Endothelial cells are fundamental to almost all physiology: foundation stones of an ancient vascular system, essential for the development and survival of animals. They are exposed to many physical forces: from external insult, the beating heart, fluid dynamics, and tissue remodelling. They have pronounced sensitivity to the frictional force of shear stress, which in physiology arises because of blood flow. In humans the force ranges from 1 to 70 dyn.cm−2 (0.1–7 Pa). It is actively sensed by endothelial cells to enable vascular development and maintain an efficient and healthy vasculature thereafter. The mechanisms by which physical forces regulate endothelial cells to determine the complexities of vascular structure and function have nevertheless been enigmatic. Important studies have revealed multiple participating proteins and sensing via Ca2+-permeable non-selective cationic channels but the nature of the sensor itself and molecular basis of the channel have been controversial and elusive.1
We recently made an intriguing discovery about endothelial mechanical sensitivity.2 Piezo1 gene, previously without known vascular relevance, was found to be important for normal shear stress-evoked Ca2+ signaling and non-selective cationic channel activity in endothelial cells. What was especially striking was that Piezo1 knock-out in the mouse was embryonic lethal just after the time when the heart started to beat and when substantial arteries should first emerge to support expansion of the embryo. We demonstrated that the lethality reflected a specific requirement for endothelial Piezo1: with endothelial-specific Piezo1 disruption, endothelial cells were present but did not remodel to form mature vascular architecture. Significance of Piezo1 in the adult mouse was also indicated through studies of haploinsufficient animals carrying 50% Piezo1 expression: there were disturbances in both nitric oxide synthase and alignment of endothelial cells to the direction of blood flow. Proteomic and other studies led us to find that downstream of Piezo1-dependent Ca2+ signaling was protease (calpain) activation, focal adhesion turnover, and spatial re-alignment of endothelial cells to the polarity of the applied force (Fig. 1). This work therefore revealed a gene of critical importance for the vascular field, new fundamental understanding of how complex life develops and new ideas for addressing health problems such as cardiovascular disease and cancer where changes in blood flow are common and often unwanted.
Figure 1.
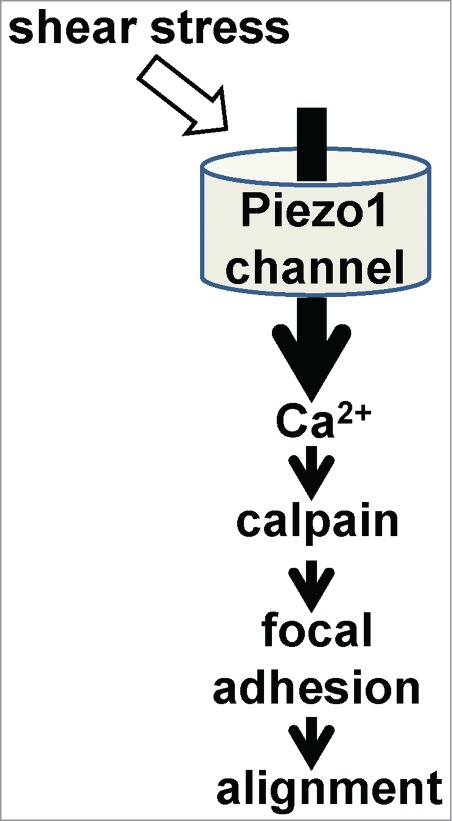
Simplified model for Piezo1 signaling in endothelial cells.
