Abstract
BACKGROUND
Primary ovarian insufficiency (POI) is characterized by marked heterogeneity, but with a significant genetic contribution. Identifying exact causative genes has been challenging, with many discoveries not replicated. It is timely to take stock of the field, outlining the progress made, framing the controversies and anticipating future directions in elucidating the genetics of POI.
METHODS
A search for original articles published up to May 2015 was performed using PubMed and Google Scholar, identifying studies on the genetic etiology of POI. Studies were included if chromosomal analysis, candidate gene screening and a genome-wide study were conducted. Articles identified were restricted to English language full-text papers.
RESULTS
Chromosomal abnormalities have long been recognized as a frequent cause of POI, with a currently estimated prevalence of 10–13%. Using the traditional karyotype methodology, monosomy X, mosaicism, X chromosome deletions and rearrangements, X-autosome translocations, and isochromosomes have been detected. Based on candidate gene studies, single gene perturbations unequivocally having a deleterious effect in at least one population include Bone morphogenetic protein 15 (BMP15), Progesterone receptor membrane component 1 (PGRMC1), and Fragile X mental retardation 1 (FMR1) premutation on the X chromosome; Growth differentiation factor 9 (GDF9), Folliculogenesis specific bHLH transcription factor (FIGLA), Newborn ovary homeobox gene (NOBOX), Nuclear receptor subfamily 5, group A, member 1 (NR5A1) and Nanos homolog 3 (NANOS3) seem likely as well, but mostly being found in no more than 1–2% of a single population studied. Whole genome approaches have utilized genome-wide association studies (GWAS) to reveal loci not predicted on the basis of a candidate gene, but it remains difficult to locate causative genes and susceptible loci were not always replicated. Cytogenomic methods (array CGH) have identified other regions of interest but studies have not shown consistent results, the resolution of arrays has varied and replication is uncommon. Whole-exome sequencing in non-syndromic POI kindreds has only recently begun, revealing mutations in the Stromal antigen 3 (STAG3), Synaptonemal complex central element 1 (SYCE1), minichromosome maintenance complex component 8 and 9 (MCM8, MCM9) and ATP-dependent DNA helicase homolog (HFM1) genes. Given the slow progress in candidate-gene analysis and relatively small sample sizes available for GWAS, family-based whole exome and whole genome sequencing appear to be the most promising approaches for detecting potential genes responsible for POI.
CONCLUSION
Taken together, the cytogenetic, cytogenomic (array CGH) and exome sequencing approaches have revealed a genetic causation in ∼20–25% of POI cases. Uncovering the remainder of the causative genes will be facilitated not only by whole genome approaches involving larger cohorts in multiple populations but also incorporating environmental exposures and exploring signaling pathways in intragenic and intergenic regions that point to perturbations in regulatory genes and networks.
Keywords: primary ovarian insufficiency, premature ovarian failure, chromosomal abnormality, gene mutation, genome-wide association studies, whole-exome sequencing, next generation sequencing
Introduction
Primary ovarian insufficiency (POI), also known as premature ovarian failure (POF) or premature menopause, is defined as cessation of menstruation before the expected age of menopause. This age is traditionally defined to be prior to 40 years and diagnosis is confirmed by elevated serum FSH levels (>40IU/l). Although frequently stated that ∼1% of the population is affected with POI before the age of 40 years and 0.1% before age 30 years, the prevalence is actually less certain (Coulam et al., 1986).
The disorder is clearly heterogeneous, with a wide spectrum of causes, namely cytogenetic, genetic, infectious or iatrogenic. Autoimmune and metabolic etiologies may or may not be genetic. Irrespective, etiology remains to be elucidated in most cases and until a decade ago few specific causes were known beyond X-chromosomal abnormalities, Fragile X mental retardation 1 (FMR1) premutation and FSH receptor (FSHR) in the Finnish population (Simpson, 1975; Aittomäki et al., 1995; Wittenberger et al., 2007). Most cases of isolated POI still appear sporadically, but ∼10–15% has an affected first-degree relative, indicating significant genetic etiology (Van Kasteren et al., 1999). Pedigrees with multiple affected relatives are not rare (recessive and dominant). Presence of POI as one component of a pleiotropic genetic disorder is also well recognized. Yet identifying precise causative genes has been challenging. Here, we enumerate known genetic causes of POI, most elucidated within the last 5–10 years.
Confusion exists concerning nomenclature, namely the use of POF or POI. It is the view of the authors that POI can be taken to encompass occult, biochemical and overt stages, whereas POF is best considered as only the final stage of POI. The designation POI is thus best reserved as alluding to the entire gamut of disorders having diminished ovarian reserve—occult, subclinical, iatrogenic. Although many authors espouse POI in lieu of POF, the canonical genetic reference—Online Mendelian inheritance in man (OMIM)—has long used, and continues to use POF, to nominate causative genes. These designations now apply to POF1–POF9 (Supplementary Table SI), and the list growing.
Methods
A search for original articles published up to May 2015 was performed using PubMed and Google Scholar to identify studies on genetic variants associated with the human disease. The key word combinations include ‘premature ovarian failure’, ‘primary ovarian insufficiency’, ‘early menopause’, ‘genetic’, ‘gene mutation’, ‘variant’ and 'genome wide study’. For a study to be included in our review, it had to focus on chromosomal analysis, candidate gene screening, or a genome-wide study in different POI cohorts. In addition, studies on mitochondrial genes causing POI and multiple malformation syndromes characterized by POI were included. Reports on the role of candidate genes in animal models were not included. Where appropriate, reference lists of identified articles were also searched for further relevant papers. However, articles identified were restricted to English language full-text papers.
Results
Chromosomal abnormalities in POI
Chromosomal abnormalities have long been recognized as a cause of POI, but percentages vary widely among reported series. This clearly reflects biases of ascertainment, for example reflecting whether a cohort was derived from a referral cytogenetic lab, a gynecologic practice, or a pediatric practice. Numerous different karyotypic anomalies have been found, ranging from numerical defects (monosomy X; X chromosomal mosaicism), X-deletions, X-autosome translocations, and X-isochromosomes and other rearrangements. Aggregate frequency of chromosomal abnormalities in reported studies is summarized in Table I. Small sample sizes as well as selection biases and differing ages of ascertainment probably account for different prevalence in different populations. However, each of the five largest studies with respect to sample size reported frequencies between 10.0 and 12.9% (Zhang et al., 2003; Lakhal et al., 2010a; Baronchelli et al., 2011; Jiao et al., 2012; Kalantari et al., 2013); thus, a prevalence of 10–13% seems reasonable.
Table I.
Frequency of chromosomal abnormalities (CA) in different population studies.
| Reference | Frequency of CA (%) | No. of CA | Sample size | Clinical characteristics | Population |
|---|---|---|---|---|---|
| Ayed et al. (2014) | 18.0 | 18 | 100 | PA, SA | Tunisian |
| Kalantari et al. (2013) | 10.05 | 18 | 179 | PA, SA | Iranian |
| Jiao et al. (2012) | 12.1 | 64 | 531 | PA, SA | Chinese (Jinan, Beijing, Shenzhen) |
| Baronchelli et al. (2011) | 10.0 | 27 | 269 | PA, SA, EM | Italian |
| Lakhal et al. (2010a) | 10.8 | 108 | 1000 | PA, SA | Tunisian |
| Ceylaner et al. (2010) | 25.3 | 19a | 75 | SA | Turkish |
| Janse et al. (2010) | 12.9 | 19 | 147 | SA | Dutch |
| Portnoi et al. (2006) | 8.8 | 8 | 90 | PA, SA | French |
| Zhang et al. (2003) | 12.5 | 13 | 104 | POI | Chinese (Chongqing) |
| Devi and Benn (1999) | 13.3 | 4 | 30 | SA | American |
| Davison et al. (1998) | 2.5 | 2 | 79 | PA, SA FSH>20 IU/l | English |
| Castillo et al. (1992) | 32.0 | 15 | 47 | POI | Chilean |
| Rebar and Connolly (1990) | 25.4 | 16 | 63 | PA, SA | American |
Chromosomal ‘abnormalities’ means visible structural changes in karyotype that are sufficiently large to cause clinical abnormalities. Variants (e.g. prominent satellites) are not included.
CA, chromosomal abnormalities; PA, primary amenorrhea; POI, primary ovarian insufficiency; SA, secondary amenorrhea; EM, early menopause.
aIncluding 2 46,XY gonadal dysgenesis (Swyer syndrome).
Numerical defects
The X chromosome has long been known to play an essential role in the maintenance of ovarian development and function. Females lacking an X chromosome as well as those showing an extra X chromosome are predisposed to developing POI.
45,X and 45,X/46,XX
Turner syndrome, often but not universally associated with X monosomy, leads to ovarian dysgenesis and accelerated follicular atresia. X monosomy without mosaicism is more typically found in primary amenorrhea and cases were almost universally understood to present with this phenotype. However, many early series were recruited from pediatric clinics, not among adult women. In 1975, Simpson (1975) reported that 3% (5/178) of 45,X patients actually menstruated. 45,X/46,XX and other forms of association also are associated with secondary amenorrhea (POI). Either haploinsufficiency of pivotal genes on the X chromosome or non-specific meiotic impairment could explain the accelerated atresia of 45,X oocytes. Variability would be expected given potential heterozygosity of alleles in genes subjected to X-inactivation.
47,XXX
47,XXX women may experience oligomenorrhea, secondary amenorrhea, and early menopause, but relative risk has not been well studied. Goswami et al. (2003) reported the prevalence of 47,XXX in 52 women with POI to be 3.8%, whereas in our much larger Chinese series we observed 1.5% (8/531) (Jiao et al., 2012). The presence of three X chromosomes plausibly leads to meiotic disturbance and, secondarily, ovarian failure. Additionally, overexpression of genes that escape X-inactivation could cause POI in 47,XXX. Mechanisms remain to be defined (Tartaglia et al., 2010). A confounder is that an association exists between 47,XXX and autoimmune diseases (Holland, 2001; Goswami et al., 2003).
X-structural abnormalities and X-autosome translocations
X chromosome deletions and X-autosome balanced translocations have long been observed in POI and were once the only approach available to localize causative genes. This strategy was illustrated in the 1970s by a region on the X chromosome appearing critical for the POI phenotype (Sarto et al., 1973). A critical region was delineated that gave boundaries for breakpoints of X-autosome translocations associated with ovarian failure. This region extends from Xq13-Xq21 (POI2) to Xq23-q27 (POI1). It has been proposed by Rizzolio and colleagues that Xq13-Xq21 governs epigenetic regulations that down-regulate oocyte-expressed autosomal genes (Rizzolio et al., 2006, 2007, 2009).
Irrespective of mechanisms involved in the critical region, almost all terminal deletions originating at Xq13 are associated with primary amenorrhea, lack of breast development and complete ovarian failure (Simpson and Rajkovic, 1999; Simpson, 2008). By contrast, terminal deletions arising at Xq25 or Xq26 are characterized by the more common phenotype being not primary amenorrhea but premature ovarian failure. The gene designation POI1 is applied to this region. The more distal deletions arise at Xq27 or Xq28 and exert a less severe effect on stature and reproductive function than do proximal deletions (Simpson, 1975; Simpson and Rajkovic, 1999). Gene(s) in this POI-causing region are considered distinct from Fragile X mental retardation 1 (FMR1), located at Xq27 and premutation of which is the most common single cause of POI.
Multiple genes on the X chromosome have been identified by X-autosomal translocations. These include Diaphanous-related formin 2 (DIAPH2, Xq22) (Bione et al., 1998), X-prolyl aminopeptidase (aminopeptidase P) 2, membrane-bound (XPNPEP2, Xq25) (Prueitt et al., 2000), Dachshund family transcription factor 2 (DACH2, Xq21.3) (Prueitt et al., 2002), Premature ovarian failure, 1B (POF1B, Xq21.1) (Lorda-Sanchez et al., 2000; Bione et al., 2004), Choroideremia (CHM, Xq21.1) (Lorda-Sanchez et al., 2000; Mansouri et al., 2008), Progesterone receptor membrane component 1 (PGRMC1, Xq24) (Mansouri et al., 2008), Collagen, type IV, alpha 6 (COL4A6, Xq22.3) (Nishimura-Tadaki et al., 2011) and Nuclear RNA export factor 5 (NXF5, Xq22.1) (Bertini et al., 2010). Some will individually be discussed below.
Autosomal rearrangements
Autosomal translocations—Robertsonian and reciprocal—have been observed in sporadic cases in Belgian, American, Japanese and Chinese women (Hens et al., 1989; Orczyk et al., 1989; Kawano et al., 1998; Jiao et al., 2012). Perturbations presumably confer haploinsufficiency or interrupt pivotal genes in these regions. Non-specific defective meiotic pairing or a position effect on contiguous genes is also a potential explanation (Simpson, 2008; Persani et al., 2009). No autosomal region appears preferentially involved, long frustrating investigators seeking to use breakpoints to localize regions containing autosomal genes of relevance. Searches for autosomal regions disrupted in X-autosome translocations have similarly not proved fruitful in identifying autosomal roles in POI.
Single genes causing non-syndromic POI
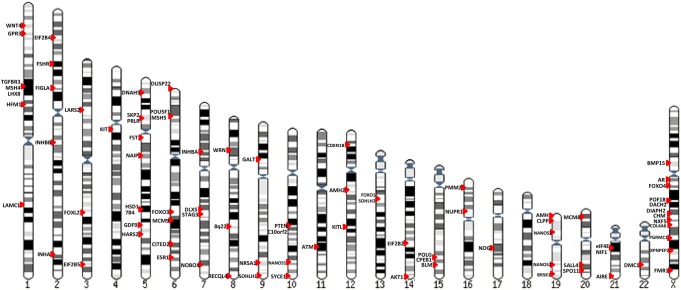
Aside from regions of interest defined by chromosome deletions and translocations, the other traditional strategy to identify candidate genes in POI is to study genes whose product is known and plays a role in human folliculogenesis or shows an organ-specific effect based on murine knockout models (candidate genes). Many genes have been interrogated for these reasons. In this section we specifically review all genes for which data warrant strong consideration as a candidate gene for POI. Table II and Supplementary Table SII contain available details of reported studies.
Table II.
Variants identified in candidate genes on the X chromosome for idiopathic and sporadic POI.
| Gene | Location | Cases (N) | Controls (N) | Ethnicity | MRa | Sequence variation | Amino acid change | FC | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| BMP15 | Xp11.2 | 50 | 214 | Caucasian North Africa Asia | 2 (4.0%) | c.242A>G | p.H81R | Tiotiu et al. (2010) | ||
| c.595G>A | p.G199R | |||||||||
| 100 | 100 | Chinese | 1 (1.0%) | c.985C>T | p.R329C | Wang et al. (2010b) | ||||
| 300 | 216 | Caucasian | 12 (4.0%) | c.13A>C | p.S5R | Yes | Slightly affects transactivation of BRE-luc in COV434 granulosa cells | Rossetti et al. (2009) | ||
| c.202C>T | p.R68W | Markedly reduces mature protein production and affects transactivation of BRE-luc in COV434 granulosa cells | ||||||||
| c.413G>A | p.R138H | |||||||||
| c.443T>C | p.L148P | |||||||||
| c.538G>A | p.A180T | No effect on protein production or transactivation | ||||||||
| 20 | 93 | Germany | None | Ledig et al. (2008) | ||||||
| 92 | 76 | Chinese | None | Zhang et al. (2007) | ||||||
| 203 | 54 | Caucasian African Asian | 3 (1.5%) | c.443T>C | p.L148P | Laissue et al. (2006) | ||||
| c.538G>A | p.A180T | |||||||||
| c.468G>A | Sense | |||||||||
| c.831T>C | Sense | |||||||||
| c.852C>T | Sense | |||||||||
| 133 | 197 | Indian | 14 (10.5%) | c.181C>T | p.R61W | Dixit et al. (2006c) and Inagaki and Shimasaki (2010) | ||||
| c.182G>A | p.R61E | |||||||||
| c.226C>T | p.R76C | Yes | Decreased mature protein production, weaker Smad1/5/8 phosphorylation in COV434 cells and decreased granulosa cell proliferation | |||||||
| c.227G>A | p.R76H | |||||||||
| c.538G>A | p.A180T | |||||||||
| c.538G>T/c.539C>T | p.A180F/S+V | |||||||||
| c.588T>A | p.N196K | |||||||||
| c.617G>A | p.R206H | Yes | Decreased mature protein production, weaker Smad1/5/8 phosphorylation in COV434 cells and decreased granulosa cell proliferation | |||||||
| c.631C>T | p.E211X | |||||||||
| c.661T>C | p.W221R | |||||||||
| c.727A>G | p.L243G | |||||||||
| c.381A>G | Sense | |||||||||
| c.*40dupG | 3′UTR | |||||||||
| 166 | 181 | Caucasian | 7 (4.2%) | c.202C>T | p.R68W | Di Pasquale et al. (2004, 2006) | ||||
| c.538G>A | p.A180T | |||||||||
| c.704A>G | p.Y235C | Yes | Diminished GC proliferation with a dominant negative effect | |||||||
| 38 | 51 | New Zealand | None | Chand et al. (2006) | ||||||
| 15 | 3 | Japanese | None | Takebayashi et al. (2000) | ||||||
| AR | Xq12 | 133 | 200 | Indian | 2 (1.5%) | c.1948A>G | p.T650A | Panda et al. (2010) | ||
| c.1972C>A | p.O658K | |||||||||
| c.1885+9C>A | Intron | |||||||||
| FOXO4 | Xq13.1 | 116 | 143 | Tunisian | None | Fonseca et al. (2012a) | ||||
| POF1B | Xq21.2 | 223 | 900 | Italian | 2 (0.9%) | c.G1477A | p.C444Y | Bione et al. (2004) | ||
| DACH2 | Xq21.3 | 257 | 1110 | Italian | 2 (0.8%) | c.G274T | p.R37L | Bione et al. (2004) | ||
| c.T1111C | p.F316S | |||||||||
| PGRMC1 | Xq22-q24 | 67 | 153 | Swedish Italian | 1 (1.5%) | c.494A>G | p.H165R | Yes | Attenuates ability to transduce progesterone's anti-apoptotic action in granulosa cells and abolishes binding capacity to CYP7A1 | Mansouri et al. (2008) |
| 196 | 200 | Chinese | 1 (0.51%) | c.556C>T | p.P186S | Wang et al. (2014b) |
MR: mutation rate; FC: functional confirmation; BMP15: bone morphogenetic protein 15; AR: androgen receptor; FOXO4: forkhead box O4; POF1B: premature ovarian failure, 1B; DACH2: dachshund family transcription factor 2; PGRMC1: progesterone receptor membrane component-1.
aOnly refer to novel missense, frameshift and nonsense mutations
Variants occurring in evolutionary conserved regions are more likely to carry functional significance. These include missense, nonsense, insertion or deletion variants and were considered as plausible causative variants with clinical significance. Thus, perturbations should yield a severe functional defect. A nonsense mutation that results in truncated protein should lead to haploinsufficiency; a splicing site mutation or insertion/deletion should result in a frameshift that leads to a different protein product; a missense mutation may change the amino acid and cause a dominant negative effect.
