://dx.doi.org/ 10.1073/pnas.2032100100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem 2002; 277:13375-8; PMID:11884385; http://dx.doi.org/ 10.1074/jbc.C200104200 [DOI] [PubMed] [Google Scholar]
- 39.Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 2008; 28:4904-17; PMID:18463244; http://dx.doi.org/ 10.1523/JNEUROSCI.0233-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 2008; 138:604-16; PMID:18381233; http://dx.doi.org/ 10.1016/j.pain.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain 2009; 146:301-7; PMID:19767149; http://dx.doi.org/ 10.1016/j.pain.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008; 59:450-61; PMID:18701070; http://dx.doi.org/ 10.1016/j.neuron.2008.05.015 [DOI] [PubMed] [Google Scholar]
- 43.Fischer MJ, Btesh J, McNaughton PA. Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J Neurosci 2013; 33:7407-14; PMID:23616546; http://dx.doi.org/ 10.1523/JNEUROSCI.3721-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanack C, Moroni M, Lima WC, Wende H, Kirchner M, Adelfinger L, Schrenk-Siemens K, Tappe-Theodor A, Wetzel C, Kuich PH, et al.. GABA blocks pathological but not acute TRPV1 pain signals. Cell 2015; 160:759-70; PMID:25679765; http://dx.doi.org/ 10.1016/j.cell.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 45.Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 2008; 133:475-85; PMID:18455988; http://dx.doi.org/ 10.1016/j.cell.2008.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang Z, Kim A, Masuch T, Park K, Weng H, Wetzel C, Dong X. Pirt functions as an endogenous regulator of TRPM8. Nat Commun 2013; 4:2179; PMID:23863968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel KN, Liu Q, Meeker S, Undem BJ, Dong X. Pirt, a TRPV1 modulator, is required for histamine-dependent and -independent itch. PLoS One 2011; 6:e20559; PMID:21655234; http://dx.doi.org/ 10.1371/journal.pone.0020559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin YC, Shin SY, Chun JN, Cho HS, Lim JM, Kim HG, So I, Kwon D, Jeon JH. TRIP database 2.0: a manually curated information hub for accessing TRP channel interaction network. PLoS One 2012; 7:e47165; PMID:23071747; http://dx.doi.org/ 10.1371/journal.pone.0047165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem 2010; 285:15167-77; PMID:20231274; http://dx.doi.org/ 10.1074/jbc.M110.106153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci 2009; 29:1568-78; PMID:19419422; http://dx.doi.org/ 10.1111/j.1460-9568.2009.06702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer MJ, Balasuriya D, Jeggle P, Goetze TA, McNaughton PA, Reeh PW, Edwardson JM. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch 2014; 466:2229-41; PMID:24643480; http://dx.doi.org/ 10.1007/s00424-014-1497-z [DOI] [PubMed] [Google Scholar]
- 52.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol 2007; 583:175-93; PMID:17584831; http://dx.doi.org/ 10.1113/jphysiol.2007.133231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain 2008; 135:271-9; PMID:17590514; http://dx.doi.org/ 10.1016/j.pain.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z, Mo GC, Wang Y, et al.. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron 2015; 85:833-46; PMID:25640077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avenali L, Narayanan P, Rouwette T, Cervellini I, Sereda M, Gomez-Varela D, Schmidt M. Annexin A2 regulates TRPA1-dependent nociception. J Neurosci 2014; 34:14506-16; PMID:25355205; http://dx.doi.org/ 10.1523/JNEUROSCI.1801-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol 2005; 6:449-61; PMID:15928709; http://dx.doi.org/ 10.1038/nrm1661 [DOI] [PubMed] [Google Scholar]
- 57.Savio-Galimberti E, Gollob MH, Darbar D. Voltage-gated sodium channels: biophysics, pharmacology, and related channelopathies. Front Pharmacol 2012; 3:124; PMID:22798951; http://dx.doi.org/ 10.3389/fphar.2012.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood JN, Boorman JP, Okuse K, Baker MD. Voltage-gated sodium channels and pain pathways. J Neurobiol 2004; 61:55-71; PMID:15362153; http://dx.doi.org/ 10.1002/neu.20094 [DOI] [PubMed] [Google Scholar]
