Abstract
BACKGROUND:
Lymphangioleiomyomas occur in 38% of patients with sporadic lymphangioleiomyomatosis (LAM) and may cause pain and increased abdominal girth, mimicking the presence of a malignancy. Lymphatic involvement in LAM is closely associated with elevated serum levels of vascular endothelium growth factor-D (VEGF-D). Because lymphangioleiomyomas undergo diurnal variation in volume, we hypothesized that daytime ingestion of food, by increasing chyle formation and lymphatic flow, is the cause of an increase in lymphangioleiomyoma volume.
METHODS:
Subjects had abdominopelvic sonograms and blood drawn for measurement of serum VEGF-D levels under nonfasting (day 1) and fasting (day 2) conditions. The size of the lymphangioleiomyomas was determined by a radiologist who was blinded to the subjects’ status. The Wilcoxon signed rank test was used to determine whether the nonfasting tumor size was different from the fasting tumor size.
RESULTS:
Thirty-five women were studied (aged 45.2 ± 8.5 years; FEV1, 82% ± 25%; diffusing capacity of the lung for carbon monoxide, 64% ± 25% predicted). Images suitable for volume measurements were obtained in 30 subjects. Fasting decreased the tumor size by 20.7 ± 39.3 cm3 (24% ± 40%, P < .001). Fasting VEGF-D levels (10,650 ± 900 pg/mL) were not significantly different from nonfasting values (12,100 ± 800 pg/mL, P = .56).
CONCLUSIONS:
Lymphangioleiomyoma volume decreased during the fasting state. Conversely, a combination of food intake and decreased chyle flow through lymphatics partially obstructed by LAM cells may account for increases in lymphangioleiomyoma size. Imaging studies performed under fasting conditions may help in determining whether an abdominal tumor is a result of LAM or malignancy.
Lymphangioleiomyomatosis (LAM) is a multisystem disorder affecting primarily women that is characterized by cystic lung destruction, abdominal angiomyolipomas, and lymphatic abnormalities comprising lymphadenopathy, lymphangioleiomyomas, and chylous effusions.1 LAM occurs sporadically, primarily as a result of mutations in TSC22,3 in patients with no evidence of genetic disease as well as in patients with tuberous sclerosis complex,4 an autosomal-dominant syndrome caused by mutations in TSC1 or TSC2.5
The lymphatic vasculature has been recognized as playing a major role in the pathophysiology of LAM. Indeed, LAM may present with thoracoabdominal lymphangioleiomyomas,6,7 chylothorax, and ascites,6,8 suggesting the presence of a malignancy.9,10 Lymphangioleiomyomas, which occur in about 38% of patients with sporadic LAM,11 are usually located along the axial lymphatics in the thorax and abdomen, retroperitoneum around the aorta, renal and superior mesenteric arteries, and pelvic region.6,7 Macroscopically, lymphangioleiomyomas consist of well-circumscribed masses with prominent cystic formations filled with chylous fluid.6 The symptoms associated with lymphangioleiomyomas may be disabling, including an increase in abdominal girth, abdominal pain suggesting an acute abdomen, obstipation, urinary frequency, incontinence, Horner syndrome, chyloptysis, malabsorption syndrome, and bladder obstruction.12‐16 Extrapulmonary lymphatic lesions may precede the appearance of pulmonary LAM.6,17
The lesions of LAM result from organ infiltration by a neoplastic cell (termed the “LAM cell”) that displays characteristics of both smooth muscle cells and melanocytes.6,18 LAM cells comprise both spindle-shaped and epithelioid smooth muscle-like cells. Both cell types react with antibodies against the smooth muscle antigens α-actin, vimentin, and desmin.6,18 The epithelioid LAM cells react with human melanin black antibody (HMB-45), a monoclonal antibody that recognizes a premelanosomal protein (gp100) encoded by the Pmel17 gene.6,18 In the lungs, LAM cells are arranged either in a haphazard pattern or in follicles, bundles, or papillary formations containing slit-like channels that are lined by lymphatic endothelial cells and may contain lymphocytes and RBCs.6,18 Aggregates of lymphoid cells may form lymphoid follicles.6,18 Lymphatic vessels may be dilated as a consequence of compression of the thin-walled lymphatic channels by proliferating LAM cells.6,18 LAM cell clusters (LCCs) have been noted in chylous effusions and elsewhere.19 These LCCs consist of LAM cells surrounded by lymphatic endothelial cells.19,20 LCCs are present in lung lymphatics, lymph nodes, uterus, and chylous fluid.19,20 LAM cells infiltrate and penetrate the lymphatic channels and pulmonary arterioles, leading to leakage of chyle and blood.6,18,20 Occlusion or infiltration of pulmonary arterioles by LAM cells may account for the hemoptysis described by patients with LAM.11 Through the lymphatic channels, LAM cells gain access to the systemic circulation, facilitating the metastatic spread of the disease.20 In summary, LAM cells appear to invade the wall of the lymphatic vessels, causing damage and leakage of chylous fluid and obstruction of chyle flow and leading to the formation of lymphangioleiomyomas.