Those regions or genes found only by genome-wide studies, including genome-wide association study, cytogenomic study, whole-exome sequencing, and next generation sequencing (NGS), are cited separately.
Genes on the X chromosome
Bone morphogenetic protein 15 (BMP15) (Xp11.2)
BMP15 is located on chromosome Xp11.2. The possible involvement of BMP15 in POI pathogenesis was initially supported by evidence from animal models. Inverdale and Hanna sheep with a naturally occurring Bmp15 mutation had increased ovulation rate and twin and triplet births in heterozygotes, but ovarian failure results from impaired follicular development beyond the primary stage in homozygotes (Galloway et al., 2000). Bmp15 knockout female mice also were subfertile, showing decreased ovulation rates, reduced litter size and decreased number of litters per lifetime (Yan et al., 2001).
In humans BMP15 was first implicated in POI by Di Pasquale et al. (2004), who reported a heterozygous p.Y235C missense mutation in each of two sisters having ovarian failure. The authors presented in vitro evidence for a dominant negative mechanism. Other variants have been identified in Caucasian, Indian and Chinese women with POI, albeit with quite different frequencies (1.5–15%) (Di Pasquale et al., 2006; Dixit et al., 2006c; Laissue et al., 2006; Ledig et al., 2008; Lakhal et al., 2009, 2010b; Rossetti et al., 2009; Wang et al., 2010b). Merely showing different frequencies between a given single nucleotide polymorphism (SNP) in POI and control is a less robust method than finding a unique perturbation with functional validation in a case (Zhang et al., 2007; Ledig et al., 2008). However, some variants found in higher frequency indeed show marked reduction of mature protein production (Rossetti et al., 2009).
Of relevance is that BMP15 is a member of the transforming growth factor (TGF) family, with dimerization occurring with other TGF proteins such as GDF9, to be discussed below. Most reported BMP15 variants are, in fact, in the region corresponding to the propeptide of the protein, which is essential for dimerization and subsequent post-translational processing into biologically active proteins.
Progesterone receptor membrane component 1 (PGRMC1) (Xq22-q24)
PGRMC1 was first described in 1998 as a putative progesterone-binding membrane receptor (Losel et al., 2008). This protein is expressed in various tissues, e.g. liver, kidney, adrenal glands, uterus and leukocytes and involves progesterone signaling in the reproductive system (Cahill, 2007; Losel et al., 2008; Mansouri et al., 2008). PGRMC1 mediates progesterone's anti-apoptotic effects on granulosa cells (Engmann et al., 2006; Peluso et al., 2006; Losel et al., 2008; Mansouri et al., 2008).
Mansouri et al. (2008) identified a mother and daughter with POI, both of whom carried an X;autosome translocation [t(X;11)(q24;q13)]. Systematic mapping of the Xq breakpoint and performing RNA expression studies revealed reduced expression of PGRMC1. Mutation screening of 67 females with idiopathic POI identified a third patient having a missense mutation (p.H165R), located in the cytochrome b5 domain. The p.H165R mutation abolishes binding of cytochrome P450 7A1 (CYP7A1) to PGRMC1 and attenuates PGRMC1's ability to mediate the anti-apoptotic action of progesterone in ovarian cells. These findings suggest that mutant or reduced levels of PGRMC1 may cause POI through impaired activation of the microsomal cytochrome P450 and increased apoptosis of ovarian cells. A recent study in Chinese patients with POI identified a novel missense mutation (C.556C>T, p. P186S), but there was no functional study to confirm a deleterious effect (Wang et al., 2014b).
Androgen receptor (AR) (Xq12)
The AR gene encodes the androgen receptor and is involved in sex differentiation and reproduction. Its perturbation in 46,XY individuals results in the well-known sex reversed phenotype of androgen insensitivity, testosterone produced by testis exerting no effect on androgen-dependent differentiation. In the ovary, AR is expressed in developing follicles, mainly granulosa cells. Deficiency of Ar in female mice results in a POI-like phenotype and dysregulation of a number of major genes critical for folliculogenesis, indicating that normal folliculogenesis requires AR-mediated androgen action (Shiina et al., 2006). An association between CAG repeat length in exon 1 of the AR gene and POI has been proposed but remains controversial (Bretherick et al., 2008; Chatterjee et al., 2009; Sugawa et al., 2009; Panda et al., 2010). An example is a repeat of two missense mutations (p.T650A and p.O658K) in Indian women with POI (Panda et al., 2010).
Forkhead box O4 (FOXO4) (Xq13.1)
The FOXO4 gene encodes a member of the O class of winged helix/forkhead transcription factor family (FOXO). FOXO4 is expressed in granulosa cells in mice and human, and is involved in the PI3K (phosphoinositide 3-kinase)/Akt (v-akt murine thymoma viral oncogene homolog 1)/Cdkn1b (cyclin-dependent kinase inhibitor 1B) molecular pathway, which suggests a functional role in ovarian physiology (Pisarska et al., 2009). Mutation screening in 116 Tunisian patients identified only one intronic variant; IVS2 + 41T>G; therefore, FOXO4 might not be a common cause of POI in the Tunisian population (Fonseca et al., 2012a).
Premature ovarian failure, 1B (POF1B) (Xq21.2)
Alluded to previously, this ‘gene’ is actually a region, but codified by OMIM. Its significance is its location within the critical POI1 region. It was found to be interrupted by a breakpoint in an X-autosome translocation in a patient with secondary amenorrhea (POI). Subsequent mutation analysis in an Italian POI cohort (N = 223) only revealed 30 SNPs (Bione et al., 2004). In a Lebanese family having five sisters with POI, Lacombe et al. (2006) established linkage to Xq21 using whole-genome SNP typing and homozygosity-by-descent mapping. Sequencing identified a homozygous p.R329Q mutation, which impaired the capacity to bind nonmuscle actin filaments, and might lead to exaggerated germ-cell apoptosis and POI.
Dachshund family transcription factor 2 (DACH2) (Xq21.3)
DACH2, also named dachshund family transcription factor 2, is located on Xq21.3. It was first identified by fine mapping of the disrupted region in an X;autosome translocation in POI patients (Prueitt et al., 2002). Subsequent mutation screening revealed two novel missense mutations—p.R37L and p.F316S—in an Italian cohort of POI patients (Bione et al., 2004). However, no subsequent evidence of involvement of DACH2 in mammalian gonads or additional mutations in other ethnic population has been reported.
Fragile X mental retardation 1 (FMR1) (Xq27.3)
One of the commonest causes of POI is a premutation of FMR1, which when fully perturbed (>200 CGG repeats) causes fragile X syndrome but paradoxically not POI. A prototype of pleiotropic single gene disorders in which POI is one component, FMR1 is discussed in the ‘Pleiotropic Single Gene Disorders Having POI’ section, along with other pleiotropic genes.
Genes on autosomes
In this section we will review autosomal genes for which data appear to warrant strong consideration as a candidate gene for POI. Supplementary Table SII contains available details on studies generating this conclusion.
Growth differentiation factor 9 (GDF9) (5q31.1)
Expressed in oocytes, GDF9 is an attractive candidate gene for POI because it is, like BMP15, a member of the TGF gene family. Increased frequencies of certain novel variants have been detected in European, Caucasian and Asian patients (Dixit et al., 2005; Laissue et al., 2006; Kovanci et al., 2007; Zhao et al., 2007), but not in Japanese and New Zealand populations (Takebayashi et al., 2000; Chand et al., 2006). All variants were heterozygous. Recently, high-resolution array comparative genomic hybridization (CGH) (2.2 kb resolution) was applied in 26 POI Swedish cases, finding one partial GDF9 gene duplication (475 bp) (Norling et al., 2014). Unfortunately, parents were not available to exclude a heritable copy number variant (CNV) less likely to carry significance. This pitfall is discussed further in ‘Genome-Wide Studies in POI’ section.
Heterozygous changes could result in a dominant negative effect, quite plausible given dimerization with fellow members of the TGF gene family (e.g. BMP15). The proportion of POI due to GDF9 perturbations is, however, unclear. If a hydrophobic amino acid replacing a hydrophilic amino acid were causative, GDF9 perturbations could account for a substantial number (1–4%) of POI cases.
Folliculogenesis specific bHLH transcription factor (FIGLA) (2p13.3)
FIGLA, also named factor in the germline, alpha, is a germ-cell specific, basic helix-loop-helix (bHLH) transcription factor, that plays a crucial role in the formation of the primordial follicle and coordinates expression of zona pellucida genes. Zhao et al. (2008) screened 100 Chinese women with POI and identified three variants in four women: missense mutation p.A4E in two women; deletion p.G6fsX66 in one woman, resulting in a frameshift that leads to haploinsufficiency; and deletion p.140delN in a fourth woman. Functional analyses by the yeast two-hybrid assay demonstrated that the p.140delN mutation disrupted FIGLA binding to the TCF3 helix-loop-helix (HLH) domain. These findings show that a subset of Chinese women with sporadic POI harbor mutations in FIGLA. Recently, another novel intronic variant was found in 219 Indian POI cases (Tosh et al., 2015). Further functional validation is warranted.
Newborn ovary homeobox gene (NOBOX) (7q35)
NOBOX is an oocyte-specific homeobox gene that plays a critical role in early folliculogenesis. The causative role was discovered by Rajkovic et al. (2004). In mice Nobox deficiency disrupted early folliculogenesis and oocyte-specific gene expression. Lack of Nobox accelerated post-natal oocyte loss and abolished the transition from primordial to growing follicles in mice. In female mice lacking Nobox, follicles are replaced by fibrous tissue in a manner similar to non-syndromic ovarian failure in women. Genes preferentially expressed in oocytes, including Pou5f1 (POU class 5 homeobox 1) and Gdf9, are also down-regulated in Nobox−/− mice. Lechowska et al. (2011) showed that POI in Nobox deficient mice results from faulty signaling between somatic and germ line components during embryonic development. In addition, the extremely unusual presence of abnormal adherens junctions between unseparated oocytes within syncytial follicles indicates that faulty communication between somatic and germ cells is involved in, or leads to, abnormalities in the cell adhesion program. Qin et al. (2007a) were the first to demonstrate that a perturbation (p.R355H) in NOBOX was responsible for human POI. The mutation disrupted NOBOX homeodomain binding to NOBOX DNA-binding element (NBE) and had a dominant negative effect. Our functional studies demonstrated that haploinsufficiency was involved in the genetic mechanism in humans for POI. Mutations in the homeobox domain of NOBOX proved not to be a common explanation for POI in Chinese women (0/200) (Qin et al., 2009) but Bouilly et al. (2011, 2015) subsequently reported that novel NOBOX loss-of-function mutations accounted for 6.2 and 5.6%, respectively, of cases in two large ‘primary ovarian insufficiency’ cohorts of Caucasian and African ancestry.
Nuclear receptor subfamily 5, group A, member 1 (NR5A1); Steroidogenic factor-1(SF-1) (9q33)
NR5A1 encodes an orphan nuclear receptor that regulates transcription of an array of genes involved in reproduction, steroidogenesis and male sexual differentiation. These include anti-Mullerian hormone (AMH), Nuclear receptor subfamily 0, group B, member 1 (DAX1), Cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A), steroidogenic acute regulatory protein (StAR), as well as genes encoding steroid hydroxylases, gonadotrophins, and aromatase. Inactivation of Nr5a1 specifically in mouse granulosa cells causes infertility associated with hypoplastic ovaries.
Philibert et al. (2010) identified NR5A1 mutations as a frequent cause of ‘primary amenorrhea’ in 46,XY phenotypic female adolescents with a low testosterone concentration. Lourenco et al. (2009) sequenced NR5A1 in four families (each having at least one family member with a 46,XY disorder of sex development and another with 46,XX POI) and 25 subjects with sporadic POI, and they identified 19 different mutations in the NR5A1 gene. Functional studies indicated that these mutations substantially impaired the transactivational activity of NR5A1. Subsequently additional mutations were identified in different ethnicities with low frequencies (Supplementary Table SII). Janse et al. (2012) sequenced the coding regions of NR5A1 in a large, well-phenotyped cohort of 356 Dutch women with POI, finding 9 different mutations in 10 patients. Functional prediction showed low to intermediate pathogenicity for all non-conserved mutations. However, the novel p.Y5D mutation, detected in a non-domain region, was presumed to result in haploinsufficiency in Chinese patients with POI (Jiao et al., 2013).
FSH receptor (FSHR) (2p21-p16)
FSH/FSHR signaling plays a key role in normal gonadal function by regulating follicular growth, estrogen production and oocyte maturation. Mutation in FSHR was the first autosomal molecular explanation for POI, elucidated prior to the contemporary era. Aittomäki (1994) and Aittomäki et al. (1995, 1996) ascertained 75 primary or secondary amenorrhea cases, and found homozygous mutations (c.566C>T, p.A189V), in the extracellular portion of this G-protein receptor, in women of six Finnish families with hypergonadotrophic ovarian dysgenesis. This mutation resulted in a dramatic reduction of binding capacity and signal transduction, but with apparently normal ligand-binding affinity (Aittomäki et al., 1995). The frequency of the c.566C>T mutation is 0.96% in a Finnish population (Jiang et al., 1998). However, subsequent screening in cohorts of different ethnicities seldom found mutations (da Fonte Kohek et al., 1998; Jiang et al., 1998; Conway et al., 1999; Takakura et al., 2001; Tong et al., 2001; Sundblad et al., 2004; Chen et al., 2006; Ledig et al., 2008; Vilodre et al., 2008; Prakash et al., 2009; Woad et al., 2013). Therefore, FSHR mutations are not uncommon in XX gonadal dysgenesis in Finland, but apparently rare elsewhere (Supplementary Table SII).
TGF, beta receptor III (TGFBR3) (1p33-p32)
Human TGFBR3 is located at 1p33-p32 and encodes the TGF-beta type III receptor. The encoded receptor is a membrane proteoglycan that often functions as a co-receptor with other TGF-beta receptor superfamily members. Two missense variants, p.E459G and p.P825L, were identified in Chinese women with idiopathic POI, both predicted to have functional and structural impacts on the TGFBR3 protein (Qin et al., 2011). Another missense mutation—p.P775S—was found in an Indian POI case (Dixit et al., 2006b).
G protein-coupled receptor 3 (GPR3) (1p36.1-p35)
The GPR3 gene, located in 1p36.1-p35 and having 2 exons, is a member of the G protein-coupled receptor family. Predominantly expressed in oocytes, GPR3 maintains meiotic arrest in antral follicles until the LH surge through pathways involved in cAMP and cGMP regulation. In Gpr3−/− mice, the majority of oocytes in antral follicles had unscheduled premature resumption of meiosis (Mehlmann et al., 2004). A synonymous variant (c.135G>A, p.V45V) was found in one Chinese patient (Zhou et al., 2010), and another study also failed to find any potential disease-associated changes in 82 North American Caucasian women with POI (Kovanci et al., 2008).
Wingless-type MMTV integration site family, member 4 (WNT4) (1p36.23-p35.1)
WNT4 encodes a secreted extracellular signaling protein that is expressed in human ovaries early in fetal development (Jaaskelainen et al., 2010), and plays a critical role in female sex determination and differentiation. In the ovaries of Wnt4 mutant mice, the rate of apoptosis was similar to that of wild type mice at birth; however, apoptotic cells progressively increased during follicular development (Jaaskelainen et al., 2010). By sequencing the coding region of WNT4 in 55 Tunisian women with POI, a synonymous variant in exon 2 (c.99G>A, p.S33S) was identified (Lakhal et al., 2012). Mutational analysis was also performed in 145 Chinese women with POI with no causative variants found (Chen et al., 2011b).
Inhibins: inhibin, alpha (INHA) (2q35); inhibin, beta A (INHBA) (7p15-p13); inhibin, beta B (INHBB) (2cen-q13)
Inhibin is a dimeric glycoprotein hormone. Belonging, like BMP-15 and GDF9, to the superfamily of TGF-β, inhibin is a negative regulator of FSH. Inhibin encompasses inhibin, alpha (INHA) (2q35), inhibin, beta A (INHBA) (7p15-p13), and inhibin, beta B (INHBB) (2cen-q13).
The missense mutation c.769G>A (p.A257T) in the INHA gene was more frequently found in patients with POI in New Zealand (7%) (Shelling et al., 2000), India (11.2%) (Dixit et al., 2004) and Italy (4.5%) (Marozzi et al., 2002). Increased susceptibility to POI was associated with impaired inhibin B bioactivity (Chand et al., 2007). The additional novel missense mutations c.275G>A (p.S92N), c.525C>G (p.H175Q) and c.545C>A (p.A182D) were exclusively identified in Indian POI patients (1.25% for each mutation) (Dixit et al., 2006a). It is unclear whether polymorphisms in the INHA promoter result in reduced inhibin expression, but the promoter variant c.-16C>T was significantly under-represented in patients with POI in New Zealand (Harris et al., 2005). The INHBA and INHBB gene encode inhibin βA and inhibin βB subunits. Causative mutations were not found in the two genes, however, except possibly for one synonymous mutation c.1032C>T in the INHBA gene (Shelling et al., 2000; Chand et al., 2010).