- 59.Ekberg J, Adams DJ. Neuronal voltage-gated sodium channel subtypes: key roles in inflammatory and neuropathic pain. Int J Biochem Cell Biol 2006; 38:2005-10; PMID:16919992; http://dx.doi.org/ 10.1016/j.biocel.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 60.Malik-Hall M, Poon WY, Baker MD, Wood JN, Okuse K. Sensory neuron proteins interact with the intracellular domains of sodium channel NaV1.8. Brain Res Mol Brain Res 2003; 110:298-304; PMID:12591166; http://dx.doi.org/ 10.1016/S0169-328X(02)00661-7 [DOI] [PubMed] [Google Scholar]
- 61.Okuse K, Malik-Hall M, Baker MD, Poon WY, Kong H, Chao MV, Wood JN. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature 2002; 417:653-6; PMID:12050667; http://dx.doi.org/ 10.1038/nature00781 [DOI] [PubMed] [Google Scholar]
- 62.Rescher U, Gerke V. S100A10/p11: family, friends and functions. Pflugers Arch 2008; 455:575-82; PMID:17638009 [DOI] [PubMed] [Google Scholar]
- 63.Poon WYL, Malik-Hall M, Wood JN, Okuse K. Identification of binding domains in the sodium channel NaV1.8 intracellular N-terminal region and annexin II light chain p11. FEBS Letters 2004; 558:114-8; PMID:14759526; http://dx.doi.org/ 10.1016/S0014-5793(03)01512-6 [DOI] [PubMed] [Google Scholar]
- 64.Foulkes T, Nassar MA, Lane T, Matthews EA, Baker MD, Gerke V, Okuse K, Dickenson AH, Wood JN. Deletion of annexin 2 light chain p11 in nociceptors causes deficits in somatosensory coding and pain behavior. J Neurosci 2006; 26:10499-507; PMID:17035534; http://dx.doi.org/ 10.1523/JNEUROSCI.1997-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Verkman AS. Aquaporin-1 tunes pain perception by interaction with Na(v)1.8 Na+ channels in dorsal root ganglion neurons. J Biol Chem 2010; 285:5896-906; PMID:20018876; http://dx.doi.org/ 10.1074/jbc.M109.090233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev 2014; 94:81-140; PMID:24382884; http://dx.doi.org/ 10.1152/physrev.00023.2013 [DOI] [PubMed] [Google Scholar]
- 67.Park J, Luo ZD. Calcium channel functions in pain processing. Channels (Austin) 2010; 4:510-7; PMID:21150297; http://dx.doi.org/ 10.4161/chan.4.6.12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simms BA, Zamponi GW. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 2014; 82:24-45; PMID:24698266; http://dx.doi.org/ 10.1016/j.neuron.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 69.Schmidtko A, Lotsch J, Freynhagen R, Geisslinger G. Ziconotide for treatment of severe chronic pain. Lancet 2010; 375:1569-77; PMID:20413151; http://dx.doi.org/ 10.1016/S0140-6736(10)60354-6 [DOI] [PubMed] [Google Scholar]
- 70.Orestes P, Osuru HP, McIntire WE, Jacus MO, Salajegheh R, Jagodic MM, Choe W, Lee J, Lee SS, Rose KE, et al.. Reversal of neuropathic pain in diabetes by targeting glycosylation of Ca(V)3.2 T-type calcium channels. Diabetes 2013; 62:3828-38; PMID: 23835327; http://dx.doi.org/ 10.2337/db13-0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia-Caballero A, Gadotti VM, Stemkowski P, Weiss N, Souza IA, Hodgkinson V, et al.. The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing Cav3.2 channel activity. Neuron 2014; 83:1144-58; PMID:25189210; http://dx.doi.org/ 10.1016/j.neuron.2014.07.036 [DOI] [PubMed] [Google Scholar]
- 72.Gadotti VM, Caballero AG, Berger ND, Gladding CM, Chen L, Pfeifer TA, Zamponi GW. Small organic molecule disruptors of Cav3.2 - USP5 interactions reverse inflammatory and neuropathic pain. Mol Pain 2015; 11:12; PMID:25889575; http://dx.doi.org/ 10.1186/s12990-015-0011-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 2005; 57:411-25; PMID:16382099; http://dx.doi.org/ 10.1124/pr.57.4.5 [DOI] [PubMed] [Google Scholar]
- 74.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci 2007; 28:220-8; PMID:17403543; http://dx.doi.org/ 10.1016/j.tips.2007.03.005 [DOI] [PubMed] [Google Scholar]