LAM cells exhibit positive immunoreactivity for vascular endothelial growth factor (VEGF) receptor-3.21 VEGF-C and VEGF-D are ligands for VEGF receptor-3, which is also expressed by lymphatic endothelial cells.20,21 The degree of lymphangioleiogenesis and VEGF-C expression has been reported to correlate with the histologic severity of lung disease.21 LAM cells also exhibit positive immunoreactivity for VEGF-D, and serum levels of VEGF-D are increased in patients with LAM, especially those with lymphatic involvement.22,23 High VEGF-D levels are associated with severity of lung disease as measured by the degree of airflow obstruction, impairment of lung diffusion capacity, and cysts on CT scans.22,23 Measurement of serum VEGF-D has been shown to be useful as a diagnostic tool and in grading the severity of the disease and the potential response to therapy.24,25
Lymphangioleiomyomas have a distinctive radiologic appearance, and diurnal variation in size of these tumors has been reported.7,26,27 In one study, CT scans taken in the evening hours showed a 140% increase in volume from morning values.24 This finding was also documented by ultrasonography.27 In the latter study, 21 of 44 patients with LAM showed morning to evening variation, with an increase in the size of the lymphangioleiomyomas ranging from 10% to 484%.27
Flow of chyle in lymphatics is determined by food intake, depending predominantly on the fat content of the diet.28 Studies of chylothorax have demonstrated a reduction in daily fluid drainage and complete resolution of effusions when patients were placed on a low-fat diet and total parenteral nutrition, with the only source of fat being medium-chain triglycerides.29‐32 Conversely, during various surgical procedures, visualization of the thoracic duct is improved by administration of milk fat.33 Consequently, it is recommended that patients with chylous effusions be placed on a low-fat diet.
The aim of this study was to determine whether the size of lymphangioleiomyomas is affected by food intake. Specifically, based on prior studies demonstrating an increase in tumor size from morning to evening time, we questioned whether the changes in tumor volume were due to overnight fasting rather than to changes in hydrostatic pressure resulting from an upright posture and ambulation. We also questioned whether changes in the size of lymphangioleiomyomas were associated with changes in serum levels of VEGF-D.
Materials and Methods
Study Subjects and Study Design
Subjects were recruited from a large cohort of patients participating in a LAM natural history and pathogenesis protocol (National Heart, Lung, and Blood Institute [NHLBI] protocol 95-H-0186). The study was approved by the NHLBI Institutional Review Board (NHLBI protocol 08-H-0016).
The study was performed in 35 patients with LAM in whom the presence of lymphangioleiomyomas had previously been ascertained by CT imaging or abdominopelvic ultrasonography. On the first day of testing, the patients were allowed to eat a regular diet at mealtimes and between-meal snacks. Patients were allowed to have dinner, which was completed by 7:00 pm, and then they fasted until study day 2 testing was completed. On the second day of the study, patients were tested both in the morning and in the evening, with the latter test being performed at the same time in the evening as study day 1. By the morning of the second day, patients had been fasting for approximately 15 h. On the second day of the study, evening testing was undertaken after 22 h of fasting. Patients were ambulatory during the intervals between assessments. The cohort did not include any night shift workers. All patients were confined to the hospital and observed by the nursing and medical staff throughout the duration of the study. Patients were scanned by the same technologist using the same machine and the same transducer frequency for both the morning and the afternoon studies. All studies were checked by the same board-certified radiologist before the patient left the department.27 Transverse, sagittal, and longitudinal diameters of the lymphangioleiomyomas were determined as previously reported27 by a radiologist who was blinded to the patients’ status. The mass sizes were quantified by multiplying the transverse, sagittal, and longitudinal diameters of the masses and applying the following mathematical equation used to calculate the volume of an ellipsoid: 4π × (T × S × L)/3.