POU class 5 homeobox 1 (POU5F1) (6p21.31)
The POU5F1 transcription factor gene, located on 6p21.31, is significantly down-regulated in Nobox knockout mice. Thus, POU5F1 becomes a potential candidate gene for POI, a downstream target of NOBOX. Wang et al. (2011) sequenced 175 Chinese POI cases and found one non-synonymous variant (p.P13T), a heterozygous hydrophobic to hydrophilic substitution.
MutS homolog 4 (MSH4) (1p31) and MSH5 (6p21.3)
MSH4 and MSH5 belong to the DNA mismatch repair gene family, playing pivotal roles in meiotic recombination. Mammalian MSH4 and MSH5 proteins form a heterodimeric complex and exert essential functions for normal chromosome synapsis during zygotene. Disruption of Msh4 or Msh5 in female mice resulted in sterility, degenerated ovaries and progressive loss of oocytes due to meiotic failure (de Vries et al., 1999; Kneitz et al., 2000). In a case–control study in a Caucasian population, in which both genes were sequenced, a heterozygous mutation p.P29S in MSH5 was found in 2 of 41 cases. The mutation was located in the Hmsh4-binding domain of MSH5 which could disrupt the integrity of the protein interaction between MSH5 and MSH4 (Mandon-Pepin et al., 2008).
Forkhead box O3 (FOXO3) (6q21)
Forkhead transcription factor FOXO3, located at 6q21, encodes a master regulator and potent suppressor of primordial follicle activation. Loss of Foxo3 function in mice leads to POI due to global follicle activation (Liu et al., 2007). The frequency of variants in POI patients differs in different ethnic groups (Supplementary Table SII), but in several populations the frequencies of FOXO3 variants are not insignificant (6% in French and 13.3% in Chinese). However, the pathological role of these variants needs to be determined by functional studies.
Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 (CITED2) (6q23.3)
CITED2 is essential for early embryonic development. This is evidenced by delayed differentiation of gonads in Cited2−/− mice (Combes et al., 2010). Fonseca and colleagues reported a novel missense mutation p.P202T in one of 116 Tunisian POI cases (Fonseca et al., 2012b). Further studies in other populations are warranted.
Spermatogenesis and oogenesis specific basic helix-loop-helix transcription factor 1 (SOHLH1) (9q34.3) and SOHLH2 (13q13.3)
As germ cell specific master-master transcription factors, SOHLH1 and SOHLH2 orchestrate different oocyte-specific genes essential for early folliculogenesis. Sohlh1/2-deficient mice exhibit atrophied ovaries devoid of follicles due to defective primordial-to-primary follicle transition (Pangas et al., 2006; Choi et al., 2008). Novel distinct heterozygous variants were identified in both SOHLH1 and SOHLH2 in large cohorts of women with POI of Han Chinese and Serbian origin (Qin et al., 2014a; Zhao et al., 2015). Plausible pathogenesis might involve disturbing the expression, transactivation or homo-/hetero-dimerization of SOHLH1 or SOHLH2 proteins. No subsequent reports exist, to our knowledge.
Phosphatase and tensin homolog (PTEN) (10q23.3)
Localized on chromosome 10q23.3, PTEN plays a causative role in early activation of primordial follicles by negatively regulating the PI3K pathway (Reddy et al., 2008). Primordial follicles become depleted in Pten null mice in early adulthood, mimicking the phenotype of POI in humans. However, no causative mutation was detected in coding regions of PTEN gene in Japanese and Chinese women with POI (Shimizu et al., 2009; Zhao et al., 2011).
Nanos homolog 1, 2, 3 (Drosophila) (NANOS1, 10q26.11; NANOS2, 19q13.32; NANOS3, 19p13.13)
The NANOS gene family is known to be required for primordial germ cell (PGC) development and maintenance. Three homologs (NANOS1, NANOS2 and NANOS3) exist. Disruption of Nanos1 in mice did not affect germ cell development, but knockout of Nanos2 or Nanos3 resulted in infertility with decreased gonad size due to loss of PGC. Nanos2 deficiency only resulted in spermatogonia loss whereas Nanos3 impaired PGC maintenance in both males and females (Tsuda et al., 2003).
Mutations in NANOS3 were investigated in 80 Chinese and 88 Caucasian women with POI (Qin et al., 2007b). No causative mutations were found in coding exons. However, one potentially relevant heterozygous mutation (c.457C>T; p.R153W) was identified in another study involving 100 Chinese POI patients (Wu et al., 2013). Functional studies showed decreased stability of NANOS3, potentially resulting in a hypomorph. And a homozygous mutation (c.358G>A, p.E120K) was found in two sisters with primary amenorrhea from 85 Brazilian women with POI. In vitro and in silico functional studies revealed that this mutation impaired the ability of NANOS3 to prevent apoptosis, suggesting a mechanism for POI involving increased PGC apoptosis during embryonic cell migration (Santos et al., 2014). Taken together, these results suggest a role for NANOS3 mutation in some cases of POI.
Cyclin-dependent kinase inhibitor 1B (CDKN1B) (12p13.1-p12)
CDKN1B, also known as P27 and KIP1, encodes a cyclin-dependent kinase inhibitor that regulates proliferation and differentiation in many tissues. It suppresses ovarian follicle endowment and activation, and promotes follicle atresia. Premature follicle depletion occurred due to accelerated activation in Cdkn1b knockout mice (Rajareddy et al., 2007). Sequence analysis of CDKN1B found one novel heterozygous mutation c.356T>C (p.I119T) in one of 87 Tunisia POI patients (Ojeda et al., 2011). However, no variants were identified in Chinese cohorts (Wang et al., 2010a; Zhao et al., 2013), suggesting that mutations in CDKN1B are not common in POI, at least in this population.
Anti-Mullerian hormone receptor, type II (AMHR2) (12q13)
AMHR2 encodes a receptor in the AMH pathway which plays a crucial role in the development and maintenance of reproductive organs in mammals. Polymorphism c.-482 A>G (rs2002555) in AMHR2 was revealed to be associated with age at menopause in interaction with parity in Dutch women, but no association was found with POI in Korean and Chinese women (Yoon et al., 2013; Qin et al., 2014b). Negative results in AMHR2 were also reported in 16 Japanese women with POI (Wang et al., 2002). However, Qin et al. (2014b) identified two novel missense mutations (p.I209N and p.L354F) in a cohort of Chinese POI women.
KIT ligand (KITLG) (12q22)
The human KITLG gene, located at 12q22, encodes the ligand of a tyrosine-kinase receptor. KIT/KITLG plays a critical role during oogenesis and folliculogenesis. Mice with a deficiency in Kitlg manifested impaired PGCs (Matsui et al., 1991). However, no perturbations were reported in the coding region of KITLG from 40 Caucasian POI patients (Hui et al., 2006).
Forkhead box O1 (FOXO1) (13q14.1)
FOXO1, another member of the forkhead family of transcription factors, is important in granulosa cell function and follicle maturation. Watkins et al. (2006) identified one 5′UTR mutation (c.-30C>T) and one missense mutation (p.P84L) in 60 New Zealand and Slovenia POI patients.
Spalt-like transcription factor 4 (SALL4) (20q13.2)
SALL4, a zinc finger transcription factor, is expressed in murine oocytes. SALL4 binds to POU5F1 and could regulate its expression. Both Sall4 and Pou5f1 are drastically down-regulated in Nobox−/− newborn ovaries (Zhang et al., 2006; Choi et al., 2007). Wang et al. (2009) screened the coding regions of SALL4 in 100 Han Chinese females with non-syndromic POI and identified two heterozygous missense mutations (p.V181M and p.T817A) in the conserved region. These may or may not be POI-associated gene variants. Further studies are needed to determine the functional effect of these variants.
Meiotic protein covalently bound to DSB (SPO11) (20q13.31)
SPO11 is involved in meiosis, forming the double-strand breaks (DSBs) that initiate meiotic recombination. Spo11−/− mice are infertile with premature depletion of oocyte because of defective meiosis. However, no novel variants were found in 41 women with non-syndromic POI (Mandon-Pepin et al., 2008). It is not clear whether an association between SPO11 mutation and sporadic POI exists in human.
DNA meiotic recombinase 1 (DMC1) (22q13.1)
Genes perturbing meiosis are logical candidates for non-syndromic POI. DMC1 encodes a member of the superfamily of recombinases, which are important for repairing double- strand DNA breaks during mitosis and meiosis. Among 41 French women with POI, Mandon-Pepin et al. (2008) found one POI case with homozygous mutation p.M200V. However, a subsequent screening revealed no mutation but two known SNPs in 192 Chinese women with POI (Wang et al., 2012).
Pleiotropic single gene disorders in POI
Distinct from non-syndromic POI, pleiotropic Mendelian disorders may manifest POI as part of their phenotypic spectrum. Indeed, the most common single genetic explanation for POI is represented by such a disorder—premutation for fragile X syndrome (Table III).
Table III.
Candidate genes responsible for Mendelian disorders that manifest POI.
| Gene | Location | Mendelian syndrome | Somatic features | Reference |
|---|---|---|---|---|
| FMR1 | Xq27.3 | Fragile X syndrome | Attention deficits, hyperactivity, social deficits, anxiety disorder, deficits in cognitive flexibility. | Reiss and Hall (2007) and Spath et al. (2010) |
| FOXL2 | 3q23 | Blepharophimosis-ptosis-epicanthus BPE type I syndrome, BPES I | BPES type I is a complex eyelid malformation associated with POI. The major features of the eyelid malformation involve (i) narrowed horizontal aperture of the eyelids (blepharophimosis), (ii) drooping of the upper eyelid (ptosis), (iii) the presence of a fold of skin arising from the lower eyelid that runs inward and upward (epicanthus inversus), and (iv) lateral displacement of the inner canthi (telecanthus). | Zlotogora et al. (1983) and Oley and Baraitser (1988) |
| GALT | 9p13 | Galactosemia | Cataracts, speech defects, poor growth, poor intellectual function, neurologic deficits (predominantly extrapyramidal findings with ataxia). | Schadewaldt et al. (2004) |
| AIRE | 21q22.3 | Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome, APECED | Candidiasis, Addison's disease, hypoparathyroidism, type 1 diabetes, alopecia, vitiligo, ectodermal dystrophy, celiac disease and other intestinal dysfunctions, chronic atrophic gastritis, chronic active hepatitis, autoimmune thyroid disorders, pernicious anemia. | Fierabracci et al. (2012) |
| EIF2B | EIF2B2 -14q24.3; EIF2B4- 2p23.3; EIF2B5- 3q27.1 |
Central nervous system leukodystrophy and ovarian failure, ovarioleukodystrophy | Neurological disorder characterized by involvement of the white matter of the central nervous system. When Leukodystrophies associated with premature ovarian failure referred to as ovarioleukodystrophy. | Mathis et al. (2008) |
| POLG | 15q25 | Progressive external ophthalmoplegia, PEO | Manifestations range from involvement limited to the eyelids and extraocular muscles. | Graziewicz et al. (2007) |
| NOG | 17q22 | Proximal symphalangism, SYM1 | Ankylosis of the proximalinterphalangeal joints. | Kosaki et al. (2004) |
| PMM2 | 16p13 | PMM2-CDG CDG-1 (a previously known as congenital disorder of glycosylation type 1a) | Cerebellar dysfunction (ataxia, dysarthria, dysmetria), non-progressive cognitive impairment, stroke-like episodes, peripheral neuropathy with or without muscle wasting, absent puberty in females, small testes in males, retinitis pigmentosa, progressive scoliosis with truncal shortening, joint contractures, and premature aging | Sparks and Krasnewich (2005) |
|
HSD17B4 HARS2 CLPP LARS2 C10orf2 |
5q21 5q31.3 19p13.3 3p21.3 10q24 |
Perrault syndrome, PS | Sensorineural deafness in both males and females, and neurological manifestations in some patients. | Jenkinson et al. (2013), Morino et al. (2014), Pierce et al. (2011), Pierce et al. (2013) and Pierce et al. (2010) |
| BLM | 15q26.1 | Bloom syndrome | Chromosomal breakage leading to early onset of aging, short stature and elevated rates of most cancers. | Ellis and German (1996) |
| ATM | 11q22-q23 | Ataxia telangiectasia, A-T | Progressive cerebellar degeneration, telangiectasias, immunodeficiency, recurrent infections, insulin-resistant diabetes, premature aging, radiosensitivity, and high risk for epithelial cancers in surviving adults. | Gatti et al. (1991) and Su and Swift (2000) |
| WRN | 8p12 | Werner syndrome | Premature aging of the skin, vasculature, and bone and elevated rates of certain cancers, particularly sarcomas. | Epstein et al. (1966) |
| RECQL4 | 8q24.3 | Rothmund–Thomson syndrome, RTS | Cutaneous rash, sparse hair, small stature, skeletal and dental abnormalities, cataracts, premature aging, and an increased risk for cancer, especially malignancies originating from bone and skin tissue. | Wang et al. (2001) |
FMR1: Fragile X mental retardation 1; FOXL2: forkhead box L2; GALT: galactose 1-phosphate uridyl transferase; AIRE: autoimmune regulator; EIF2B: eukaryotic translation initiation factor; POLG: polymerase (DNA directed), gamma; NOG: noggin; PMM2: Phosphomannomutase 2; HSD17B4: Hydroxysteroid (17-beta) dehydrogenase 4; HARS2: Histidyl-tRNA synthetase 2, mitochondrial; CLPP: caseinolytic mitochondrial matrix peptidase proteolytic subunit; LARS2: leucyl-tRNA synthetase 2, mitochondrial; C10orf2: Chromosome 10 open reading frame 2; BLM: Bloom syndrome, RecQ helicase-like; ATM: ATM serine/threonine kinase; WRN: Werner syndrome, RecQ helicase-like; RECQL4: RecQ protein-like 4.
Fragile X syndrome: familial mental retardation 1 (FMR1) (Xq27.3)
Perturbations of FMR1 are responsible for fragile X syndrome. Clinical features include mental retardation, characteristic facial features with large ears and prominent jaw, connective tissue findings (joint laxity), large testes after puberty, and behavioral abnormalities. Fragile X syndrome occurs in males when CGG repeats number above 200. In females ∼70% of women with >200 CGG repeats show intellectual disability (de Vries et al., 1996). The incidence of fragile X syndrome in males is approximately 1 in 4000, and in females 1 in 8000 (ACOG committee opinion, 2006a)
The normal number of CGG repeats in FMR1 is 32. Thereafter, there is a stage (premutation) in which 54–200 CGG repeats exist. Pathogenic effects including mental retardation and ataxia may exist, as well as POI. About 15–20% of women with a FMR1 premutation develop POI (Wittenberger et al., 2007). Conversely, 5% of sporadic cases and 10–15% of familial cases in the Caucasian population are explained by FMR1 premutations. For reasons that are not clear, the number of CGG repeats significantly correlates with risk of POI only within selected ranges. There is only a slightly increased risk of expansion associated with 40–79 repeats; but higher risk with 80–99 repeats, yet no further increased risk occurs after >100 repeats, and as noted POI is not observed with the full mutation (>200 CGG) (Allingham-Hawkins et al., 1999). One possible explanation is that certain genes are suppressed in the 54–100 premutation range and link to POI, whereas other genes become suppressed only with higher numbers of CGG repeats. Perhaps, then, phenotypes in the two groups differ because some ovarian genes are inhibitory and others are the converse. Thus, ovarian function may be initially suppressed but later return to normal function.
Given the comparatively higher frequency of premutation of FMR1 in POI than the general population (Supplemental Table SIII), FMR1 testing has become part of the work-up for women with POI. It is formally recommended in Europe (Foresta et al., 2002; European Society of Human Genetics; European Society of Human Reproduction and Embryology, 2006b). In other populations the prevalence is lower. Guo et al. (2014) reported that only 2 premutation carriers were found in 379 sporadic Chinese POI cases (0.49%); none were found in 402 controls. The frequency in Chinese women is thus considerably lower than in Caucasian women (3.3–6.7%). Frequencies are 1.56% in Japanese (Ishizuka et al., 2011) and 4.8% in Slovenian women (Gersak et al., 2003).
Blepharophimosis-ptosis-epicanthus syndrome (BPES): forkhead box L2 (FOXL2) (3q23)
Blepharophimosis-ptosis-epicanthus syndrome (BPES) is a pleiotropic autosomal dominant syndrome in which FOXL2 is perturbed and premature ovarian failure occurs (Crisponi et al., 2001). That FOXL2 plays a key if not the pivotal role in ovarian development initially came from study of BPES kindreds. More than one hundred unique FOXL2 mutations have now been described in BPES in different populations (Beysen et al., 2004). By contrast, constitutional mutations are uncommon but reported in non-syndromic POI. Perhaps 2–3% of isolated POI cases have a FOXL2 mutation (De Baere et al., 2002; Harris et al., 2002) (Supplementary Table SII).
Galactosemia: galactose 1-phosphate uridyl transferase (GALT) (9p13)
Galactosemia is caused by deficiency of galactose 1-phosphate uridyl transferase (GALT). Ovarian failure is a common long-term complication in girls and women with galactosemia, first described by Kaufman and coworkers (Kaufman et al., 1979, 1981), who observed POI in 12 of 18 (67%) galactosemic women. Later Waggoner et al. (1990) reported that 17% (8/47) galactosemic women presented with ovarian failure. However, with regard to duarte galactosemia, a mild variant of GALT deficiency, no apparent ovarian dysfunction was reported (Badik et al., 2011). Pathogenesis involves excess galactose toxicity that impairs folliculogenesis, induces resistance to gonadotrophins and accelerates follicular atresia (Fridovich-Keil et al., 2011; Banerjee et al., 2012).
Carbohydrate-deficient glycoprotein syndrome type I (CDG-Ia): phosphomannomutase 2 (PMM2) (16p13)
In type 1 carbohydrate-deficient glycoprotein deficiency, also named phosphomannomutase deficiency, mannose-6-phosphate cannot be converted to mannose-1-phosphate. This lipid linked oligosaccharide is necessary for formation of secretory glycoproteins. Neurologic abnormalities and ovarian failure occur (de Zegher and Jaeken, 1995; Kristiansson et al., 1995). Located on 16p13, PMM2 is typically caused by a missense mutation (Bjursell et al., 1997).