- 75.Hidalgo P, Neely A. Multiplicity of protein interactions and functions of the voltage-gated calcium channel beta-subunit. Cell Calcium 2007; 42:389-96; PMID:17629941; http://dx.doi.org/ 10.1016/j.ceca.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 76.Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function, and drug binding. J Bioenerg Biomembr 2003; 35:639-47; PMID:15000524; http://dx.doi.org/ 10.1023/B:JOBB.0000008028.41056.58 [DOI] [PubMed] [Google Scholar]
- 77.Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin–calcium channel alpha2-delta [Cavalpha2-delta] ligands. Pain 2009; 142:13-6; PMID:19128880; http://dx.doi.org/ 10.1016/j.pain.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 78.Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, et al.. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci 2009; 29:4076-88; PMID:19339603; http://dx.doi.org/ 10.1523/JNEUROSCI.0356-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hendrich J, Bauer CS, Dolphin AC. Chronic pregabalin inhibits synaptic transmission between rat dorsal root ganglion and dorsal horn neurons in culture. Channels (Austin) 2012; 6:124-32; PMID:22627148; http://dx.doi.org/ 10.4161/chan.19805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, et al.. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(+) channel complex. Nat Med 2011; 17:822-9; PMID:21642979; http://dx.doi.org/ 10.1038/nm.2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feldman P, Khanna R. Challenging the catechism of therapeutics for chronic neuropathic pain: Targeting CaV2.2 interactions with CRMP2 peptides. Neurosci Lett 2013; 557(Pt A):27-36; PMID:23831344; http://dx.doi.org/ 10.1016/j.neulet.2013.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. J Biol Chem 2009; 284:31375-90; PMID:19755421; http://dx.doi.org/ 10.1074/jbc.M109.009951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chi XX, Schmutzler BS, Brittain JM, Wang Y, Hingtgen CM, Nicol GD, Khanna R. Regulation of N-type voltage-gated calcium channels (Cav2.2) and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J Cell Sci 2009; 122:4351-62; PMID:19903690; http://dx.doi.org/ 10.1242/jcs.053280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson SM, Schmutzler BS, Brittain JM, Dustrude ET, Ripsch MS, Pellman JJ, Yeum TS, Hurley JH, Hingtgen CM, White FA, et al.. Inhibition of transmitter release and attenuation of anti-retroviral-associated and tibial nerve injury-related painful peripheral neuropathy by novel synthetic Ca2+ channel peptides. J Biol Chem 2012; 287:35065-77; PMID:22891239; http://dx.doi.org/ 10.1074/jbc.M112.378695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piekarz AD, Due MR, Khanna M, Wang B, Ripsch MS, Wang R, Meroueh SO, Vasko MR, White FA, Khanna R. CRMP-2 peptide mediated decrease of high and low voltage-activated calcium channels, attenuation of nociceptor excitability, and anti-nociception in a model of AIDS therapy-induced painful peripheral neuropathy. Mol Pain 2012; 8:54; PMID:22828369; http://dx.doi.org/ 10.1186/1744-8069-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, et al.. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci U S A 2010; 107:14950-7; PMID:20668236; http://dx.doi.org/ 10.1073/pnas.1005940107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khanna R, Zougman A, Stanley EF. A proteomic screen for presynaptic terminal N-type calcium channel (CaV2.2) binding partners. J Biochem Mol Biol 2007; 40:302-14; PMID:17562281; http://dx.doi.org/ 10.5483/BMBRep.2007.40.3.302 [DOI] [PubMed] [Google Scholar]
- 88.Collins BC, Gillet LC, Rosenberger G, Röst HL, Vichalkovski A, Gstaiger M, Aebersold R. Quantifying protein interaction dynamics by SWATH mass spectrometry: application to the 14-3-3 system. Nature Methods 2013; 10:1246-53; PMID:24162925; http://dx.doi.org/ 10.1038/nmeth.2703 [DOI] [PubMed] [Google Scholar]
- 89.Carlton SM. Peripheral excitatory amino acids. Curr Opin Pharmacol 2001; 1:52-6; PMID:11712535; http://dx.doi.org/ 10.1016/S1471-4892(01)00002-9 [DOI] [PubMed] [Google Scholar]