Statistical Analysis
The primary end point was the change in lymphangioleiomyoma size (volume) between nonfasting and fasting states. We hypothesized that these changes were associated with alterations in serum levels of some biomarkers, especially VEGF-D. Accordingly, we measured serum VEGF-D levels on the evening of both day 1 and day 2. For each patient, the difference between lesion size in the fasting and nonfasting states was calculated, and the Wilcoxon signed rank test was used to test whether the nonfasting lymphangioleiomyoma size was different from the fasting one. Because it is likely that the measurement in the fasting state would be correlated with the volume change or percent volume change, we performed secondary analyses of the primary end point using an analysis of covariance, where the baseline measurement was used to adjust for the effect of nonfasting on lymphangioleiomyoma size. All values are shown as mean ± SD.
Results
Patient Characteristics
Thirty-five patients were enrolled in the study. Their mean age at the time of the study was 42.8 ± 8 years. Their age when initial symptoms were experienced was 38.2 ± 8.4 years, and the age at diagnosis was 41.5 ± 8.2 years. Thirty patients were white, three black, and two Asian. Ten patients were on supplemental oxygen therapy. Eighteen diagnoses were made by tissue biopsy specimen, 12 by lymphangioleiomyoma biopsy specimen, and six by lung biopsy specimen. The remaining 17 diagnoses were made by a combination of characteristic lung cysts, as evidenced on CT imaging: 14 lymphangioleiomyomas, two chylous effusions, and one tuberous sclerosis complex. Pulmonary function tests at enrollment are shown on Table 1. Patients showed decreases in FEV1 and diffusing capacity of the lung for carbon monoxide.
TABLE 1 ] .
Pulmonary Function of 35 Patients With LAM at the Time of Enrollment
| Parameter | Value | % Predicted |
| FVC, L | 3.1 ± 1.7 | 90.8 ± 20.3 |
| FEV1, L | 2.0 ± 0.6 | 76.8 ± 26.4 |
| FEV1/FVC, % | 64 ± 15 | … |
| TLC, L | 4.8 ± 0.9 | 92.9 ± 17.3 |
| FRC, L | 2.6 ± 0.7 | 94.7 ± 21.7 |
| RV, L | 1.6 ± 0.5 | 95.3 ± 26.3 |
| RV/TLC, % | 34 ± 7 | … |
| Dlco, mL/min/mm Hg | 13.4 ± 5.3 | 64.1 ± 25.6 |
Data are presented as mean ± SD. Dlco = diffusing capacity of the lung for carbon monoxide; FRC = functional residual capacity; LAM = lymphangioleiomyomatosis; RV = residual volume; TLC = total lung capacity.
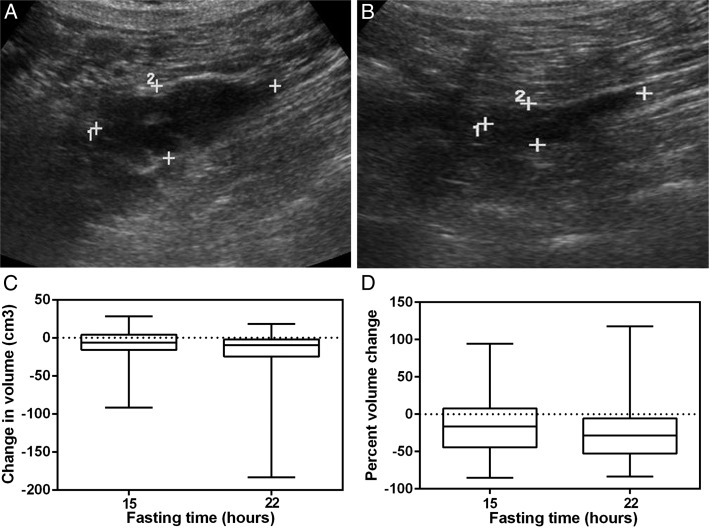
Tumor size could not be estimated in five patients. There were 30 patients with tumor size measurements at 22 h (nonfasting to fasting evening measurement) and 29 with measurements at 15 h (evening day 1 nonfasting to morning day 2 fasting). Figure 1 and Table 2 show that mean ± SD absolute tumor changes from day 2 evening fasting to day 1 evening nonfasting were −20.7 ± 39.3 cm3. The median (interquartile range [IQR]) values were −9.4 (−19.5 to −2.9, P < .001). Mean absolute tumor changes for day 1 evening nonfasting to day 2 morning fasting were −14.0 ± 29 cm3. The median values were −6.3 (IQR, −15.6 to −2.7; P = .014). We also calculated the percent change, defined as (fasting − nonfasting)/nonfasting, to adjust for the baseline nonfasting measurement (Fig 1D). Mean percent tumor changes for day 1 evening nonfasting to day 2 evening fasting were −24% ± 40% (P < .001). Mean percent tumor changes for day 1 evening nonfasting to day 2 morning fasting were −15% ± 42% (P = .037). An illustrative example of a reduction in lymphangioleiomyoma volume after fasting for 22 h is shown in Figures 1A and 1B.
