Abstract
BACKGROUND:
The National Heart, Lung, and Blood Institute-sponsored IPF Clinical Research Network (IPFnet) studies enrolled subjects with idiopathic pulmonary fibrosis (IPF) to evaluate drug therapies in treatment trials. An adjudication committee (AC) provided a structured review of cases in which there was uncertainty or disagreement regarding diagnosis or clinical event classification. This article describes the diagnosis and adjudication processes.
METHODS:
The diagnostic process was based on review of clinical data and high-resolution CT scans with central review of lung biopsies when available. The AC worked closely with the data coordinating center to obtain clinical, radiologic, and histologic data and to communicate with the clinical centers. The AC used a multidisciplinary discussion model with four clinicians, one radiologist, and one pathologist to adjudicate diagnosis and outcome measures.
RESULTS:
The IPFnet trials screened 1,015 subjects; of these, 23 cases required review by the AC to establish eligibility. The most common diagnosis for exclusion was suspected chronic hypersensitivity pneumonitis. The AC reviewed 88 suspected acute exacerbations (AExs), 93 nonelective hospitalizations, and 16 cases of bleeding. Determination of AEx presented practical challenges to adjudicators, as necessary clinical data were often not collected, particularly when subjects were evaluated outside of the primary study site.
CONCLUSIONS:
The IPFnet diagnostic process was generally efficient, but a multidisciplinary adjudication committee was critical to assure correct phenotype for study enrollment. The AC was key in adjudicating all adverse outcomes in two IPFnet studies terminated early because of safety issues. Future clinical trials in IPF should consider logistical and cost issues as they incorporate AExs and hospitalizations as outcome measures.
TRIAL REGISTRY:
ClinicalTrials.gov; No.: NCT00517933, NCT00650091, NCT00957242; URL: www.clinicaltrials.gov
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive lung disease of unknown cause characterized by the histopathologic and radiologic pattern of usual interstitial pneumonia (UIP), with a median survival after diagnosis between 2 and 5 years.1 In 2005, the National Heart, Lung, and Blood Institute formed the IPF Clinical Research Network (IPFnet) with the goal of finding effective therapies for patients at all stages of the disease. The network designed three randomized clinical trials exploring the role of sildenafil (Sildenafil Trial of Exercise Performance in Idiopathic Pulmonary Fibrosis [STEP-IPF]), warfarin (Anticoagulant Effectiveness in Idiopathic Pulmonary Fibrosis [ACE-IPF]), and the combination of prednisone, azathioprine, and N-acetylcysteine (NAC) or NAC alone (Evaluating the Effectiveness of Prednisone, Azathioprine, and N-acetylcysteine in Patients With Idiopathic Pulmonary Fibrosis [PANTHER-IPF]) for the treatment of IPF. Descriptions of the studies are given later. As the network aimed to study only IPF cases, case definition was a major concern. Furthermore, other important outcome measures required rigorous definition and review. The purpose of this article is to illustrate how the IPFnet established and conducted the adjudication process for the IPFnet studies and to review its results.
Materials and Methods
Role of the Adjudication Committee
The adjudication committee (AC) of the IPFnet was composed of four clinicians (J. d. A., M. S., H. R. C., and R. J. K.), one thoracic radiologist (D. L.), and one lung pathologist (T. C.). It was established by the IPFnet Steering Committee and had the primary mission of adjudicating study eligibility when requested by the clinical center. The AC also reviewed suspected instances of acute exacerbations (AExs), nonelective hospitalizations, deaths, and, for ACE-IPF specifically, all bleeding events. AC meetings followed a multidisciplinary discussion format.2 The AC relied upon the IPFnet Data Coordinating Center at Duke Clinical Research Institute to gather the necessary data from clinical sites, to identify suspected end-point events, to organize the AC conference calls, and to convey the decisions of the committee to the site principal investigators (PIs). The AC worked with other IPFnet committees, statisticians, and data entry personnel to help operationalize entry criteria and to provide input on key sections of the case report forms.