Proximal symphalangism (SYM1) and multiple synostoses syndrome (SYNS1): noggin (NOG) (17q22)
NOG encodes a secreted polypeptide that binds to and inactivates members of the TGF-β superfamily (i.e. BMP2, 4, 7, 14 and GDF5). NOG is expressed in various tissues including female reproductive organs. NOG mutations are known to explain proximal symphalangism (SYM1) and multiple synostoses syndrome (SYNS1) (Gong et al., 1999). In addition, Kosaki et al. (2004) described a heterozygous mutation (p.E48K) in NOG in a female presenting with SYM1 and also having POI. However, Laissue et al. (2007) concluded the relationship between NOG mutations and non-syndromic POI was not clear, having screened the coding sequence of NOG in 100 non-syndromic sporadic POI patients and identifying only one heterozygous mutation (p.G92E) (Supplementary Table SII). Actually this experience mirrors the situation involving other pleiotropic genes causing POI.
Autoimmune regulation/autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED): autoimmune regulator (AIRE) (21q22.3)
The AIRE gene can if perturbed lead to multi-system abnormalities: alopecia, vitiligo, keratopathy, malabsorption, hepatitis and mucocutaneous candidiasis. Ovarian hypoplasia often occurs, usually in the third decade (Wang et al., 1998). Many different AIRE perturbations have been found in this autosomal dominant disorder, not only nonsense mutations but also frame shifts. No particular mutation leads to ovarian failure as distinct from other autoimmune phenomena. AIRE mutations have not yet been sought in women with isolated POI (non-syndromic).
Ovarian leukodystrophy: eukaryotic translation initiation factor (EIF2B): subunit 2 beta, 39 kDa (EIF2B2) (14q24.3); subunit 4 delta, 67 kDa (EIF2B4) (2p23.3); subunit 5 epsilon, 82 kDa (EIF2B5) (3q27.1)
‘Vanishing white matter’ leads to variable but progressive neurological degeneration. Ovarian failure may coexist (Schiffmann et al., 1997; Boltshauser et al., 2002). That the causative gene (EIF2B) allows denatured stress-related proteins to accumulate makes plausible the relevance to oogenesis, given ongoing oocyte degeneration. Fogli et al. (2003) found variants in EIF2B2, EIF2B4 and EIF2B5 in seven POI cases with neurologic abnormalities, but in 93 non-syndromic POI none were found positive (Fogli et al., 2004). Therefore, EIF2B genes have not yet been shown to be responsible for non-syndromic POI.
Perrault syndrome: hydroxysteroid (17-beta) dehydrogenase 4, HSD17B4, 5q21; histidyl-tRNA synthetase 2, mitochondrial, HARS2, 5q31.3; leucyl-tRNA synthetase 2, mitochondrial, LARS2, 3p21.3; caseinolytic mitochondrial matrix peptidase proteolytic subunit, CLPP, 19p13.3; chromosome 10 open reading frame 2, C10orf2, 10q24
Perrault syndrome is a well-recognized pleiotropic autosomal recessive disorder characterized by ovarian failure in females, progressive sensorineural deafness in both males and females, and in some patients, neurological manifestations. Only recently have the underlying genes been identified and proved to be heterogeneous. In a family of mixed European ancestry with two sisters presenting with Perrault syndrome, compound heterozygous variants - c.650A>G (p.Y217C) and c.1704T>A (p.Y568X), in HSD17B4 were found. Then with similar genomic strategies with linkage analysis or whole-exome sequencing (WES), mutations in HARS2, LARS2, CLPP and C10orf2 have been found in the context of Perrault syndrome (Pierce et al., 2011, 2013; Jenkinson et al., 2013; Morino et al., 2014). All these genes are essential for normal mitochondrial function. This group of causative genes is detailed in ‘Mitochondrial Genes Causing POI’ Section.
Other pleiotropic disorders
Table III lists other nuclear genes causing syndromes in which POI is a feature. These include POLG associated with progressive external ophthalmoplegia (PEO, detailed in ‘Mitochondrial Genes Causing POI’ section), BLM with Bloom syndrome, AIRE with Ataxia telangiectasia, WRN with Werner syndrome and RECQL4 with Rothmund–Thomson syndrome. A common feature of several is chromosomal breakage, best exemplified by Bloom syndrome (Simpson and Elias, 2003; Simpson, 2014).
In aggregate these and other conditions in Table III explain no more than 1% of cases of POI. From a scientific perspective however, elucidating the role these genes play in reproduction offers novel clues to integrity needed for normal ovarian differentiation. Clinicians caring for females with these syndromes should thoroughly investigate and evaluate any reported menstrual irregularities.
Mitochondrial genes causing POI
Perturbations of mitochondrial genes or nuclear genes affecting mitochondria are good candidates for POI because the mature oocyte has the greatest number of mitochondria of any human cell. Mature oocytes readily accumulate mitochondria during oogenesis, mitochondrial biogenesis playing an essential role in oocyte maturation, fertilization and embryo development. Dysregulation of mitochondrial dynamics contributes to excess oxidative stress and initiation of apoptosis, thus accelerating follicle depletion. A marked quantitative decrease of mitochondrial DNA (mt DNA) in oocytes and peripheral blood cells has been well documented in women with ovarian insufficiency (May-Panloup et al., 2005; Bonomi et al., 2012). Thus, any gene affecting mitochondria involving muscular and neurological disturbance is a candidate, because these systems are so dependent on mitochondrial integrity. Genes governing mitochondrial functions may be located in the nucleus, like those discussed in ‘Single Genes Causing Non-syndromic POI’ section, or in mitochondria itself (mt DNA). To date, those of relevance to POI have been nuclear genes.
Progressive external ophthalmoplegia (PEO): polymerase (DNA directed), gamma (POLG) (15q25)
mtDNA mutations usually affect muscular and neurological systems. In progressive external ophthalmoplegia (PEO) clinical features are proximal myopathy, sensory ataxia and parkinsonism. The causative mutation lies in the gene encoding polymerase gamma (POLG), which is responsible for mtDNA replication and repair. In three of seven families studied by Luoma et al. (2004), POI cosegregated with PEO. A p.Y955C mutation in POLG was found in two of these three families. p.Y955C (tyrosine to cytosine) affects a highly conserved region, rending functional plausibility. Compound heterozygosity (N468D/A1105T) was observed by Pagnamenta et al. (2006) in another 3-generation family in which multiple family members were affected with both PEO and POI. Mutation screening of POLG in isolated POI, has, however, yielded few perturbations. A single p.R953C mutation was found in 201 POI cases (0.5%) cumulatively reported by Tong et al. (2010). No novel mutations were identified in Italian and British women with POI (Pagnamenta et al., 2006; Bonomi et al., 2012; Duncan et al., 2012) (Supplementary Table SII). Therefore, POLG variation is not a common explanation for sporadic POI in the absence of clinical suspicion for other mitochondrial-associated physical signs.
Perrault syndrome: HARS2, 5q31.3; LARS2, 3p21.3; CLPP, 19p13.3; C10orf2, 10q24
As reviewed in ‘Pleiotropic Single Gene Disorders Having POI’ Section, ovarian failure is one of the characterized features in Perrault syndrome in females. The genes listed encode mitochondrial tRNA synthetase, chambered protease and primase-helicase and have been found to harbor mutations responsible for POI in Perrault syndrome (Pierce et al., 2011, 2013; Jenkinson et al., 2013; Morino et al., 2014). Through WES in a consanguineous Palestinian family and a nonconsanguineous Slovenian family with Perrault Syndrome, homozygous mutation c.1565C>A (p.T522N) and compound heterozygous mutation c.1077delT and c.1886C>T (p.T629M) in the LARS2 gene were identified, respectively (Pierce et al., 2013). WES was also performed in a nonconsanguineous family of mixed European ancestry, showing compound heterozygous mutations c.598C>G (p.L200V) and c.1102G>T (p.V368L) in the HARS2 gene (Pierce et al., 2011). The homozygous mutations c.433A>C (p.T145P), c.440G>C (p.C147S) and c.270+4A>G in the CLPP gene were observed in three consanguineous Pakistani families with Perrault Syndrome (Jenkinson et al., 2013). In a Japanese family, affected individuals carried compound heterozygous p.R391H and p.N585S in the C10orf2 gene essential for replication of mtDNA; meanwhile compound heterozygous mutations p.W441G and p.V507I were responsible for this disease in another family of European ancestry (Morino et al., 2014).
In aggregate, the above supports a critical role for genes controlling mitochondria in the maintenance of ovarian function and their roles in non-syndromic POI warrant further investigation.
Multiple malformation syndromes characterized by POI
In other syndromes, POI or primary ovarian failure (gonadal dysgenesis) is accepted as one component but the causative gene has not been found. Supplementary Table SIV lists these conditions. Of particular interest is POI associated with cerebellar ataxia (Simpson, 2013).
Genome-wide studies in POI
Contemporary genetic strategies applied to locate susceptible loci or genes causing POI have extended beyond suspected candidate gene interrogations to genome-wide approaches. Approaches include linkage analysis in families with multiple affected members, CGH for CNV, genome-wide association studies (GWAS), genome-wide sequencing of exomes (WES) and, in the future, whole genome sequencing (WGS).
GWAS
In GWAS, also known as whole genome association study (WGAS), one examines many common genetic variants in different individuals to see if any variant is associated with a trait. In GWAS one searches agnostically through the entire genome to identify variants (SNPs) more common in cases than controls of similar ethnicity. No a priori expectations exist. Six GWAS have been performed to identify variants associated with POI, but not all adhered to accepted criteria. Sample sizes were often very small and replicates not sought (Table IV). The first GWAS showed association with the PTH-responsive B1 gene (PTHB1) in a small discovery set of 24 women and 24 controls (Kang et al., 2008). Knauff et al. (2009) identified an association of an intron SNP in ADAM metallopeptidase with thrombospondin type 1 motif, 19 (ADAMTS19) with POI in a discovery set of only 99 Dutch women and 181 controls; no replication set existed. Laminin, gamma 1 (LAMC1) was then reported to be significantly associated with POI in Korea in 122 cases versus 242 controls (Pyun et al., 2012).
Table IV.
Genome-wide association studies for POI.
| Kang et al. (2008) | Knauff et al. (2009) | Qin et al. (2012b) | Pyun et al. (2012) | Oldenburg et al. (2008) | Caburet et al. (2012) | |
|---|---|---|---|---|---|---|
| Ethnicity | Korean | Caucasian (Dutch) | Chinese | Korean | Dutch | Middle-Eastern |
| Discovery set | ||||||
| No. of cases | 24 | 99 | 391 | 24 | 10 | 5 |
| No. of controls | 24 | 181 | 895 | 24 | 5 | 4 |
| Associations in discovery set |
PTHB1 at 7p14 showed strongest association. Ht1 GAAAG: POI-susceptible haplotype; Ht2 TGTGC: POI-resistant haplotype. |
rs246246 mapped to ADAMTS19 intron | 8q22.3 (10−6) |
22 SNPs in LAMC1 associated with POI | Susceptible locus: 5q14.1-q15 | Susceptible loci: 7p21.1-15.3, 7q21.3-22.2 |
| Replication set | ||||||
| No. of cases | 101 | 60 | 400 | 98 | – | – |
| No. of controls | 87 | 90 | 800 | 218 | – | – |
| Results of replication set | PTHB1 associated with POI; Ht1 confers susceptibility to POI. | Association not confirmed. | Frequencies of 9 SNPs and 1 haplotype were higher in POI than in control. | – | Sequencing three candidate genes DLX5, DLX6 and DSS1 did not reveal causal mutations |
PTHB1: Bardet–Biedl syndrome 9 (BBS9); ADAMTS19: ADAM metallopeptidase with thrombospondin type 1 motif, 19; LAMC1: laminin, gamma 1; DLX5, 6: distal-less homeobox 5 and 6; DSS1: split hand/foot malformation (ectrodactyly) type 1.
Our group conducted the largest GWAS with an initial discovery set of 391 cases versus 895 controls; the independent replication set consisted of 400 cases and 800 controls. The most significant association was at 8q22.3 (1.6 × 10−6–3.86 × 10−6). This falls short of the canonical 10−8 expected to confer unequivocal significance. This region does not contain a protein coding candidate gene (Qin et al., 2012b). However, it is now appreciated that 90% of significant GWAS associations are in intragenic or intergenic regions, portions of the 98.5% of the genome not coding for protein. These regions include a host of regulatory genes and networks (ENCODE). 8q22.3 may be an important yet undefined long-distance regulatory region affecting ovarian differentiation and oogenesis. Disruption might lead to ovarian failure, analogous to disruption of the region upstream of Sex determining region Y-box 9 (SOX9) that causes XY sex reversal in mice and humans (Qin et al., 2003, 2004). Replication in independent cohorts needs to be performed to determine potential causative roles.
GWAS have revealed multiple loci potentially associated with POI in Chinese, Korean, and Dutch women. However, in each it was difficult to implicate specific novel genes, and in none did significance exceed 10−6; positive findings were not always replicated. The most likely explanation for these data is limitation based on small sample size. In no study did sample number exceed 1000, thus lacking statistical power sufficient to detect a modest association when evaluating hundreds of thousands of SNPs. That POI is a rarer condition than polycystic ovary syndrome or endometriosis makes it difficult to accrue the requisite sample size possible in studying those disorders (Uno et al., 2010; Chen et al., 2011a; Shi et al., 2012).
GWAS involving family linkage analysis
Table IV includes two genome-wide association linkage studies involving single extended families. In a Dutch POI family subjected to a genome-wide linkage scan 5q14.1-q15 was found to be a susceptibility region (Oldenburg et al., 2008). Caburet et al. (2012) performed genome-wide linkage and homozygosity analysis in a large consanguineous Middle-Eastern POI-affected family showing autosomal inheritance. Two regions—7p21.1-p15.3 and 7q21.3-q22.2—were identified as candidate regions by homozygosity mapping. However, sequencing the three most plausible candidate genes in this region—DLX5, DLX6 and DSS1—failed to reveal mutations.
GWAS involving age of menopause
Insights derived from shared genetic susceptibility between POI and either age at natural menopause (AANM) or early menopause (EM) represent another potential path identifying novel entry points for unraveling genetic mechanism involved in POI. Thus, we gathered 36 SNPs shown from previous GWAS studies seeking SNPs associated with AANM or EM, plus 3 additional SNPs in ESR1 and 2 additional SNPs in PTHB1. Differential association was then sought in 371 POI and 800 controls, all of Chinese Han origin (Qin et al., 2012c). Three SNPs, rs2278493 in hexokinase 3 (HK3), rs2234693 in estrogen receptor 1 (ESR1) and rs12611091 in BR serine/threonine kinase 1 (BRSK1), showed nominally significant association with POI. Thus, a plausible relationship could exist between POI and ESR1, BRSK1, HK3.
Cytogenomic studies (CNV)
There is increasing interest toward whole genome studies based on CNVs. Using CGH, one can efficiently search for small duplications or deletions potentially associated with a complex trait (such as POI). The purpose of identifying CNVs is that they may contain or be linked to a causative gene for the disorder studied. Seven array CGH studies have identified CNVs associated with POI (Supplementary Table SV).
In 2009, Aboura et al. (2009) reported that 8 previously known CNVs in 99 POI French patients located near 5 potential candidate genes—Dynein, axonemal, heavy chain 5 (DNAH5), NLR family, apoptosis inhibitory protein (NAIP), Dual specificity phosphatase 22 (DUSP22), Nuclear protein, transcriptional regulator, 1 (NUPR1), and AKT1. However, this and all other CGH studies have the major pitfall of usually lacking information on parents. A large, de novo, CNV deletion is more likely to be pathogenic than an inherited CNV. Another array CGH study involving 74 German patients with POI or ovarian dysgenesis identified 44 losses or gains at autosomes and X chromosome that might explain POI (Ledig et al., 2010). McGuire et al. (2011) identified 17 novel microduplications and 7 novel microdeletions among 89 POI patients, all but one located at autosomes. Included were two novel microdeletions causing haploinsufficiency for Synaptonemal complex central element protein 1 (SYCE1) and Cytoplasmic polyadenylation element binding protein 1 (CPEB1), genes known to cause ovarian failure in knockout mouse models (Bolcun-Filas et al., 2009; Novoa et al., 2010). Recently, a high-resolution array CGH identified eleven unique CNVs in 11 patients with POI. Among these CNVs, a tandem duplication of 475 bp containing 3 NOBOX-binding elements and an E-box important for GDF9 gene regulation in the promoter of GDF9 is likely causative of POI (Norling et al., 2014).
Using a complete X chromosome tiling path array, Quilter et al. (2010) found 15 novel discrete X chromosome intervals in 20/42 (48%) women with POI in the UK. However, in patients from New Zealand, Dudding et al. (2010) detected only two microduplications (Xp22.33 and Xq13.3) in a low frequency of 4%. Knauff et al. (2011) found one CNV in Xq21.3 to be associated with POI, specifically in a region where Protocadherin 11 X-linked (PCDH11X) and TGFB-induced factor homeobox 2-like, X-linked (TGIF2LX) are located. Interestingly, no deletions were found in other regions, several considered on the basis of traditional cytogenetic studies to be pivotal (Simpson and Rajkovic, 1999).
Differing array CGH platforms and definitions for pathogenicity probably explain some of the discrepant results among the above studies. Also problematic is the limited sample size and lack of parental studies to exclude a CNV ostensibly associated with POI merely having been transmitted from a normal mother.
WES
Low prevalence and impaired fecundity resulting in limited numbers of POI pedigrees without associated somatic anomalies (non-syndromic) has led to increasing application of WES, another agnostic approach. WES has identified several genes in POI not previously anticipated.