Figure 1 –
A, B, Changes in the size of a lymphangioleiomyoma in a 44-y-old woman with lymphangioleiomyomatosis after fasting for 22 h. Numbers 1 and 2 indicate cross marks for longitudinal and transverse diameters, respectively. The diameters decreased after fasting for 22 h. A, Image of a pelvic lymphangioleiomyoma with an estimated volume of 57 cm3. B, Twenty-two hours after fasting, the volume of the tumor decreased to 18 cm3. C, Box and whisker plot showing changes in lymphangioleiomyoma volume in 30 patients after fasting for 15 and 22 h, respectively. The bottom line of each box represents the first quartile, the top line represents the third quartile, and the line across the boxes represents the second quartile (median). The upper and lower whiskers indicate the maximum and minimum changes. D, Changes in lymphangioleiomyoma volume expressed as a percentage of nonfasting volume with median and interquartile range changes. Mean lymphangioleiomyoma volume was significantly lower at both 15 h (P = .014) and 22 h (P < .001) of fasting.
TABLE 2 ] .
Changes in Lymphangioleiomyoma Size in 30 Patients With LAM
| Variable | Day 1 Nonfasting Evening | Day 2 Fasting Morning | Day 2 Fasting Evening |
| No. patients | 30 | 29 | 30 |
| Time, h | 0 | 15 | 22 |
| Tumor volume, cm3 | 66 ± 80 | 58 ± 63 | 44 ± 48 |
| Volume change, cm3 | … | −14 ± 29a | −21 ± 39b |
| Tumor volume, % | 100 | 86 ± 41 | 76 ± 39 |
| Volume change, % | … | −15 ± 42c | −24 ± 40b |
| VEGF-D level, pg/mL | 12,100 ± 800 | … | 10,650 ± 900 |
Data are presented as mean ± SD unless otherwise indicated. VEGF = vascular endothelial growth factor. See Table 1 legend for expansion of other abbreviation.
P = .014.
P < .001.
P = .037.
There was no statistically significant difference in tumor volume between day 2 morning and day 2 evening (P = .176). Twenty of 29 patients (69%) had some reduction in the tumor size the morning after fasting overnight. Additionally, 18 of 29 patients (62.1%) had further tumor reduction from morning to evening on the fasting day. Overall, 23 of 30 (76.7%) had some tumor reduction 22 h after fasting. Of the 23 patients with a tumor reduction, the median change was −35% (IQR, −55% to −28%). Twenty of 30 patients had a reduction of at least 15% in the 22-h fasting period (median change, −28%; IQR, −51% to −10%; P = .016). The median in tumor size in the seven patients who had an increase in tumor volume was 20% (IQR, 17%-23%).