- 90.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10:895-926; PMID:19712899; http://dx.doi.org/ 10.1016/j.jpain.2009.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152:S2-15; PMID:20961685; http://dx.doi.org/ 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fundytus ME. Glutamate receptors and nociception: implications for the drug treatment of pain. CNS Drugs 2001; 15:29-58; PMID:11465012; http://dx.doi.org/ 10.2165/00023210-200115010-00004 [DOI] [PubMed] [Google Scholar]
- 93.Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med 2008; 14:1325-32; PMID:19011637; http://dx.doi.org/ 10.1038/nm.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garry EM, Moss A, Delaney A, O'Neill F, Blakemore J, Bowen J, Husi H, Mitchell R, Grant SG, Fleetwood-Walker SM. Neuropathic sensitization of behavioral reflexes and spinal NMDA receptor/CaM kinase II interactions are disrupted in PSD-95 mutant mice. Curr Biol 2003; 13:321-8; PMID:12593798; http://dx.doi.org/ 10.1016/S0960-9822(03)00084-8 [DOI] [PubMed] [Google Scholar]
- 95.Tao F, Tao YX, Mao P, Johns RA. Role of postsynaptic density protein-95 in the maintenance of peripheral nerve injury-induced neuropathic pain in rats. Neuroscience 2003; 117:731-9; PMID:12617977; http://dx.doi.org/ 10.1016/S0306-4522(02)00801-1 [DOI] [PubMed] [Google Scholar]
- 96.Tao YX, Huang YZ, Mei L, Johns RA. Expression of PSD-95/SAP90 is critical for N-methyl-D-aspartate receptor-mediated thermal hyperalgesia in the spinal cord. Neuroscience 2000; 98:201-6; PMID:10854750; http://dx.doi.org/ 10.1016/S0306-4522(00)00193-7 [DOI] [PubMed] [Google Scholar]
- 97.Tao F, Su Q, Johns RA. Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice. Mol Ther 2008; 16:1776-82; PMID:18781143; http://dx.doi.org/ 10.1038/mt.2008.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tao F, Tao YX, Gonzalez JA, Fang M, Mao P, Johns RA. Knockdown of PSD-95/SAP90 delays the development of neuropathic pain in rats. Neuroreport 2001; 12:3251-5; PMID:11711866; http://dx.doi.org/ 10.1097/00001756-200110290-00022 [DOI] [PubMed] [Google Scholar]
- 99.D'Mello R, Marchand F, Pezet S, McMahon SB, Dickenson AH. Perturbing PSD-95 interactions with NR2B-subtype receptors attenuates spinal nociceptive plasticity and neuropathic pain. Mol Ther 2011; 19:1780-92; PMID:21427709; http://dx.doi.org/ 10.1038/mt.2011.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Florio SK, Loh C, Huang SM, Iwamaye AE, Kitto KF, Fowler KW, Treiberg JA, Hayflick JS, Walker JM, Fairbanks CA, et al.. Disruption of nNOS-PSD95 protein-protein interaction inhibits acute thermal hyperalgesia and chronic mechanical allodynia in rodents. Br J Pharmacol 2009; 158:494-506; PMID:19732061; http://dx.doi.org/ 10.1111/j.1476-5381.2009.00300.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 2004; 5:317-28; PMID:15034556; http://dx.doi.org/ 10.1038/nrn1368 [DOI] [PubMed] [Google Scholar]
- 102.Tappe A, Klugmann M, Luo C, Hirlinger D, Agarwal N, Benrath J, Ehrengruber MU, During MJ, Kuner R. Synaptic scaffolding protein Homer1a protects against chronic inflammatory pain. Nat Med 2006; 12:677-81; PMID:16715092; http://dx.doi.org/ 10.1038/nm1406 [DOI] [PubMed] [Google Scholar]
- 103.Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol 2000; 10:370-4; PMID:10851183; http://dx.doi.org/ 10.1016/S0959-4388(00)00087-8 [DOI] [PubMed] [Google Scholar]
- 104.Tappe-Theodor A, Fu Y, Kuner R, Neugebauer V. Homer1a signaling in the amygdala counteracts pain-related synaptic plasticity, mGluR1 function and pain behaviors. Mol Pain 2011; 7:38; PMID:21595930; http://dx.doi.org/ 10.1186/1744-8069-7-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y, Wu J, Wu Z, Lin Q, Yue Y, Fang L. Regulation of AMPA receptors in spinal nociception. Mol Pain 2010; 6:5; PMID:20092646; http://dx.doi.org/ 10.1186/1744-8069-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuner R. Central mechanisms of pathological pain. Nat Med 2010; 16:1258-66; PMID:20948531; http://dx.doi.org/ 10.1038/nm.2231 [DOI] [PubMed] [Google Scholar]