In POI associated with somatic features, causative perturbations have been found by WES for HSB17B4, LARS2, CLPP and C10orf2 in Perrault syndrome (Pierce et al., 2010, 2013; Jenkinson et al., 2013; Morino et al., 2014) (detailed in ‘Mitochondrial Genes Causing POI’ section and Table V). Up to the present, there have been six WES conducted in non-syndromic POI pedigrees. Interestingly, almost all plausible candidate genes identified were involved in meiosis and DNA repair (Table V).
Table V.
Whole-exome sequencing for syndromic or isolated POI.
| Gene | Location | Mutation | Function | Compound Het/Homo/Het | Reference |
|---|---|---|---|---|---|
| HSB17B4 | 5q21 | c.650A>G (p.Y217C); c.1704T>A (p.Y568X) | Reduced expression with mutant protein | Compound heterozygous | Pierce et al. (2010) |
| CLPP | 19p13.3 | c.433A>C (p.T145P), | Alter the structure of the CLPP barrel chamber that captures unfolded proteins and exposes them to proteolysis | Homozygous | Jenkinson et al. (2013) |
| c.440G>C (p.C147S) | |||||
| c.270+4A>G | Weakens donor splice-site function | ||||
| LARS2 | 3p21.3 | c.1565C>A (p.T522N) | Partially functional in Yeast complementation assay | Homozygous | Pierce et al. (2013) |
| c.1077delT; c.1886C>T (p.T629M) |
Nonfunctional in Yeast complementation assay | Compound heterozygous | |||
| C10orf2 | 10q24 | c.1172G>A (p.R391H) c.1754A>G (p.N585S) | Affect interactions of the linker domain Likely affect enzyme activity |
Compound heterozygous | Morino et al. (2014) |
| c.1321T>G (p.W441G) c.1519G>A (p.V507I) | Affect interactions of the linker domain | Compound heterozygous | |||
| HFM1 | 1p22.2 | c.1686-1G>C c.2651T>G (p.I884S) |
Compound heterozygous | Wang et al. (2014a) | |
| c.2206G>A (p.G736S); c.3929_3930 delinsG, (p.P1310R fs*41) | Compound heterozygous | ||||
| MCM9 | 6q22.31 | c.1732+2T>C | Abnormal splicing and truncated protein that are unable to be recruited to sites necessary for DNA damage | Homozygous | Wood-Trageser et al. (2014) |
| c.394C>T (p.R132*) | Repair of chromosome breaks impaired in lymphocytes | Homozygous | |||
| STAG3 | 7q22.1 | c.968delC (p.F187fs*7) | Homozygous | Caburet et al. (2014) | |
| SYCE1 | 10q26.3 | c.613C>T (p.Q205*) | Homozygous | de Vries et al. (2014) | |
| MCM8 | 20p12.3 | c.446C>G (p.P149R) | Impedes the repair of MMC-induced chromosomal breaks; inhibits MCM8 recruitment to sites of DNA damage; impairs DNA binding ability at the N-terminus | Homozygous | AlAsiri et al. (2015) |
| eIF4ENIF1 | 22q11.2 | c.1286C>G (p.S429*) | Heterozygous | Kasippillai et al. (2013) |
HSD17B4: Hydroxysteroid (17-beta) dehydrogenase 4; CLPP: Caseinolytic mitochondrial matrix peptidase proteolytic subunit; LARS2: leucyl-tRNA synthetase 2, mitochondrial; C10orf2: Chromosome 10 open reading frame 2; HFM1: ATP-dependent DNA helicase homolog; MCM8, MCM9: minichromosome maintenance complex component 8 and 9; STAG3: stromal antigen 3; SYCE1: synaptonemal complex central element; eIF4ENIF1: eukaryotic translation initiation factor 4E nuclear import factor 1.
ATP-dependent DNA helicase homolog (HFM1) (1p22.2)
HFM1, a meiotic gene encoding DNA helicase preferentially expressed in germ-line tissues such as testis and ovary, is necessary for homologous recombination and synapsis during meiosis (Tanaka et al., 2006). Hfm1−/− female mice had significantly reduced ovaries and follicle numbers and an increase in stromal cells (Guiraldelli et al., 2013). The recent report by Wang et al. (2014a) identified a compound heterozygous mutation (c.1686-1G>C and c.2651T>G, p.I884S) in the HFM1 gene in two affected Chinese sisters. Screening for HFM1 mutations in a cohort of 69 Chinese women with sporadic POI identified another patient with compound heterozygous mutations (c.2206G>A, p.G736S and c.3929_3930 delinsG, p.P1310R fs*41). These variants were not found in 316 matched controls or databases.
Minichromosome maintenance complex component 8 and 9 (MCM8, 20p12.3; MCM9, 6q22.31)
MCM8 and MCM9, recently discovered members of the highly conserved mini-chromosome maintenance proteins (MCM), are genes implicated in homologous recombination and repair of double-stranded DNA breaks. The MCM8/MCM9 complex is required for the resolution of dsDNA breaks that occur during homologous recombination in pachytene of meiosis I. Failure to resolve breaks predictably leads to oocyte death and small or absent ovaries. Mcm8 and Mcm9-deficient mice are infertile and have small gonads due to germ cell depletion (Lutzmann et al., 2012). Recently, Rajkovic and colleagues (AlAsiri et al., 2015; Wood-Trageser et al., 2014) discovered homozygous mutations in MCM8 and MCM9 genes in consanguineous families with POI (primary amenorrhea), using WES. One of the two mutations found in MCM9 is c.1732+2T>C, which resulted in abnormal alternative splicing and truncated forms of MCM9 that are unable to be recruited to sites of DNA damage. The other mutation c.394C>T (p.R132*) results in loss of function of MCM9. It is suspected that preferential sensitivity of germline meiosis to MCM9 functional deficiency and compromised DNA repair in the somatic component most likely account for the ovarian failure and short stature. A homozygous mutant c.446C>G (p.P149R) found in MCM8 inhibited recruitment of MCM8 to sites of DNA damage and led to genomic instability. ASNP rs16991615 in MCM8 was also found associated with the age of natural menopause in the GWAS previously discussed (Chen et al., 2012). The role the novel MCM8/MCM9 pathway plays in women with idiopathic POI needs to be explored further.
Stromal antigen 3 (STAG3) (7q22.1)
STAG3 encodes a subunit of cohesin, a large protein complex that is essential for proper pairing and segregation of chromosomes during meiosis. A homozygous frameshift mutation resulting from a 1-bp deletion (c.968delC, p.F187fs*7) in STAG3 was identified in a highly consanguineous Palestinian pedigree; in which a 10-Mb region on 7q21.3–22.2 and a 3-Mb region on 7p21.1–15.3 had been previously identified to show significant linkage with the POI phenotype (Caburet et al., 2012, 2014). Deficiency of Stag3 in female mice results in severe and early ovarian dysgenesis, with distinctive lack of oocytes and ovarian follicles. Early meiotic arrest and the centromeric chromosomal cohesion defects were observed in Stag3−/−fetal oocytes providing further evidence that Stag3 is essential for assembly of the meiotic cohesin ring and the synaptonemal complex (Caburet et al., 2014).
Synaptonemal complex central element 1 (SYCE1) (10q26.3)
The SYCE1 gene encodes a component of the synaptonemal complex where paired chromosome homologs closely associate in meiosis (synapsis) before crossover. Syce1−/− mice were infertile with smaller gonads, and showed loss of follicles in ovaries and postmeiotic cells in testis. Early meiosis arrest at prophase\xE2\x85followed by cell apoptosis could explain the phenotype (Bolcun-Filas et al., 2009). Different microdeletions of SYCE1 have also been reported in Caucasian and Chinese POI patients (McGuire et al., 2011; Zhen et al., 2013). In a consanguineous Israeli Arab family, a homozygous nonsense mutation (c.613C>T, p.Q205*) in SYCE1 was identified in two sisters with primary amenorrhea, presumably inherited in autosomal recessive fashion (de Vries et al., 2014).
Eukaryotic translation initiation factor 4E nuclear import factor 1 (eIF4ENIF1) (22q11.2)
eIF4ENIF1, a transport protein, plays an important role in repressing translation through eIF4E. Both genes appear to play important roles in ovarian germ cell development (Villaescusa et al., 2006). A heterozygous nonsense mutation (c.1286C>G, p.S429*) was identified in eIF4ENIF1, segregating with ovarian insufficiency (menopause age 29–35 years) in a large French Canadian family. No additional mutations were identified in eIF4ENIF1 or eIF4E in 38 unrelated women with isolated POI. Haploinsufficiency or nuclear sequestration might disrupt the development of a normal oocyte complement (de Vries et al., 2014).
The whole exome studies in familial POI mentioned above mainly involve genes crucial during meiosis, such as generating and repairing DSBs, chromosome synapsis and recombination, and sister chromatid cohesion. This implies that perturbation of genes encoding proteins that regulate meiosis can result in autosomal recessive primary ovarian insufficiency in humans. Despite some negative candidate gene results with meiotic genes (DMC1, SPO11 and MSH4) in POI, WES results still provide reasons to pursue other genes participating in meiosis and DNA repair in ubiquitous pathways.
NGS
Future studies on targeted candidate genes of POI can be anticipated using NGS. A recent abstract (Fonseca et al., 2015) reported a study of 12 POI cases having non-syndromic POI and 176 control women whose menopause had occurred after age 50 years. A further 345 women from the same ethnic origin were stated to be recruited to assess allele frequency for potentially deleterious sequence variants. In the 12 POF cases, complete coding regions of 70 candidate genes were fully sequenced with mutations claimed in ADAMTS19, BMPR2 and LHCGR genes. Full details are awaited.
Regulatory genes and networks
Seeking POF genes has to date largely focused on coding variants, presuming plausible protein disruption. However, only 1.5% of the genome is protein-coding. Indeed, many POF-associated variants in whole genome studies map within, or in linkage disequilibrium to, intronic or intergenic regions; thus, these regions likely contain causative regulatory genes or networks. In our own POI GWAS, for example, ‘gene desert’ 8q22.3 was the region of most significance by association (Qin et al., 2012b).
Non-coding variants must be more robustly interrogated. Perturbations sought should include involving non-coding RNA (microRNA [miRNA], long non-coding RNA [lncRNA]), disruption or creation of alternative splicing or transcription factor-binding sites, and epigenetic modifications (DNA methylation patterns, chromatin modification). Large epigenetic consortia, such as ENCODE (http://genome.ucsc.edu/ENCODE/), Roadmap (http://www.roadmapepigenomics.org/) and iHEC (http://www.ihec-epigenomes.org/) point to approaches used to characterize the regulatory landscape of susceptibility regions for specific cell types. Unfortunately, ENCODE (Birney et al., 2007; ENCODE Project Consortium, 2012; Stamatoyannopoulos et al., 2012) bypassed the reproductive track (males and females), and thus an ovarian ENCODE does not yet exist.
In order to generate the reproductive ENCODE, human ovarian tissue of specific cell types must be studied. Then, differences in regulatory genes between affected and normal individuals can be determined. Massive parallel sequencing using RNA-seq, or Methyl-seq will facilitate a systematic study of the transcriptome in ovaries in relation to genotypes and variants in POI. Chromatin immunoprecipitation followed by sequencing (ChIP-Seq) and RNA-Seq can also help identify novel transcriptional and epigenetic regions and, hence, mechanisms that potentially underlie POI. To analyze the complete proteomes and quantification of post-translational modifications, mass spectrometry would be the most attractive approach.
Having accumulated the requisite information, network and pathway-based analyses of assembled datasets can be assembled using systems biology approaches. Cascades of transcriptional regulation can be evaluated simultaneously, identifying overlap among genes or putative networks that currently constrain key variables in model systems.
The ultimate proof that an identified variant has a pathological effect traditionally relies on constitutive or conditional knock in/out or cell lines with a mutation signature. Increasingly, other approaches will be utilized. Patient-specific induced pluripotent stem cells (iPSCs) lines might offer an individually targeted genetic model system to identify and manipulate pathologic pathway. Use of iPSCs should be of particular value in assessing regulatory genes, which may have a quantitative deleterious effect rather than ‘all or none’ qualitative effect, observed or assumed, in missense or nonsense protein-coding mutations.
Conclusion: current status of genes causing POI
The POI—causative genes surveyed and found to date allow us to reach several conclusions.
First, many genes have emerged as POI candidates (Fig. 1), but in non-syndromic POI only a minority have been proven equivocally causative by functional validation. These include BMP15, PGRMC1, and FMR1 premutation on the X chromosome and on autosomes GDF9, FIGLA, FSHR, NOBOX, NR5A1, NANOS3, STAG3, SYCE1, MCM8/9 and HFM 1. No perturbations have been found in a dozen other plausible candidates for which murine knockouts show ovarian failure, but this may simply reflect small sample sizes or interrogation restricted to a single ethnic group.
Figure 1.
Schematic representation of chromosomal location of plausible genes associated with primary ovarian insufficiency.
Second, notable differences in frequency exist among different populations. This is predictable for any genetic condition, and in POI this has already been observed in FSHR, BMP15, NOBOX, FOXL2, TGFBR3, CDKN1B and FOXO3A (Supplementary Table SII). Future genetic studies should involve different ethnic groups and larger sample sizes. Clinically, caution should apply when counseling on the basis of data derived from an ethnic group different from that of the counseled individual.
Third, causative POI genes are increasingly being shown not only to be restricted to expression in ovaries but also expressed ubiquitously. True, POF5 (NOBOX) and POF6 (FIGLA) are restricted to the ovaries. However, PTEN is a major regulator of the PI3K pathway involved in systemic cell proliferation, survival, migration and metabolism. PTEN also plays a vital role in the activation of primordial follicles. The spectrum of candidate genes potentially causing POI is being enriched.
Fourth, many genes that currently appear isolated in function actually may be interrelated within yet to be defined pathways. It is logical to stratify by gene function in ostensibly distinct systems: endocrine, folliculogenesis, cell cycle, meiosis, mitochondrial, as examples. More difficult are gene-gene or protein-protein interactions, acting in ways not yet evident. This review thus unavoidably overestimates the role that protein-coding genes play to the detriment of upstream and downstream regulatory genes. Until recently, exome studies had to be restricted to sequencing individual candidate genes. Continued advances in sequencing techniques and contemporary bioinformatives will facilitate finding additional genes responsible for POI in other portions of the genome.
Fifth, elucidating the etiology and molecular basis of POI is of paramount importance not only in understanding ovarian physiology but also in providing genetic counseling and fertility guidance. Once additional variants are detected, it will be increasingly possible to predict the age of menopause. Women having certain perturbations of POI can be offered the option of oocyte cryopreservation, with later thawing and use in assisted reproductive technology at the appropriate age.
Supplementary data
Supplementary data are available at http://humupd.oxfordjournals.org/.
Authors' roles
Y.Q. contributed to literature searching and drafting the article. X.J. participated in literature searching and creating figures and tables. J.L.S. contributed the study design and revised the article critically. Z.-J.C. supervised the study design and revision.
Funding
This work was supported by the National Basic Research Program of China (973 program-2012CB944700); the National Natural Science Foundation of China (81270662, 81471509, 81370692, 81370687); the Key Program of National Natural Science Foundation of China (81430029). Funding to pay the Open Access publication charges for this article was provided by National Basic Research Program of China (973 program-2012CB944700).
Conflict of interest
None declared.