Discussion
Dysregulation of lymphangiogenesis plays an important role in the pathogenesis of LAM.20 The disease phenotype is associated with lung nodules infiltrated with lymphatics, lymphatic vessels showing LAM cells penetrating through the walls, and chylous effusions.19‐25 These forms of LAM are characterized by the presence of vascular abnormalities and evidence of arteriolar and venular channels as well as lymphatic channel infiltration by LAM cells, resulting in both vascular obstruction and vascular wall disruption.6,18 In the lungs, the consequences of these abnormalities are twofold. First, an increase in vascular permeability leads to lung hemorrhage, hemosiderosis, and an increased number of alveolar hemosiderin-laden macrophages in the lungs.34 These pathologic findings are associated with symptoms of hemoptysis at rest and during exercise or sexual intercourse and greater severity and rate of progression of lung disease.11,34 Second, there is evidence for an increase in lung water, presumably chyle,35‐37 showing lung infiltrates on imaging studies and suggesting pulmonary edema that shifts with body position and resolves after treatment with mammalian target of rapamycin inhibitors.35‐37
The lymphangioleiomyomas appear to be the result of lymphatic infiltration by LAM cells, resulting in blockade and the accumulation of chyle and cystic structures. Many factors affect the formation and size of these tumors, such as fat intake. Lymphangioleiomyomas are associated with severity of pulmonary function impairment and overexpression of growth factors (eg, VEGF-D). As in the case of pulmonary disease, treatment with mammalian target of rapamycin inhibitors reduces the size of the tumors, with significant resolution of chylous effusions, while simultaneously causing a reduction in serum VEGF-D levels.25,35
The current study shows that fasting also reduces the size of lymphangioleiomyomas. This was evident after 15 and 22 h of fasting regardless of whether the measurements within the fasting period were done in the morning or the evening. However, changes in the volume of lymphangioleiomyomas were not associated with alterations in serum VEGF-D levels. Studies done after 22 h of fasting showed that the size of the lymphangioleiomyomas either continued to decrease from morning to evening or remained unchanged. These findings are not consistent with those previously reported in fed subjects showing that the volume of lymphangioleiomyomas increases from morning to evening.26,27 These data suggest that the diurnal increase in lymphangioleiomyoma size may have been caused by increased chyle production after meals, resulting from an increase in lymph flow through the abdomen and pelvis.
This study has several limitations. Not all patients experienced a reduction in tumor size during fasting; seven experienced a small increase in tumor volume, and of the 35 enrolled patients, five previously known to have lymphangioleiomyomas showed no evidence of measurable tumors, suggesting that spontaneous changes in lymphangioleiomyoma size may have occurred independently of food intake or body position. The possible explanations for the lymphangioleiomyoma volume increase in seven of the 35 patients are several. First, there may have been some inaccuracy and lack of precision in the measurement of lymphangioleiomyoma volume. This would be more likely to occur in patients in whom the shape of the lymphangioleiomyomas could not be established by the measurement of only three diameters and may have led to an underestimation of the volume changes. Second, the structure and composition of the tumors may not be uniform and similar in all patients with LAM. In some cases, lymphangioleiomyomas mainly comprise predominantly fluid-filled structures. In these instances, reduced food intake may be more likely to cause a reduction in tumor size. Conversely, in patients in whom the main component of the tumor consists of cellular elements, fasting may be less likely to affect the tumor volume. Inadvertent violation of the fasting state by patients could also have affected the volume of the tumors. Finally, an increase in lymphangioleiomyoma size associated with hormonal changes of the menstrual cycle may have occurred in some patients.38 Although the present study was performed in hospitalized patients who were ambulatory, it is possible that increases in lymphatic flow through the pelvis and abdomen because of physical activity and the upright position may increase lymphatic pressure in dependent body regions. These effects could have been diminished by recumbent positioning.
Because in some patients the reduction in tumor volume was small, the clinical significance of a small reduction in volume of a still undiagnosed tumor does not preclude the need for further diagnostic tests. More accurate measurement of the tumor size by MRI or CT imaging along with more prolonged fasting with complete elimination of fat intake may improve the utility of the method. Although only 76% of patients had a decrease in tumor size, some had a sufficiently large reduction in tumor volume to strongly suggest that the tumor was very unlikely to be malignant.
Conclusions
The size of a lymphangioleiomyoma may decrease in volume during the fasting state perhaps because of decreased chyle flow. Conversely, it is conceivable that lymphangioleiomyoma volume may increase with food intake due to problems with chyle flow through lymphatics partially obstructed or disrupted by LAM cells. Along with prior observations that lymphangioleiomyoma volume may increase from morning to evening,26,27 the current findings suggest that imaging studies performed in the morning after fasting may help in determining whether an abdominal tumor seen on CT scan or ultrasound may be a result of LAM or perhaps a malignancy, such as ovarian cancer or sarcoma.