In 2010, relationships of Piezo1 to ion channels, transmembrane Ca2+ flux, and mechanical sensitivity were first suggested.3,4 The primary molecular hypothesis for Piezo1 has since been that it assembles as a tetramer to form a mechanically-activated machine with a central ion pore that allows transmembrane Na+ and Ca2+ flux into cells.5 A role in Ca2+ release has been suggested,4 although it could not be detected in endothelial cells.2 Amino acid sequence analysis suggests no relationship to other ion channel or putative ion channel proteins, except Piezo2, and there is currently no clear insight into the amino acid residues participating in the ion permeation pathway or mechanical sensitivity. Structural information for an extracellular loop has been obtained at 2.5Å resolution, supporting the suggestion that Piezo proteins lack close relatives.6
Piezo1 is at least 286 kDa, so the tetramer is over a million Daltons. It is tempting to think that such a large assembly might be capable of ion channel and non-ion channel functions, although all of the effects linked to Piezo1 in endothelial cells were also sensitive to the spider toxin GsMTx4,2 an inhibitor of Piezo1 channels.7 There is however evidence of Piezo1 activity in the absence of applied exogenous mechanical force: regulation of β1-integrin in epithelial cells4 and regulation of cell migration and nitric oxide synthase in endothelial cells.2 Such effects may reflect independence of mechanical force or relationships of Piezo1 to intrinsic forces in cells, or between cells and their substrates.
Mutations in human Piezo1 gene (PIEZO1) are linked to hereditary hemolytic anaemias.8 They are found to alter the kinetics of Piezo1 channel activation and inactivation.8 Although often considered to be gain-of-function mutations, the M2225R mutation slowed activation in response to mechanical strain8 and inhibited activation by shear stress.2 The finding of Piezo1 sensitivity to shear stress2 could have important implications for understanding Piezo1 in erythrocytes, which routinely experience shear stress.
Endothelial Piezo1 stands out in being critical for vascular development but contributions of other proteins as additional shear stress sensors or sensors of other mechanical forces in endothelial cells is likely and there is good evidence for it.1 The breadth of forces experienced by endothelial cells is substantial and so we can expect involvement of other proteins as sensors, back-up mechanisms, regulators and amplifiers, or components of integrated multi-protein complexes with Piezo1. A central player in shear stress sensing has been documented as the CD31 protein.1 There are also other Ca2+-permeable channels to consider: notably those formed from TRPP2 (polycystin 2), TRPV4 and P2X4 proteins.1
Determination of the reason for lethality in Piezo1-disrupted mice and exposure of a critical role specifically for endothelial Piezo12 provide important opportunities for achieving better understanding of the processes underlying maturation of arteries during vascular development and for revealing relationships between seemingly subtle physiological forces and the architecture and function of the vasculature. There is much work ahead to understand this profoundly important ion channel protein.
Funding
Supported by the Wellcome Trust, Medical Research Council UK, and British Heart Foundation.
References
- 1. Ando J, Yamamoto K. Flow detection and calcium signaling in vascular endothelial cells. Cardiovasc Res 2013; 99:260-268; PMID:23572234; http://dx.doi.org/ 10.1093/cvr/cvt084 [DOI] [PubMed] [Google Scholar]
- 2. Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014; 515:279-82; http://dx.doi.org/ 10.1038/nature13701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010; 330:55-60; PMID:20813920; http://dx.doi.org/ 10.1126/science.1193270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McHugh BJ, Buttery R, Lad Y, Banks S, Haslett C, Sethi T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J Cell Sci 2010; 123:51-61; PMID:20016066; http://dx.doi.org/ 10.1242/jcs.056424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, et al. . Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012; 483:176-181; PMID:22343900; http://dx.doi.org/ 10.1038/nature10812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamajaya A, Kaiser JT, Lee J, Reid M, Rees DC. The structure of a conserved piezo channel domain reveals a topologically distinct beta sandwich fold. Structure 2014; PMID:25242456; http://dx.doi.org/ 10.1016/j.str.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 2011; 50:6295-6300; PMID:21696149; http://dx.doi.org/ 10.1021/bi200770q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci U S A 2013; 110:E1162-1168; PMID:23487776; http://dx.doi.org/ 10.1073/pnas.1219777110 [DOI] [PMC free article] [PubMed] [Google Scholar]