- 107.Tong CK, MacDermott AB. Both Ca2+-permeable and -impermeable AMPA receptors contribute to primary synaptic drive onto rat dorsal horn neurons. J Physiol 2006; 575:133-44; PMID:16763002; http://dx.doi.org/ 10.1113/jphysiol.2006.110072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vikman KS, Rycroft BK, Christie MJ. Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol 2008; 586:515-27; PMID:18033811; http://dx.doi.org/ 10.1113/jphysiol.2007.145581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Voitenko N, Gerber G, Youn D, Randic M. Peripheral inflamation-induced increase of AMPA-mediated currents and Ca2+ transients in the presence of cyclothiazide in the rat substantia gelatinosa neurons. Cell Calcium 2004; 35:461-9; PMID:15003855; http://dx.doi.org/ 10.1016/j.ceca.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 110.Garry EM, Moss A, Rosie R, Delaney A, Mitchell R, Fleetwood-Walker SM. Specific involvement in neuropathic pain of AMPA receptors and adapter proteins for the GluR2 subunit. Mol Cell Neurosci 2003; 24:10-22; PMID:14550765; http://dx.doi.org/ 10.1016/S1044-7431(03)00134-9 [DOI] [PubMed] [Google Scholar]
- 111.Katano T, Furue H, Okuda-Ashitaka E, Tagaya M, Watanabe M, Yoshimura M, Ito S. N-ethylmaleimide-sensitive fusion protein (NSF) is involved in central sensitization in the spinal cord through GluR2 subunit composition switch after inflammation. Eur J Neurosci 2008; 27:3161-70; PMID:18598260; http://dx.doi.org/ 10.1111/j.1460-9568.2008.06293.x [DOI] [PubMed] [Google Scholar]
- 112.Nissenbaum J. From mouse to humans: discovery of the CACNG2 pain susceptibility gene. Clin Genet 2012; 82:311-20; PMID:22775325; http://dx.doi.org/ 10.1111/j.1399-0004.2012.01924.x [DOI] [PubMed] [Google Scholar]
- 113.Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, et al.. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci 2009; 29:3206-19; PMID:19279258; http://dx.doi.org/ 10.1523/JNEUROSCI.4514-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci 2004; 5:952-62; PMID:15550950; http://dx.doi.org/ 10.1038/nrn1556 [DOI] [PubMed] [Google Scholar]
- 115.Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron 2005; 47:407-21; PMID:16055064; http://dx.doi.org/ 10.1016/j.neuron.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 116.Li X, Zou Y, Luo H, Weng Y, Guo Q, Huang C. Spatiotemporal changes in NSF expression of DRG neurons in a rat model of spinal nerve ligation. J Mol Neurosci 2014; 53:645-53; PMID:24443234; http://dx.doi.org/ 10.1007/s12031-014-0231-9 [DOI] [PubMed] [Google Scholar]
- 117.Tao F, Skinner J, Su Q, Johns RA. New role for spinal Stargazin in alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated pain sensitization after inflammation. J Neurosci Res 2006; 84:867-73; PMID:16791853; http://dx.doi.org/ 10.1002/jnr.20973 [DOI] [PubMed] [Google Scholar]
- 118.Guo R, Zhao Y, Zhang M, Wang Y, Shi R, Liu Y, Xu J, Wu A, Yue Y, Wu J, et al.. Down-regulation of Stargazin inhibits the enhanced surface delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor GluR1 subunit in rat dorsal horn and ameliorates postoperative pain. Anesthesiology 2014; 121:609-19; PMID:25093662; http://dx.doi.org/ 10.1097/ALN.0000000000000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nissenbaum J, Devor M, Seltzer Z, Gebauer M, Michaelis M, Tal M, Dorfman R, Abitbul-Yarkoni M, Lu Y, Elahipanah T, et al.. Susceptibility to chronic pain following nerve injury is genetically affected by CACNG2. Genome Res 2010; 20:1180-90; PMID:20688780; http://dx.doi.org/ 10.1101/gr.104976.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Watanabe N, Osada H. Phosphorylation-dependent protein-protein interaction modules as potential molecular targets for cancer therapy. Curr Drug Targets 2012; 13:1654-8; PMID:23030498; http://dx.doi.org/ 10.2174/138945012803530035 [DOI] [PubMed] [Google Scholar]
- 121.Moreira IS, Fernandes PA, Ramos MJ. Hot spots–a review of the protein-protein interface determinant amino-acid residues. Proteins 2007; 68:803-12; PMID:17546660; http://dx.doi.org/ 10.1002/prot.21396 [DOI] [PubMed] [Google Scholar]
- 122.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015; 520:511-7; PMID:25855297; http://dx.doi.org/ 10.1038/nature14367 [DOI] [PMC free article] [PubMed] [Google Scholar]