Supplementary Material
References
- Aboura A, Dupas C, Tachdjian G, Portnoi MF, Bourcigaux N, Dewailly D, Frydman R, Fauser B, Ronci-Chaix N, Donadille B et al. Array comparative genomic hybridization profiling analysis reveals deoxyribonucleic acid copy number variations associated with premature ovarian failure. J Clin Endocrinol Metab 2009;11:4540–4546. [DOI] [PubMed] [Google Scholar]
- ACOG committee opinion. No. 338: Screening for fragile X syndrome. Obstet Gynecol 2006a;6:1483–1485. [DOI] [PubMed] [Google Scholar]
- Aittomäki K. The genetics of XX gonadal dysgenesis. Am J Hum Genet 1994;5:844–851. [PMC free article] [PubMed] [Google Scholar]
- Aittomäki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lehvaslaiho H, Engel AR et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 1995;6:959–968. [DOI] [PubMed] [Google Scholar]
- Aittomäki K, Herva R, Stenman UH, Juntunen K, Ylostalo P, Hovatta O, de la Chapelle A. Clinical features of primary ovarian failure caused by a point mutation in the follicle-stimulating hormone receptor gene. J Clin Endocrinol Metab 1996;10:3722–3726. [DOI] [PubMed] [Google Scholar]
- AlAsiri S, Basit S, Wood-Trageser MA, Yatsenko SA, Jeffries EP, Surti U, Ketterer DM, Afzal S, Ramzan K, Faiyaz-Ul Haque M et al. Exome sequencing reveals MCM8 mutation underlies ovarian failure and chromosomal instability. J Clin Invest 2015;125:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet 1999;4:322–325. [PMC free article] [PubMed] [Google Scholar]
- Ayed W, Amouri A, Hammami W, Kilani O, Turki Z, Harzallah F, Bouayed-Abdelmoula N, Chemkhi I, Zhioua F, Slama CB. Cytogenetic abnormalities in Tunisian women with premature ovarian failure. C R Biol 2014;12:691–694. [DOI] [PubMed] [Google Scholar]
- Badik JR, Castaneda U, Gleason TJ, Spencer JB, Epstein MP, Ficicioglu C, Fitzgerald K, Fridovich-Keil JL. Ovarian function in Duarte galactosemia. Fertil Steril 2011;2:469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Chakraborty P, Saha P, Bandyopadhyay SA, Banerjee S, Kabir SN. Ovotoxic effects of galactose involve attenuation of follicle-stimulating hormone bioactivity and up-regulation of granulosa cell p53 expression. PLoS One 2012;2:e30709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronchelli S, Conconi D, Panzeri E, Bentivegna A, Redaelli S, Lissoni S, Saccheri F, Villa N, Crosti F, Sala E et al. Cytogenetics of premature ovarian failure: an investigation on 269 affected women. J Biomed Biotechnol 2011;370195. [DOI] [PMC free article] [PubMed]
- Bertini V, Ghirri P, Bicocchi MP, Simi P, Valetto A. Molecular cytogenetic definition of a translocation t(X;15) associated with premature ovarian failure. Fertil Steril 2010;3:1097. [DOI] [PubMed] [Google Scholar]
- Beysen D, Vandesompele J, Messiaen L, De Paepe A, De Baere E. The human FOXL2 mutation database. Hum Mutat 2004;3:189–193. [DOI] [PubMed] [Google Scholar]
- Bione S, Sala C, Manzini C, Arrigo G, Zuffardi O, Banfi S, Borsani G, Jonveaux P, Philippe C, Zuccotti M et al. A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am J Hum Genet 1998;3:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bione S, Rizzolio F, Sala C, Ricotti R, Goegan M, Manzini MC, Battaglia R, Marozzi A, Vegetti W, Dalpra L et al. Mutation analysis of two candidate genes for premature ovarian failure, DACH2 and POF1B. Hum Reprod 2004;12:2759–2766. [DOI] [PubMed] [Google Scholar]
- Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 2007;7146:799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell C, Stibler H, Wahlstrom J, Kristiansson B, Skovby F, Stromme P, Blennow G, Martinsson T. Fine mapping of the gene for carbohydrate-deficient glycoprotein syndrome, type I (CDG1): linkage disequilibrium and founder effect in Scandinavian families. Genomics 1997;3:247–253. [DOI] [PubMed] [Google Scholar]
- Bolcun-Filas E, Hall E, Speed R, Taggart M, Grey C, de Massy B, Benavente R, Cooke HJ. Mutation of the mouse Syce1 gene disrupts synapsis and suggests a link between synaptonemal complex structural components and DNA repair. PLoS Genet 2009;2:e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltshauser E, Barth PG, Troost D, Martin E, Stallmach T. ‘Vanishing white matter’ and ovarian dysgenesis in an infant with cerebro-oculo-facio-skeletal phenotype. Neuropediatrics 2002;2:57–62. [DOI] [PubMed] [Google Scholar]
- Bonomi M, Somigliana E, Cacciatore C, Busnelli M, Rossetti R, Bonetti S, Paffoni A, Mari D, Ragni G, Persani L. Blood cell mitochondrial DNA content and premature ovarian aging. PLoS One 2012;8:e42423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouilly J, Bachelot A, Broutin I, Touraine P, Binart N. Novel NOBOX loss-of-function mutations account for 6.2% of cases in a large primary ovarian insufficiency cohort. Hum Mutat 2011;10:1108–1113. [DOI] [PubMed] [Google Scholar]
- Bouilly J, Roucher-Boulez F, Gompel A, Bry-Gauillard H, Azibi K, Beldjord C, Dode C, Bouligand J, Guiochon Mantel A, Hecart AC et al. New NOBOX mutations identified in a large cohort of women with primary ovarian insufficiency decrease KIT-L expression. J Clin Endocrinol Metab 2015;3:994–1001. [DOI] [PubMed] [Google Scholar]
- Bretherick KL, Hanna CW, Currie LM, Fluker MR, Hammond GL, Robinson WP. Estrogen receptor alpha gene polymorphisms are associated with idiopathic premature ovarian failure. Fertil Steril 2008;2:318–324. [DOI] [PubMed] [Google Scholar]
- Caburet S, Zavadakova P, Ben-Neriah Z, Bouhali K, Dipietromaria A, Charon C, Besse C, Laissue P, Chalifa-Caspi V, Christin-Maitre S et al. Genome-wide linkage in a highly consanguineous pedigree reveals two novel Loci on chromosome 7 for non-syndromic familial premature ovarian failure. PLoS One 2012;3:e33412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caburet S, Arboleda VA, Llano E, Overbeek PA, Barbero JL, Oka K, Harrison W, Vaiman D, Ben-Neriah Z, Garcia-Tunon I et al. Mutant cohesin in premature ovarian failure. N Engl J Med 2014;10:943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol 2007;1–5:16–36. [DOI] [PubMed] [Google Scholar]
- Castillo S, Lopez F, Tobella L, Salazar S, Daher V. The cytogenetics of premature ovarian failure. Rev Chil Obstet Ginecol 1992;5:341–345. [PubMed] [Google Scholar]
- Ceylaner G, Altinkaya SO, Mollamahmutoglu L, Ceylaner S. Genetic abnormalities in Turkish women with premature ovarian failure. Int J Gynaecol Obstet 2010;2:122–124. [DOI] [PubMed] [Google Scholar]
- Chand AL, Ponnampalam AP, Harris SE, Winship IM, Shelling AN. Mutational analysis of BMP15 and GDF9 as candidate genes for premature ovarian failure. Fertil Steril 2006;4:1009–1012. [DOI] [PubMed] [Google Scholar]
- Chand AL, Ooi GT, Harrison CA, Shelling AN, Robertson DM. Functional analysis of the human inhibin alpha subunit variant A257T and its potential role in premature ovarian failure. Hum Reprod 2007;12:3241–3248. [DOI] [PubMed] [Google Scholar]
- Chand AL, Harrison CA, Shelling AN. Inhibin and premature ovarian failure. Hum Reprod Update 2010;1:39–50. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Singh R, Kadam S, Maitra A, Thangaraj K, Meherji P, Modi D. Longer CAG repeat length in the androgen receptor gene is associated with premature ovarian failure. Hum Reprod 2009;12:3230–3235. [DOI] [PubMed] [Google Scholar]
- Chen XN, Chen GA, Li MZ. Follicular stimulating hormone receptor gene C566T mutation in premature ovarian failure. Zhonghua Fu Chan Ke Za Zhi 2006;5:315–318. [PubMed] [Google Scholar]
- Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Li Z, You L, Zhao J, Liu J, Liang X et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet 2011a;1:55–59. [DOI] [PubMed] [Google Scholar]
- Chen B, Suo P, Wang B, Wang J, Yang L, Zhou S, Zhu Y, Ma X, Cao Y. Mutation analysis of the WNT4 gene in Han Chinese women with premature ovarian failure. Reprod Biol Endocrinol 2011b;1:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CT, Fernandez-Rhodes L, Brzyski RG, Carlson CS, Chen Z, Heiss G, North KE, Woods NF, Rajkovic A, Kooperberg C et al. Replication of loci influencing ages at menarche and menopause in Hispanic women: the Women's Health Initiative SHARe Study. Hum Mol Genet 2012;6:1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A. Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod 2007;2:312–319. [DOI] [PubMed] [Google Scholar]
- Choi Y, Yuan D, Rajkovic A. Germ cell-specific transcriptional regulator sohlh2 is essential for early mouse folliculogenesis and oocyte-specific gene expression. Biol Reprod 2008;6:1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes AN, Spiller CM, Harley VR, Sinclair AH, Dunwoodie SL, Wilhelm D, Koopman P. Gonadal defects in Cited2-mutant mice indicate a role for SF1 in both testis and ovary differentiation. Int J Dev Biol 2010;4:683–689. [DOI] [PubMed] [Google Scholar]
- Conway GS, Conway E, Walker C, Hoppner W, Gromoll J, Simoni M. Mutation screening and isoform prevalence of the follicle stimulating hormone receptor gene in women with premature ovarian failure, resistant ovary syndrome and polycystic ovary syndrome. Clin Endocrinol 1999;1:97–99. [DOI] [PubMed] [Google Scholar]
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol 1986;4:604–606. [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 2001;2:159–166. [DOI] [PubMed] [Google Scholar]
- da Fonte Kohek MB, Batista MC, Russell AJ, Vass K, Giacaglia LR, Mendonca BB, Latronico AC. No evidence of the inactivating mutation (C566T) in the follicle-stimulating hormone receptor gene in Brazilian women with premature ovarian failure. Fertil Steril 1998;3:565–567. [DOI] [PubMed] [Google Scholar]
- Davison RM, Quilter CR, Webb J, Murray A, Fisher AM, Valentine A, Serhal P, Conway GS. A familial case of X chromosome deletion ascertained by cytogenetic screening of women with premature ovarian failure. Hum Reprod 1998;11:3039–3041. [DOI] [PubMed] [Google Scholar]
- De Baere E, Lemercier B, Christin-Maitre S, Durval D, Messiaen L, Fellous M, Veitia R. FOXL2 mutation screening in a large panel of POF patients and XX males. J Med Genet 2002;8:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi A, Benn PA. X-chromosome abnormalities in women with premature ovarian failure. J Reprod Med 1999;4:321–324. [PubMed] [Google Scholar]
- de Vries BB, Wiegers AM, Smits AP, Mohkamsing S, Duivenvoorden HJ, Fryns JP, Curfs LM, Halley DJ, Oostra BA, van den Ouweland AM et al. Mental status of females with an FMR1 gene full mutation. Am J Hum Genet 1996;5:1025–1032. [PMC free article] [PubMed] [Google Scholar]
- de Vries SS, Baart EB, Dekker M, Siezen A, de Rooij DG, de Boer P, te Riele H. Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev 1999;5:523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries L, Behar DM, Smirin-Yosef P, Lagovsky I, Tzur S, Basel-Vanagaite L. Exome sequencing reveals SYCE1 mutation associated with autosomal recessive primary ovarian insufficiency. J Clin Endocrinol Metab 2014;10:E2129–E2132. [DOI] [PubMed] [Google Scholar]
- de Zegher F, Jaeken J. Endocrinology of the carbohydrate-deficient glycoprotein syndrome type 1 from birth through adolescence. Pediatr Res 1995;4 Pt 1:395–401. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet 2004;1:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, Einaudi S, Radetti G, Russo G, Sacco M et al. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab 2006;5:1976–1979. [DOI] [PubMed] [Google Scholar]
- Dixit H, Deendayal M, Singh L. Mutational analysis of the mature peptide region of inhibin genes in Indian women with ovarian failure. Hum Reprod 2004;8:1760–1764. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty B, Singh L. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause 2005;6:749–754. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao KL, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty BN, Singh L. Expansion of the germline analysis for the INHA gene in Indian women with ovarian failure. Hum Reprod 2006a;6:1643–1644. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao KL, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty BN, Singh L. Mutational analysis of the betaglycan gene-coding region in susceptibility for ovarian failure. Hum Reprod 2006b;8:2041–2046. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty B, Singh L. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet 2006c;4:408–415. [DOI] [PubMed] [Google Scholar]
- Dudding TE, Lawrence O, Winship I, Froyen G, Vandewalle J, Scott R, Shelling AN. Array comparative genomic hybridization for the detection of submicroscopic copy number variations of the X chromosome in women with premature ovarian failure. Hum Reprod 2010;12:3159–3160; author reply 3160–3151. [DOI] [PubMed] [Google Scholar]
- Duncan AJ, Knight JA, Costello H, Conway GS, Rahman S. POLG mutations and age at menopause. Hum Reprod 2012;7:2243–2244. [DOI] [PubMed] [Google Scholar]
- Ellis NA, German J. Molecular genetics of Bloom's syndrome. Hum Mol Genet 1996;1457–1463. [DOI] [PubMed]
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012;7414:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engmann L, Losel R, Wehling M, Peluso JJ. Progesterone regulation of human granulosa/luteal cell viability by an RU486-independent mechanism. J Clin Endocrinol Metab 2006;12:4962–4968. [DOI] [PubMed] [Google Scholar]
- Epstein CJ, Martin GM, Schultz AL, Motulsky AG. Werner's syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine 1966;3:177–221. [DOI] [PubMed] [Google Scholar]
- European Society of Human Genetics; European Society of Human Reproduction and Embryology. The need for interaction between assisted reproduction technology and genetics. Recommendations of the European Societies of Human Genetics and Human Reproduction and Embryology. Eur J Hum Genet 2006b;5:509–511. [DOI] [PubMed] [Google Scholar]
- Fierabracci A, Bizzarri C, Palma A, Milillo A, Bellacchio E, Cappa M. A novel heterozygous mutation of the AIRE gene in a patient with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome (APECED). Gene 2012;1:113–117. [DOI] [PubMed] [Google Scholar]
- Fogli A, Rodriguez D, Eymard-Pierre E, Bouhour F, Labauge P, Meaney BF, Zeesman S, Kaneski CR, Schiffmann R, Boespflug-Tanguy O. Ovarian failure related to eukaryotic initiation factor 2B mutations. Am J Hum Genet 2003;6:1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogli A, Gauthier-Barichard F, Schiffmann R, Vanderhoof VH, Bakalov VK, Nelson LM, Boespflug-Tanguy O. Screening for known mutations in EIF2B genes in a large panel of patients with premature ovarian failure. BMC Womens Health 2004;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca DJ, Garzon E, Lakhal B, Braham R, Ojeda D, Elghezal H, Saad A, Restrepo CM, Laissue P. Screening for mutations of the FOXO4 gene in premature ovarian failure patients. Reprod Biomed Online 2012a;3:339–341. [DOI] [PubMed] [Google Scholar]
- Fonseca DJ, Ojeda D, Lakhal B, Braham R, Eggers S, Turbitt E, White S, Grover S, Warne G, Zacharin M et al. CITED2 mutations potentially cause idiopathic premature ovarian failure. Transl Res 2012b;5:384–388. [DOI] [PubMed] [Google Scholar]
- Fonseca DJ, Patiño LC 1, Suárez YC, de Jesús Rodríguez A, Mateus HE, Jiménez KM, Ortega-Recalde O, Díaz-Yamal I, Laissue P. Next generation sequencing in women affected by nonsyndromic premature ovarian failure displays new potential causative genes and mutations. Fertil Steril 2015;104:154–162. [DOI] [PubMed] [Google Scholar]
- Foresta C, Ferlin A, Gianaroli L, Dallapiccola B. Guidelines for the appropriate use of genetic tests in infertile couples. Eur J Hum Genet 2002;5:303–312. [DOI] [PubMed] [Google Scholar]
- Fridovich-Keil JL, Gubbels CS, Spencer JB, Sanders RD, Land JA, Rubio-Gozalbo E. Ovarian function in girls and women with GALT-deficiency galactosemia. J Inherit Metab Dis 2011;2:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 2000;3:279–283. [DOI] [PubMed] [Google Scholar]
- Gatti RA, Boder E, Vinters HV, Sparkes RS, Norman A, Lange K. Ataxia-telangiectasia: an interdisciplinary approach to pathogenesis. Medicine 1991;2:99–117. [PubMed] [Google Scholar]
- Gersak K, Meden-Vrtovec H, Peterlin B. Fragile X premutation in women with sporadic premature ovarian failure in Slovenia. Hum Reprod 2003;8:1637–1640. [DOI] [PubMed] [Google Scholar]
- Gong Y, Krakow D, Marcelino J, Wilkin D, Chitayat D, Babul-Hirji R, Hudgins L, Cremers CW, Cremers FP, Brunner HG et al. Heterozygous mutations in the gene encoding noggin affect human joint morphogenesis. Nat Genet 1999;3:302–304. [DOI] [PubMed] [Google Scholar]
- Goswami R, Goswami D, Kabra M, Gupta N, Dubey S, Dadhwal V. Prevalence of the triple X syndrome in phenotypically normal women with premature ovarian failure and its association with autoimmune thyroid disorders. Fertil Steril 2003;4:1052–1054. [DOI] [PubMed] [Google Scholar]
- Graziewicz MA, Bienstock RJ, Copeland WC. The DNA polymerase gamma Y955C disease variant associated with PEO and parkinsonism mediates the incorporation and translesion synthesis opposite 7,8-dihydro-8-oxo-2′-deoxyguanosine. Hum Mol Genet 2007;22:2729–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraldelli MF, Eyster C, Wilkerson JL, Dresser ME, Pezza RJ. Mouse HFM1/Mer3 is required for crossover formation and complete synapsis of homologous chromosomes during meiosis. PLoS Genet 2013;3:e1003383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Qin Y, Jiao X, Li G, Simpson JL, Chen ZJ. FMR1 premutation is an uncommon explanation for premature ovarian failure in Han Chinese. PLoS One 2014;7:e103316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Chand AL, Winship IM, Gersak K, Aittomaki K, Shelling AN. Identification of novel mutations in FOXL2 associated with premature ovarian failure. Mol Hum Reprod 2002;8:729–733. [DOI] [PubMed] [Google Scholar]