Acknowledgments
Author contributions: A. M. T.-D. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. A. M. T.-D. and J. M. contributed to the study design, data analysis, and writing and final approval of the manuscript; A. M. J. and P. J.-W. contributed to the data collection, review of clinical data, and final approval of the manuscript; T. S. contributed to the abdominal and pelvic ultrasonographic studies and final approval of the manuscript; C. G. G. contributed to the serum VEGF-D assays and final approval of the manuscript; and M. S. contributed to the statistical analysis and final approval of the manuscript.
Conflict of interest: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- IQR
interquartile range
- LAM
lymphangioleiomyomatosis
- LCC
lymphangioleiomyomatosis cell cluster
- NHLBI
National Heart, Lung, and Blood Institute
- VEGF
vascular endothelial growth factor
Footnotes
FUNDING/SUPPORT: This study was supported by the Intramural Research Program, National Institutes of Health, National Heart, Lung, and Blood Institute [Grant NHLBI 08-H-0016].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Ryu JH, Moss J, Beck GJ, et al. ; NHLBI LAM Registry Group. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173(1):105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62(4):810-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97(11):6085-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss J, Avila NA, Barnes PM, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001;164(4):669-671. [DOI] [PubMed] [Google Scholar]
- 5.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657-668. [DOI] [PubMed] [Google Scholar]
- 6.Matsui K, Tatsuguchi A, Valencia J, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases. Hum Pathol. 2000;31(10):1242-1248. [DOI] [PubMed] [Google Scholar]
- 7.Avila NA, Kelly JA, Chu SC, Dwyer AJ, Moss J. Lymphangioleiomyomatosis: abdominopelvic CT and US findings. Radiology. 2000;216(1):147-153. [DOI] [PubMed] [Google Scholar]
- 8.Ryu JH, Doerr CH, Fisher SD, Olson EJ, Sahn SA. Chylothorax in lymphangioleiomyomatosis. Chest. 2003;123(2):623-627. [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal VR, Baird J, Fleming J, Miller DS, Sharma S, Molberg K. Localized retroperitoneal lymphangioleiomyomatosis mimicking malignancy. A case report and review of the literature. Arch Pathol Lab Med. 2003;127(7):879-882. [DOI] [PubMed] [Google Scholar]
- 10.Llopis I, Arandiga R, Real E, et al. Lymphangiomyomatosis mimicking an abdominal lymphoma. Haematologica. 2002;87(10):EIM23. [PubMed] [Google Scholar]
- 11.Taveira-DaSilva AM, Jones AM, Julien-Williams P, Yao J, Stylianou M, Moss J. Severity and outcome of cystic lung disease in women with tuberous sclerosis complex. Eur Respir J. 2015;45(1):171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H-C, Wang J, Tsang Y-M, Lin M-C, Li Y-W. Lymphangioleiomyomatosis initially presenting with abdominal pain: a case report. Clin Imaging. 2003;27(3):166-170. [DOI] [PubMed] [Google Scholar]
- 13.Wong Y-Y, Yeung T-K, Chu WC. Atypical presentation of lymphangioleiomyomatosis as acute abdomen: CT diagnosis. AJR Am J Roentgenol. 2003;181(1):284-285. [DOI] [PubMed] [Google Scholar]
- 14.Scales CD, Jr, Springhart WP, Young MD, Anger JT, Leder RA, Preminger GM. Lymphangioleiomyomatosis presenting as bladder outlet obstruction. Urology. 2005;65(3):589-590. [DOI] [PubMed] [Google Scholar]
- 15.Lin C-C, Lee T-C, Liu K-L, Lin JT, Lin MT, Wang HP. Education and imaging. Gastrointestinal: lymphangioleiomyomatosis with protein-losing enteropathy. J Gastroenterol Hepatol. 2006;21(12):1860. [DOI] [PubMed] [Google Scholar]
- 16.Kahn SU, Mehta AC. Chyloptyses found in a patient with lymphangioleiomyomatosis. Chest. 1995;108(6):1775. [DOI] [PubMed] [Google Scholar]
- 17.Ernst JC, Sohaey R, Cary JM. Pelvic lymphangioleiomyomatosis. Atypical precursor to pulmonary disease. Chest. 1994;106(4):1267-1269. [DOI] [PubMed] [Google Scholar]