- Harris SE, Chand AL, Winship IM, Gersak K, Nishi Y, Yanase T, Nawata H, Shelling AN. INHA promoter polymorphisms are associated with premature ovarian failure. Mol Hum Reprod 2005;11:779–784. [DOI] [PubMed] [Google Scholar]
- Hens L, Devroey P, Van Waesberghe L, Bonduelle M, Van Steirteghem AC, Liebaers I. Chromosome studies and fertility treatment in women with ovarian failure. Clin Genet 1989;2:81–91. [DOI] [PubMed] [Google Scholar]
- Holland CM. 47, XXX in an adolescent with premature ovarian failure and autoimmune disease. J Pediatr Adolesc Gynecol 2001;2:77–80. [DOI] [PubMed] [Google Scholar]
- Hui ES, Udofa EA, Soto J, Vanderhoof VH, Zachman K, Tong ZB, Nelson LM. Investigation of the human stem cell factor KIT ligand gene, KITLG, in women with 46,XX spontaneous premature ovarian failure. Fertil Steril 2006;5:1502–1507. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Shimasaki S. Impaired production of BMP-15 and GDF-9 mature proteins derived from proproteins WITH mutations in the proregion. Mol Cell Endocrinol 2010;1–2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka B, Okamoto N, Hamada N, Sugishita Y, Saito J, Takahashi N, Ogata T, Itoh MT. Number of CGG repeats in the FMR1 gene of Japanese patients with primary ovarian insufficiency. Fertil Steril 2011;5:1170–1174. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen M, Prunskaite-Hyyrylainen R, Naillat F, Parviainen H, Anttonen M, Heikinheimo M, Liakka A, Ola R, Vainio S, Vaskivuo TE et al. WNT4 is expressed in human fetal and adult ovaries and its signaling contributes to ovarian cell survival. Mol Cell Endocrinol 2010;1-2:106–111. [DOI] [PubMed] [Google Scholar]
- Janse F, Knauff EA, Niermeijer MF, Eijkemans MJ, Laven JS, Lambalk CB, Fauser BC, Goverde AJ. Similar phenotype characteristics comparing familial and sporadic premature ovarian failure. Menopause 2010;4:758–765. [DOI] [PubMed] [Google Scholar]
- Janse F, de With LM, Duran KJ, Kloosterman WP, Goverde AJ, Lambalk CB, Laven JS, Fauser BC, Giltay JC; Dutch Primary Ovarian Insufficiency Consortium. Limited contribution of NR5A1 (SF-1) mutations in women with primary ovarian insufficiency (POI). Fertil Steril 2012;1:141–146. [DOI] [PubMed] [Google Scholar]
- Jenkinson EM, Rehman AU, Walsh T, Clayton-Smith J, Lee K, Morell RJ, Drummond MC, Khan SN, Naeem MA, Rauf B et al. Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am J Hum Genet 2013;4:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Aittomaki K, Nilsson C, Pakarinen P, Iitia A, Torresani T, Simonsen H, Goh V, Pettersson K, de la Chapelle A et al. The frequency of an inactivating point mutation (566C-->T) of the human follicle-stimulating hormone receptor gene in four populations using allele-specific hybridization and time-resolved fluorometry. J Clin Endocrinol Metab 1998;12:4338–4343. [DOI] [PubMed] [Google Scholar]
- Jiao X, Qin C, Li J, Qin Y, Gao X, Zhang B, Zhen X, Feng Y, Simpson JL, Chen ZJ. Cytogenetic analysis of 531 Chinese women with premature ovarian failure. Hum Reprod 2012;7:2201–2207. [DOI] [PubMed] [Google Scholar]
- Jiao X, Qin Y, Li G, Zhao S, You L, Ma J, Simpson JL, Chen ZJ. Novel NR5A1 missense mutation in premature ovarian failure: detection in Han Chinese indicates causation in different ethnic groups. PLoS One 2013;9:e74759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantari H, Madani T, Zari Moradi S, Mansouri Z, Almadani N, Gourabi H, Mohseni Meybodi A. Cytogenetic analysis of 179 Iranian women with premature ovarian failure. Gynecol Endocrinol 2013;6:588–591. [DOI] [PubMed] [Google Scholar]
- Kang H, Lee SK, Kim MH, Song J, Bae SJ, Kim NK, Lee SH, Kwack K. Parathyroid hormone-responsive B1 gene is associated with premature ovarian failure. Hum Reprod 2008;6:1457–1465. [DOI] [PubMed] [Google Scholar]
- Kasippillai T, MacArthur DG, Kirby A, Thomas B, Lambalk CB, Daly MJ, Welt CK. Mutations in eIF4ENIF1 are associated with primary ovarian insufficiency. J Clin Endocrinol Metab 2013;9:E1534–E1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman F, Kogut MD, Donnell GN, Koch H, Goebelsmann U. Ovarian failure in galactosaemia. Lancet 1979;8145:737–738. [DOI] [PubMed] [Google Scholar]
- Kaufman FR, Kogut MD, Donnell GN, Goebelsmann U, March C, Koch R. Hypergonadotropic hypogonadism in female patients with galactosemia. N Engl J Med 1981;17:994–998. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Narahara H, Matsui N, Miyakawa I. Premature ovarian failure associated with a Robertsonian translocation. Acta Obstet Gynecol Scand 1998;4:467–469. [PubMed] [Google Scholar]
- Knauff EA, Franke L, van Es MA, van den Berg LH, van der Schouw YT, Laven JS, Lambalk CB, Hoek A, Goverde AJ, Christin-Maitre S et al. Genome-wide association study in premature ovarian failure patients suggests ADAMTS19 as a possible candidate gene. Hum Reprod 2009;9:2372–2378. [DOI] [PubMed] [Google Scholar]
- Knauff EA, Blauw HM, Pearson PL, Kok K, Wijmenga C, Veldink JH, van den Berg LH, Bouchard P, Fauser BC, Franke L. Copy number variants on the X chromosome in women with primary ovarian insufficiency. Fertil Steril 2011;5:1584–1588. [DOI] [PubMed] [Google Scholar]
- Kneitz B, Cohen PE, Avdievich E, Zhu L, Kane MF, Hou H Jr, Kolodner RD, Kucherlapati R, Pollard JW, Edelmann W. MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev 2000;9:1085–1097. [PMC free article] [PubMed] [Google Scholar]
- Kosaki K, Sato S, Hasegawa T, Matsuo N, Suzuki T, Ogata T. Premature ovarian failure in a female with proximal symphalangism and Noggin mutation. Fertil Steril 2004;4:1137–1139. [DOI] [PubMed] [Google Scholar]
- Kovanci E, Rohozinski J, Simpson JL, Heard MJ, Bishop CE, Carson SA. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril 2007;1:143–146. [DOI] [PubMed] [Google Scholar]
- Kovanci E, Simpson JL, Amato P, Rohozinski J, Heard MJ, Bishop CE, Carson SA. Oocyte-specific G-protein-coupled receptor 3 (GPR3): no perturbations found in 82 women with premature ovarian failure (first report). Fertil Steril 2008;4:1269–1271. [DOI] [PubMed] [Google Scholar]
- Kristiansson M, Soop M, Saraste L, Sundqvist KG, Suontaka AM, Blomback M. Cytokine and coagulation characteristics of retrieved blood after arthroplasty. Intensive Care Med 1995;12:989–995. [DOI] [PubMed] [Google Scholar]
- Lacombe A, Lee H, Zahed L, Choucair M, Muller JM, Nelson SF, Salameh W, Vilain E. Disruption of POF1B binding to nonmuscle actin filaments is associated with premature ovarian failure. Am J Hum Genet 2006;1:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R et al. Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur J Endocrinol 2006;5:739–744. [DOI] [PubMed] [Google Scholar]
- Laissue P, Christin-Maitre S, Bouchard P, Fellous M, Veitia RA. Mutations in the NOG gene are not a common cause of nonsyndromic premature ovarian failure. Clin Endocrinol (Oxf) 2007;6:900. [DOI] [PubMed] [Google Scholar]
- Lakhal B, Laissue P, Braham R, Elghezal H, Saad A, Fellous M, Veitia RA. A novel BMP15 variant, potentially affecting the signal peptide, in a familial case of premature ovarian failure. Clin Endocrinol (Oxf) 2009;5:752–753. [DOI] [PubMed] [Google Scholar]
- Lakhal B, Braham R, Berguigua R, Bouali N, Zaouali M, Chaieb M, Veitia RA, Saad A, Elghezal H. Cytogenetic analyses of premature ovarian failure using karyotyping and interphase fluorescence in situ hybridization (FISH) in a group of 1000 patients. Clin Genet 2010a;2:181–185. [DOI] [PubMed] [Google Scholar]
- Lakhal B, Laissue P, Braham R, Elghezal H, Saad A, Fellous M, Veitia RA. BMP15 and premature ovarian failure: causal mutations, variants, polymorphisms? Clin Endocrinol (Oxf) 2010b;3:425–426. [DOI] [PubMed] [Google Scholar]
- Lakhal B, Ben-Hadj-Khalifa S, Bouali N, Philipert P, Audran F, Braham R, Hatem E, Sultan C, Saad A. Mutational screening of SF1 and WNT4 in Tunisian women with premature ovarian failure. Gene 2012;2:298–301. [DOI] [PubMed] [Google Scholar]
- Lechowska A, Bilinski S, Choi Y, Shin Y, Kloc M, Rajkovic A. Premature ovarian failure in nobox-deficient mice is caused by defects in somatic cell invasion and germ cell cyst breakdown. J Assist Reprod Genet 2011;7:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledig S, Ropke A, Haeusler G, Hinney B, Wieacker P. BMP15 mutations in XX gonadal dysgenesis and premature ovarian failure. Am J Obstet Gynecol 2008;1:84. [DOI] [PubMed] [Google Scholar]
- Ledig S, Ropke A, Wieacker P. Copy number variants in premature ovarian failure and ovarian dysgenesis. Sex Dev 2010;4–5:225–232. [DOI] [PubMed] [Google Scholar]
- Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 2007;1:199–209. [DOI] [PubMed] [Google Scholar]
- Lorda-Sanchez IJ, Ibanez AJ, Sanz RJ, Trujillo MJ, Anabitarte ME, Querejeta ME, Rodriguez de Alba M, Gimenez A, Infantes F, Ramos C et al. Choroideremia, sensorineural deafness, and primary ovarian failure in a woman with a balanced X-4 translocation. Ophthalmic Genet 2000;3:185–189. [PubMed] [Google Scholar]
- Losel RM, Besong D, Peluso JJ, Wehling M. Progesterone receptor membrane component 1—many tasks for a versatile protein. Steroids 2008;9–10:929–934. [DOI] [PubMed] [Google Scholar]
- Lourenco D, Brauner R, Lin L, De Perdigo A, Weryha G, Muresan M, Boudjenah R, Guerra-Junior G, Maciel-Guerra AT, Achermann JC et al. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med 2009;12:1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K et al. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet 2004;9437:875–882. [DOI] [PubMed] [Google Scholar]
- Lutzmann M, Grey C, Traver S, Ganier O, Maya-Mendoza A, Ranisavljevic N, Bernex F, Nishiyama A, Montel N, Gavois E et al. MCM8- and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol cell 2012;4:523–534. [DOI] [PubMed] [Google Scholar]
- Mandon-Pepin B, Touraine P, Kuttenn F, Derbois C, Rouxel A, Matsuda F, Nicolas A, Cotinot C, Fellous M. Genetic investigation of four meiotic genes in women with premature ovarian failure. Eur J Endocrinol 2008;1:107–115. [DOI] [PubMed] [Google Scholar]
- Mansouri MR, Schuster J, Badhai J, Stattin EL, Losel R, Wehling M, Carlsson B, Hovatta O, Karlstrom PO, Golovleva I et al. Alterations in the expression, structure and function of progesterone receptor membrane component-1 (PGRMC1) in premature ovarian failure. Hum Mol Genet 2008;23:3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marozzi A, Porta C, Vegetti W, Crosignani PG, Tibiletti MG, Dalpra L, Ginelli E. Mutation analysis of the inhibin alpha gene in a cohort of Italian women affected by ovarian failure. Hum Reprod 2002;7:1741–1745. [DOI] [PubMed] [Google Scholar]
- Mathis S, Scheper GC, Baumann N, Petit E, Gil R, van der Knaap MS, Neau JP. The ovarioleukodystrophy. Clin Neurol Neurosurg 2008;10:1035–1037. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Toksoz D, Nishikawa S, Nishikawa S, Williams D, Zsebo K, Hogan BL. Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature 1991;6346:750–752. [DOI] [PubMed] [Google Scholar]
- May-Panloup P, Chretien MF, Jacques C, Vasseur C, Malthiery Y, Reynier P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod 2005;3:593–597. [DOI] [PubMed] [Google Scholar]
- McGuire MM, Bowden W, Engel NJ, Ahn HW, Kovanci E, Rajkovic A. Genomic analysis using high-resolution single-nucleotide polymorphism arrays reveals novel microdeletions associated with premature ovarian failure. Fertil Steril 2011;5:1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 2004;5703:1947–1950. [DOI] [PubMed] [Google Scholar]
- Morino H, Pierce SB, Matsuda Y, Walsh T, Ohsawa R, Newby M, Hiraki-Kamon K, Kuramochi M, Lee MK, Klevit RE et al. Mutations in Twinkle primase-helicase cause Perrault syndrome with neurologic features. Neurology 2014;22:2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura-Tadaki A, Wada T, Bano G, Gough K, Warner J, Kosho T, Ando N, Hamanoue H, Sakakibara H, Nishimura G et al. Breakpoint determination of X;autosome balanced translocations in four patients with premature ovarian failure. J Hum Genet 2011;2:156–160. [DOI] [PubMed] [Google Scholar]
- Norling A, Hirschberg AL, Rodriguez-Wallberg KA, Iwarsson E, Wedell A, Barbaro M. Identification of a duplication within the GDF9 gene and novel candidate genes for primary ovarian insufficiency (POI) by a customized high-resolution array comparative genomic hybridization platform. Hum Reprod 2014;8:1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Gallego J, Ferreira PG, Mendez R. Mitotic cell-cycle progression is regulated by CPEB1 and CPEB4-dependent translational control. Nat Cell Biol 2010;5:447–456. [DOI] [PubMed] [Google Scholar]
- Ojeda D, Lakhal B, Fonseca DJ, Braham R, Landolsi H, Mateus HE, Restrepo CM, Elghezal H, Saad A, Laissue P. Sequence analysis of the CDKN1B gene in patients with premature ovarian failure reveals a novel mutation potentially related to the phenotype. Fertil Steril 2011;8:2658–2660. [DOI] [PubMed] [Google Scholar]
- Oldenburg RA, van Dooren MF, de Graaf B, Simons E, Govaerts L, Swagemakers S, Verkerk JM, Oostra BA, Bertoli-Avella AM. A genome-wide linkage scan in a Dutch family identifies a premature ovarian failure susceptibility locus. Hum Reprod 2008;12:2835–2841. [DOI] [PubMed] [Google Scholar]
- Oley C, Baraitser M. Blepharophimosis, ptosis, epicanthus inversus syndrome (BPES syndrome). J Med Genet 1988;1:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orczyk GP, Pehrson J, Leventhal JM. Premature ovarian failure in a 35-year-old woman with a Robertsonian translocation. Int J Fertil 1989;3:184–187. [PubMed] [Google Scholar]
- Pagnamenta AT, Taanman JW, Wilson CJ, Anderson NE, Marotta R, Duncan AJ, Bitner-Glindzicz M, Taylor RW, Laskowski A, Thorburn DR et al. Dominant inheritance of premature ovarian failure associated with mutant mitochondrial DNA polymerase gamma. Hum Reprod 2006;10:2467–2473. [DOI] [PubMed] [Google Scholar]
- Panda B, Rao L, Tosh D, Dixit H, Padmalatha V, Kanakavalli M, Raseswari T, Deenadayal M, Gupta N, Chakrabarty B et al. Germline study of AR gene of Indian women with ovarian failure. Gynecol Endocrinol 2010;8:572–578. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci USA 2006;21:8090–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone's antiapoptotic action. Endocrinology 2006;6:3133–3140. [DOI] [PubMed] [Google Scholar]
- Persani L, Rossetti R, Cacciatore C, Bonomi M. Primary Ovarian Insufficiency: X chromosome defects and autoimmunity. J Autoimmun 2009;1:35–41. [DOI] [PubMed] [Google Scholar]
- Philibert P, Leprieur E, Zenaty D, Thibaud E, Polak M, Frances AM, Lespinasse J, Raingeard I, Servant N, Audran F et al. Steroidogenic factor-1 (SF-1) gene mutation as a frequent cause of primary amenorrhea in 46,XY female adolescents with low testosterone concentration. Reprod Biol Endocrinol 2010;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SB, Walsh T, Chisholm KM, Lee MK, Thornton AM, Fiumara A, Opitz JM, Levy-Lahad E, Klevit RE, King MC. Mutations in the DBP-deficiency protein HSD17B4 cause ovarian dysgenesis, hearing loss, and ataxia of Perrault Syndrome. Am J Hum Genet 2010;2:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SB, Chisholm KM, Lynch ED, Lee MK, Walsh T, Opitz JM, Li W, Klevit RE, King MC. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc Natl Acad Sci USA 2011;16:6543–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SB, Gersak K, Michaelson-Cohen R, Walsh T, Lee MK, Malach D, Klevit RE, King MC, Levy-Lahad E. Mutations in LARS2, encoding mitochondrial leucyl-tRNA synthetase, lead to premature ovarian failure and hearing loss in Perrault syndrome. Am J Hum Genet 2013;4:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarska MD, Kuo FT, Tang D, Zarrini P, Khan S, Ketefian A. Expression of forkhead transcription factors in human granulosa cells. Fertil Steril 2009;4 Suppl:1392–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoi MF, Aboura A, Tachdjian G, Bouchard P, Dewailly D, Bourcigaux N, Frydman R, Reyss AC, Brisset S, Christin-Maitre S. Molecular cytogenetic studies of Xq critical regions in premature ovarian failure patients. Hum Reprod 2006;9:2329–2334. [DOI] [PubMed] [Google Scholar]
- Prakash GJ, Kanth VV, Shelling AN, Rozati R, Sujatha M. Absence of 566C>T mutation in exon 7 of the FSHR gene in Indian women with premature ovarian failure. Int J Gynaecol Obstet 2009;3:265–266. [DOI] [PubMed] [Google Scholar]
- Prueitt RL, Ross JL, Zinn AR. Physical mapping of nine Xq translocation breakpoints and identification of XPNPEP2 as a premature ovarian failure candidate gene. Cytogenet Cell Genet 2000;1-2:44–50. [DOI] [PubMed] [Google Scholar]
- Prueitt RL, Chen H, Barnes RI, Zinn AR. Most X;autosome translocations associated with premature ovarian failure do not interrupt X-linked genes. Cytogenet Genome Res 2002;1–2:32–38. [DOI] [PubMed] [Google Scholar]
- Pyun JA, Cha DH, Kwack K. LAMC1 gene is associated with premature ovarian failure. Maturitas 2012;4:402–406. [DOI] [PubMed] [Google Scholar]
- Qin Y, Poirier C, Truong C, Schumacher A, Agoulnik AI, Bishop CE. A major locus on mouse chromosome 18 controls XX sex reversal in Odd Sex (Ods) mice. Hum Mol Genet 2003;5:509–515. [DOI] [PubMed] [Google Scholar]
- Qin Y, Kong LK, Poirier C, Truong C, Overbeek PA, Bishop CE. Long-range activation of Sox9 in Odd Sex (Ods) mice. Hum Mol Genet 2004;12:1213–1218. [DOI] [PubMed] [Google Scholar]
- Qin Y, Choi Y, Zhao H, Simpson JL, Chen ZJ, Rajkovic A. NOBOX homeobox mutation causes premature ovarian failure. Am J Hum Genet 2007a;3:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Zhao H, Kovanci E, Simpson JL, Chen ZJ, Rajkovic A. Mutation analysis of NANOS3 in 80 Chinese and 88 Caucasian women with premature ovarian failure. Fertil Steril 2007b;5:1465–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Shi Y, Zhao Y, Carson SA, Simpson JL, Chen ZJ. Mutation analysis of NOBOX homeodomain in Chinese women with premature ovarian failure. Fertil Steril 2009;4(Suppl):1507–1509. [DOI] [PubMed] [Google Scholar]
- Qin CR, Chen SL, Yao JL, Wu WQ, Xie JS. Identification of novel missense mutations of the TGFBR3 gene in Chinese women with premature ovarian failure. Reprod Biomed Online 2011;6:697–703. [DOI] [PubMed] [Google Scholar]
- Qin CR, Chen SL, Yao JL, Li T, Wu WQ. Haplotype and mutation analysis of the TGFBR3 gene in Chinese women with idiopathic premature ovarian failure. Gynecol Endocrinol 2012a;1:63–67. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhao H, Xu J, Shi Y, Li Z, Qiao J, Liu J, Qin C, Ren C, Li J et al. Association of 8q22.3 locus in Chinese Han with idiopathic premature ovarian failure (POF). Hum Mol Genet 2012b;2:430–436. [DOI] [PubMed] [Google Scholar]
- Qin Y, Sun M, You L, Wei D, Sun J, Liang X, Zhang B, Jiang H, Xu J, Chen Z. ESR1, HK3 and BRSK1 gene variants are associated with both age at natural menopause and premature ovarian failure. Orphanet J Rare Dis 2012c;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Jiao X, Dalgleish R, Vujovic S, Li J, Simpson JL, Al-Azzawi F, Chen ZJ. Novel variants in the SOHLH2 gene are implicated in human premature ovarian failure. Fertil Steril 2014a;4:1104–1109. [DOI] [PubMed] [Google Scholar]
- Qin C, Yuan Z, Yao J, Zhu W, Wu W, Xie J. AMH and AMHR2 genetic variants in Chinese women with primary ovarian insufficiency and normal age at natural menopause. Reprod Biomed Online 2014b;3:311–318. [DOI] [PubMed] [Google Scholar]
- Quilter CR, Karcanias AC, Bagga MR, Duncan S, Murray A, Conway GS, Sargent CA, Affara NA. Analysis of X chromosome genomic DNA sequence copy number variation associated with premature ovarian failure (POF). Hum Reprod 2010;8:2139–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, Shen Y, Berthet C, Peng SL, Kaldis P et al. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol 2007;9:2189–2202. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004;5687:1157–1159. [DOI] [PubMed] [Google Scholar]
- Rebar RW, Connolly HV. Clinical features of young women with hypergonadotropic amenorrhea. Fertil Steril 1990;5:804–810. [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang WL, Hamalainen T, Peng SL et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008;5863:611–613. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Hall SS. Fragile X syndrome: assessment and treatment implications. Child Adolesc Psychiatr Clin N Am 2007;3:663–675. [DOI] [PubMed] [Google Scholar]
- Rizzolio F, Bione S, Sala C, Goegan M, Gentile M, Gregato G, Rossi E, Pramparo T, Zuffardi O, Toniolo D. Chromosomal rearrangements in Xq and premature ovarian failure: mapping of 25 new cases and review of the literature. Hum Reprod 2006;6:1477–1483. [DOI] [PubMed] [Google Scholar]
- Rizzolio F, Sala C, Alboresi S, Bione S, Gilli S, Goegan M, Pramparo T, Zuffardi O, Toniolo D. Epigenetic control of the critical region for premature ovarian failure on autosomal genes translocated to the X chromosome: a hypothesis. Hum Genet 2007;3-4:441–450. [DOI] [PubMed] [Google Scholar]
- Rizzolio F, Pramparo T, Sala C, Zuffardi O, De Santis L, Rabellotti E, Calzi F, Fusi F, Bellazzi R, Toniolo D. Epigenetic analysis of the critical region I for premature ovarian failure: demonstration of a highly heterochromatic domain on the long arm of the mammalian X chromosome. J Med Genet 2009;9:585–592. [DOI] [PubMed] [Google Scholar]
- Rossetti R, Di Pasquale E, Marozzi A, Bione S, Toniolo D, Grammatico P, Nelson LM, Beck-Peccoz P, Persani L. BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum Mutat 2009;5:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MG, Machado AZ, Martins CN, Domenice S, Costa EM, Nishi MY, Ferraz-de-Souza B, Jorge SA, Pereira CA, Soardi FC et al. Homozygous inactivating mutation in NANOS3 in two sisters with primary ovarian insufficiency. Biomed Res Int 2014;787465. [DOI] [PMC free article] [PubMed]
- Sarto GE, Therman E, Patau K. X inactivation in man: a woman with t(Xq--;12q+). Am J Hum Genet 1973;3:262–270. [PMC free article] [PubMed] [Google Scholar]
- Schadewaldt P, Kamalanathan L, Hammen HW, Wendel U. Age dependence of endogenous galactose formation in Q188R homozygous galactosemic patients. Mol Genet Metab 2004;1:31–44. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Tedeschi G, Kinkel RP, Trapp BD, Frank JA, Kaneski CR, Brady RO, Barton NW, Nelson L, Yanovski JA. Leukodystrophy in patients with ovarian dysgenesis. Ann Neurol 1997;5:654–661. [DOI] [PubMed] [Google Scholar]
- Shelling AN, Burton KA, Chand AL, van Ee CC, France JT, Farquhar CM, Milsom SR, Love DR, Gersak K, Aittomaki K et al. Inhibin: a candidate gene for premature ovarian failure. Hum Reprod 2000;12:2644–2649. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhao H, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet 2012;9:1020–1025. [DOI] [PubMed] [Google Scholar]
- Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA 2006;1:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Kimura F, Takebayashi K, Fujiwara M, Takakura K, Takahashi K. Mutational analysis of the PTEN gene in women with premature ovarian failure. Acta Obstet Gynecol Scand 2009;7:824–825. [DOI] [PubMed] [Google Scholar]
- Simpson JL. Gonadal dysgenesis and abnormalities of the human sex chromosomes: current status of phenotypic-karyotypic correlations. Birth Defects Orig Artic Ser 1975;4:23–59. [PubMed] [Google Scholar]
- Simpson JL. Genetic and phenotypic heterogeneity in ovarian failure: overview of selected candidate genes. Ann N Y Acad Sci 2008;1135:146–154. [DOI] [PubMed] [Google Scholar]
- Simpson JL. Disorders of the gonads, genital tract, and genitalia. In: Rimoin DL, Connor JM, Pyertiz RE, Korf BR (eds). Emery and Rimoin's Principles and Practice of Medical Genetics. New York: Churchill-Livingstone, 2013, 4700 pp. [Google Scholar]
- Simpson JL. Genetics of female infertility due to anomalies of the ovary and mullerian ducts. Methods Mol Biol 2014;1154:39–73. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Elias S. Genetics in Obstetrics and Gynecology, 3rd edn Philadelphia: Saunders, 2003. [Google Scholar]
- Simpson JL, Rajkovic A. Ovarian differentiation and gonadal failure. Am J Med Genet 1999;4:186–200. [DOI] [PubMed] [Google Scholar]
- Sparks SE, Krasnewich DM. PMM2-CDG CDG-Ia GeneReviews™ [Internet] http://www.ncbi.nlm.nih.gov/books/NBK1110/. 2005.
- Spath MA, Nillesen WN, Smits AP, Feuth TB, Braat DD, van Kessel AG, Yntema HG. X chromosome inactivation does not define the development of premature ovarian failure in fragile X premutation carriers. Am J Med Genet A 2010;2:387–393. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos JA, Snyder M, Hardison R, Ren B, Gingeras T, Gilbert DM, Groudine M, Bender M, Kaul R, Canfield T et al. An encyclopedia of mouse DNA elements (Mouse ENCODE). Genome Biol 2012;8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Swift M. Mortality rates among carriers of ataxia-telangiectasia mutant alleles. Ann Intern Med 2000;10:770–778. [DOI] [PubMed] [Google Scholar]
- Sugawa F, Wada Y, Maruyama T, Uchida H, Ishizuka B, Ogata T. Premature ovarian failure and androgen receptor gene CAG repeat lengths weighted by X chromosome inactivation patterns. Fertil Steril 2009;2:649–652. [DOI] [PubMed] [Google Scholar]
- Sundblad V, Chiauzzi VA, Escobar ME, Dain L, Charreau EH. Screening of FSH receptor gene in Argentine women with premature ovarian failure (POF). Mol Cell Endocrinol 2004;1-2:53–59. [DOI] [PubMed] [Google Scholar]
- Takakura K, Takebayashi K, Wang HQ, Kimura F, Kasahara K, Noda Y. Follicle-stimulating hormone receptor gene mutations are rare in Japanese women with premature ovarian failure and polycystic ovary syndrome. Fertil Steril 2001;1:207–209. [DOI] [PubMed] [Google Scholar]
- Takebayashi K, Takakura K, Wang HQ, Kimura F, Kasahara K, Noda Y. Mutation analysis of the growth differentiation factor-9 and-9B genes in patients with premature ovarian failure and polycystic ovary syndrome. Fertil Steril 2000;5:976–979. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Miyamoto N, Shouguchi-Miyata J, Ikeda JE. HFM1, the human homologue of yeast Mer3, encodes a putative DNA helicase expressed specifically in germ-line cells. DNA Seq 2006;3:242–246. [DOI] [PubMed] [Google Scholar]
- Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47,XXX). Orphanet J Rare Dis 2010;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiotiu D, Alvaro Mercadal B, Imbert R, Verbist J, Demeestere I, De Leener A, Englert Y, Vassart G, Costagliola S, Delbaere A. Variants of the BMP15 gene in a cohort of patients with premature ovarian failure. Hum Reprod 2010;6:1581–1587. [DOI] [PubMed] [Google Scholar]
- Tong Y, Liao WX, Roy AC, Ng SC. Absence of mutations in the coding regions of follicle-stimulating hormone receptor gene in Singapore Chinese women with premature ovarian failure and polycystic ovary syndrome. Horm Metab Res 2001;4:221–226. [DOI] [PubMed] [Google Scholar]
- Tong ZB, Sullivan SD, Lawless LM, Vanderhoof V, Zachman K, Nelson LM. Five mutations of mitochondrial DNA polymerase-gamma (POLG) are not a prevalent etiology for spontaneous 46,XX primary ovarian insufficiency. Fertil Steril 2010;7:2932–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh D, Rani HS, Murty US, Deenadayal A, Grover P. Mutational analysis of the FIGLA gene in women with idiopathic premature ovarian failure. Menopause 2015;22:520–526. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y. Conserved role of nanos proteins in germ cell development. Science 2003;5637:1239–1241. [DOI] [PubMed] [Google Scholar]
- Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet 2010;8:707–710. [DOI] [PubMed] [Google Scholar]
- Van Kasteren YM, Hundscheid RD, Smits AP, Cremers FP, van Zonneveld P, Braat DD. Familial idiopathic premature ovarian failure: an overrated and underestimated genetic disease? Hum Reprod 1999;10:2455–2459. [DOI] [PubMed] [Google Scholar]
- Villaescusa JC, Allard P, Carminati E, Kontogiannea M, Talarico D, Blasi F, Farookhi R, Verrotti AC. Clast4, the murine homologue of human eIF4E-Transporter, is highly expressed in developing oocytes and post-translationally modified at meiotic maturation. Gene 2006;367:101–109. [DOI] [PubMed] [Google Scholar]
- Vilodre LC, Kohek MB, Spritzer PM. Screening of follicle-stimulating hormone receptor gene in women with premature ovarian failure in southern Brazil and associations with phenotype. J Endocrinol Invest 2008;6:552–557. [DOI] [PubMed] [Google Scholar]
- Waggoner DD, Buist NR, Donnell GN. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis 1990;6:802–818. [DOI] [PubMed] [Google Scholar]
- Wang CY, Davoodi-Semiromi A, Huang W, Connor E, Shi JD, She JX. Characterization of mutations in patients with autoimmune polyglandular syndrome type 1 (APS1). Hum Genet 1998;6:681–685. [DOI] [PubMed] [Google Scholar]
- Wang LL, Levy ML, Lewis RA, Chintagumpala MM, Lev D, Rogers M, Plon SE. Clinical manifestations in a cohort of 41 Rothmund-Thomson syndrome patients. Am J Med Genet 2001;1:11–17. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Takakura K, Takebayashi K, Noda Y. Mutational analysis of the mullerian-inhibiting substance gene and its receptor gene in Japanese women with polycystic ovary syndrome and premature ovarian failure. Fertil Steril 2002;6:1329–1330. [DOI] [PubMed] [Google Scholar]
- Wang B, Li L, Ni F, Song J, Wang J, Mu Y, Ma X, Cao Y. Mutational analysis of SAL-Like 4 (SALL4) in Han Chinese women with premature ovarian failure. Mol Hum Reprod 2009;9:557–562. [DOI] [PubMed] [Google Scholar]
- Wang B, Ni F, Li L, Wei Z, Zhu X, Wang J, Cao Y, Ma X. Analysis of cyclin-dependent kinase inhibitor 1B mutation in Han Chinese women with premature ovarian failure. Reprod Biomed Online 2010a;2:212–214. [DOI] [PubMed] [Google Scholar]
- Wang B, Wen Q, Ni F, Zhou S, Wang J, Cao Y, Ma X. Analyses of growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) mutation in Chinese women with premature ovarian failure. Clin Endocrinol (Oxf) 2010b;1:135–136. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang B, Song J, Suo P, Ni F, Chen B, Ma X, Cao Y. New candidate gene POU5F1 associated with premature ovarian failure in Chinese patients. Reprod Biomed Online 2011;3:312–316. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun M, Qin Y, Xia T, Ma J, Chen ZJ. Mutations in DMC1 are not responsible for premature ovarian failure in Chinese women. Reprod Biomed Online 2012;2:175–178. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang W, Jiang H, Wu BL. Mutations in HFM1 in recessive primary ovarian insufficiency. N Engl J Med 2014a;10:972–974. [DOI] [PubMed] [Google Scholar]
- Wang JL, Li SL, Qin YY, Chen ZJ. Analysis of progesterone receptor membrane component 1 mutation in Han Chinese women with premature ovarian failure. Reprod Biomed Online 2014b;5:640–643. [DOI] [PubMed] [Google Scholar]
- Watkins WJ, Umbers AJ, Woad KJ, Harris SE, Winship IM, Gersak K, Shelling AN. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil Steril 2006;5:1518–1521. [DOI] [PubMed] [Google Scholar]
- Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril 2007;3:456–465. [DOI] [PubMed] [Google Scholar]
- Woad KJ, Prendergast D, Winship IM, Shelling AN. FSH receptor gene variants are rarely associated with premature ovarian failure. Reprod Biomed Online 2013;4:396–399. [DOI] [PubMed] [Google Scholar]
- Wood-Trageser MA, Gurbuz F, Yatsenko SA, Jeffries EP, Kotan LD, Surti U, Ketterer DM, Matic J, Chipkin J, Jiang H et al. MCM9 mutations are associated with ovarian failure, short stature, and chromosomal instability. Am J Hum Genet 2014;6:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wang B, Dong Z, Zhou S, Liu Z, Shi G, Cao Y, Xu Y. A NANOS3 mutation linked to protein degradation causes premature ovarian insufficiency. Cell Death Dis 2013;4:e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 2001;6:854–866. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Choi YM, Hong MA, Kim JJ, Lee GH, Hwang KR, Moon SY. Association study of anti-Mullerian hormone and anti-Mullerian hormone type II receptor polymorphisms with idiopathic primary ovarian insufficiency. Hum Reprod 2013;12:3301–3305. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tang YY, Guo YP. A study of hypergonadotropic secondary amenorrhea with cytogenetics. J Chongqing Med Univ 2003;28:151–152. [Google Scholar]
- Zhang J, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, Lou Y, Yang J, Ma Y, Chai L et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol 2006;10:1114–1123. [DOI] [PubMed] [Google Scholar]
- Zhang P, Shi YH, Wang LC, Chen ZJ. Sequence variants in exons of the BMP-15 gene in Chinese patients with premature ovarian failure. Acta Obstet Gynecol Scand 2007;5:585–589. [DOI] [PubMed] [Google Scholar]
- Zhao H, Qin Y, Kovanci E, Simpson JL, Chen ZJ, Rajkovic A. Analyses of GDF9 mutation in 100 Chinese women with premature ovarian failure. Fertil Steril 2007;5:1474–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chen ZJ, Qin Y, Shi Y, Wang S, Choi Y, Simpson JL, Rajkovic A. Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am J Hum Genet 2008;6:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Qin Y, Ma J, Zhao H, Li J, Wang L, Ren C, Che L, Chen ZJ. PTEN gene analysis in premature ovarian failure patients. Acta Obstet Gynecol Scand 2011;6:678–679. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Wei D, Mu Y, Qin Y, Li G, Cui L, Chen ZJ. Mutational analysis of SKP2 and P27 in Chinese Han women with premature ovarian failure. Reprod Biomed Online 2013;1:104–106. [DOI] [PubMed] [Google Scholar]
- Zhao S, Li G, Dalgleish R, Vujovic S, Jiao X, Li J, Simpson JL, Qin Y, Ivanisevic M, Ivovic M et al. Transcription factor SOHLH1 potentially associated with primary ovarian insufficiency. Fertil Steril 2015;103:548–553. [DOI] [PubMed] [Google Scholar]
- Zhen XM, Sun YM, Qiao J, Li R, Wang LN, Liu P. Genome-wide copy number scan in Chinese patients with premature ovarian failure. Beijing Da Xue Xue Bao 2013;6:841–847. [PubMed] [Google Scholar]
- Zhou S, Wang B, Ni F, Wang J, Cao Y, Ma X. GPR3 may not be a potential candidate gene for premature ovarian failure. Reprod Biomed Online 2010;1:53–55. [DOI] [PubMed] [Google Scholar]
- Zlotogora J, Sagi M, Cohen T. The blepharophimosis, ptosis, and epicanthus inversus syndrome: delineation of two types. Am J Hum Genet 1983;5:1020–1027. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.