- 18.Ferrans VJ, Yu ZX, Nelson WK, et al. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nippon Med Sch. 2000;67(5):311-329. [DOI] [PubMed] [Google Scholar]
- 19.Mitani K, Kumasaka T, Takemura H, et al. Cytologic, immunocytochemical and ultrastructural characterization of lymphangioleiomyomatosis cell clusters in chylous effusions of patients with lymphangioleiomyomatosis. Acta Cytol. 2009;53(4):402-409. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R, Kitaichi M, Inoue Y, Kotloff R, McCormack FX. Lymphatic manifestations of lymphangioleiomyomatosis. Lymphology. 2014;47(3):106-117. [PubMed] [Google Scholar]
- 21.Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004;28(8):1007-1016. [DOI] [PubMed] [Google Scholar]
- 22.Seyama K, Kumasaka T, Souma S, et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol. 2006;4(3):143-152. [DOI] [PubMed] [Google Scholar]
- 23.Glasgow CG, Avila NA, Lin JP, Stylianou MP, Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest. 2009;135(5):1293-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young LR, Vandyke R, Gulleman PM, et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138(3):674-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young L, Lee HS, Inoue Y, et al. ; MILES Trial Group. Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med. 2013;1(6):445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avila NA, Bechtle J, Dwyer AJ, Ferrans VJ, Moss J. Lymphangioleiomyomatosis: CT of diurnal variation of lymphangioleiomyomas. Radiology. 2001;221(2):415-421. [DOI] [PubMed] [Google Scholar]
- 27.Avila NA, Dwyer AJ, Murphy-Johnson DV, Brooks P, Moss J. Sonography of lymphangioleiomyoma in lymphangioleiomyomatosis: demonstration of diurnal variation in lesion size. AJR Am J Roentgenol. 2005;184(2):459-464. [DOI] [PubMed] [Google Scholar]
- 28.Sitrin MD. Digestion and absorption of dietary triglycerides. In: Leung PS, ed. The Gastrointestinal System: Gastrointestinal, Nutritional and Hepatobiliary Physiology. Dordrecht, The Netherlands: Springer Science + Business Media Dordrecht; 2014:159-178. [Google Scholar]
- 29.Marts BC, Naunheim KS, Fiore AC, Pennington DG. Conservative versus surgical management of chylothorax. Am J Surg. 1992;164(5):532-534. [DOI] [PubMed] [Google Scholar]
- 30.Chan GM, Lechtenberg E. The use of fat-free human milk in infants with chylous pleural effusion. J Perinatol. 2007;27(7):434-436. [DOI] [PubMed] [Google Scholar]
- 31.Epaud R, Dubern B, Larroquet M, et al. Therapeutic strategies for idiopathic chylothorax. J Pediatr Surg. 2008;43(3):461-465. [DOI] [PubMed] [Google Scholar]
- 32.Bibby AC, Maskell NA. Nutritional management in chyle leaks and chylous effusions. Br J Community Nurs. 2014;19(suppl 10):S6-S8. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, Feng M, Khan MA, Wang H, Tan L, Wang Q. A simple method minimizes chylothorax after minimally invasive esophagectomy. J Am Coll Surg. 2014;218(1):108-112. [DOI] [PubMed] [Google Scholar]
- 34.Matsui K, Beasley MB, Nelson WK, et al. Prognostic significance of pulmonary lymphangioleiomyomatosis histologic score. Am J Surg Pathol. 2001;25(4):479-484. [DOI] [PubMed] [Google Scholar]
- 35.Taveira-DaSilva AM, Hathaway O, Stylianou M, Moss J. Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus. Ann Intern Med. 2011;154(12):797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moua T, Olson EJ, Jean HC, Ryu JH. Resolution of chylous pulmonary congestion and respiratory failure in lymphangioleiomyomatosis with sirolimus therapy. Am J Respir Crit Care Med. 2012;186(4):389-390. [DOI] [PubMed] [Google Scholar]
- 37.Tobino K, Johkoh T, Fujimoto K, et al. Computed tomographic features of lymphangioleiomyomatosis: evaluation in 138 patients. Eur J Radiol. 2015;84(3):534-541. [DOI] [PubMed] [Google Scholar]
- 38.Sandrini A, Silverstone E, Yates DH. Menstrual cycle variation of retroperitoneal lymphangioleiomyomas in lymphangioleiomyomatosis. Intern Med J. 2011;41(12):832-835. [DOI] [PubMed] [Google Scholar]