IPFnet Protocols Overview
STEP-IPF Trial:
This was a double-blind, placebo-controlled, randomized trial evaluating the benefits and risks of sildenafil in an IPF population with advanced disease defined by diffusing capacity of the lung for carbon monoxide < 35% predicted.3 It enrolled patients between September 2007 and March 2009. The primary outcome was defined by an improvement of 20% in 6-min walk distance from baseline to 12 weeks. This study showed that sildenafil had significant benefit in gas exchange, dyspnea, and quality of life in patients with advanced IPF but did not meet the primary end point.3
PANTHER-IPF Study:
The study initially used a three-arm factorial design with 1:1:1 randomization to prednisone + azathioprine + NAC vs NAC alone vs placebo. Approximately 390 subjects with mild to moderate IPF were to be enrolled. The primary outcome measure was the change in FVC over a 60-week period. At a prespecified interim analysis, the Data and Safety Monitoring Board recommended termination of the three-drug arm of the study because of excess mortality.4 The trial continued as a two-arm design (NAC vs placebo) without other changes and enrolled 133 and 131 patients in the NAC and placebo arms, respectively. The study enrolled patients from December 2009 until mid-October 2011 (prealert period) and then from January 2012 to July 2012 (postalert period). Compared with placebo, NAC offered no benefit for the preservation of FVC in the studied population.5
ACE-IPF Trial:
The ACE-IPF trial compared warfarin to placebo in subjects with IPF and enrolled patients between October 2009 and July 2011.6 The primary outcome measure was a composite of death, hospitalization (nonbleeding, nonelective), or ≥ 10% absolute decline in FVC. Because of a low probability of benefit and an increased probability of death in the warfarin group, the Data and Safety Monitoring Board recommended stopping the study after 145 of the planned 256 subjects were enrolled.6
Diagnosis of IPF
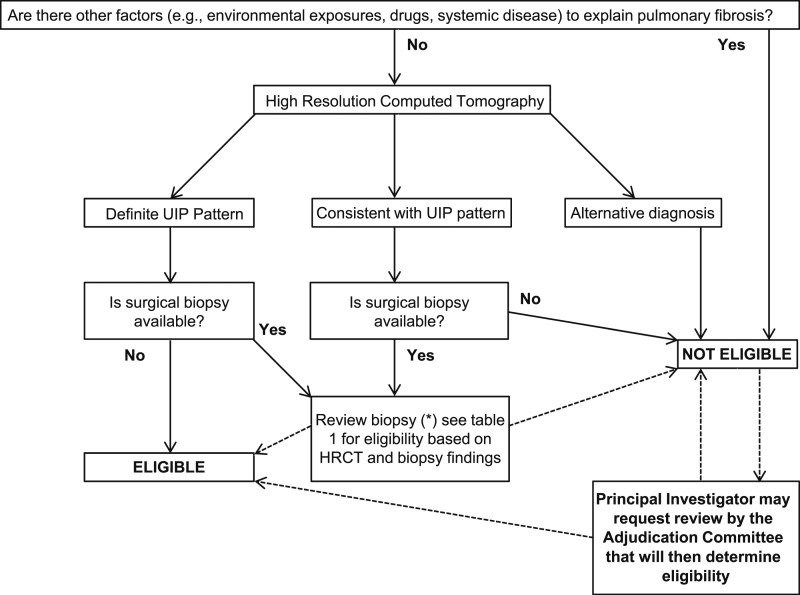
Only subjects with IPF were eligible for enrollment into the IPFnet studies. Subjects with a potential cause for interstitial lung disease, such as occupational and environmental exposures, use of drugs known to cause pulmonary fibrosis, and evidence of underlying connective tissue disease, were excluded. Each site conducted a multidisciplinary review of clinical and physiologic features, high-resolution CT (HRCT) scan of the chest, and available surgical lung biopsy (SLB) specimens to establish an IPF case. An algorithm for study eligibility was provided to guide enrollment into the studies (Fig 1). Local expert radiologists were identified by each site’s PI, and all had to complete lung imaging training set prior to activation of each clinical center. A layer of quality control was added by having a central review of the first 10 HRCT scans from each site. Local radiologists classified each study as “definite UIP,” “consistent with UIP,” or “inconsistent with UIP.”
Figure 1 –
General algorithm to define eligibility. HRCT = high-resolution CT; UIP = usual interstitial pneumonia.
In the appropriate clinical setting, the diagnosis of IPF was established by the demonstration of a “definite UIP” pattern on HRCT images. Patients with HRCT scans classified as “inconsistent with UIP” were ineligible for enrollment. In cases where the HRCT scan was not diagnostic, review of an SLB was required. Biopsy specimens were reviewed by the clinical center pathologist and by a member of the central pathology review committee. Clinical center pathologists were required to complete an IPF histology training module prior to activation of the center. Biopsy specimens were classified as definite UIP, probable UIP, or possible UIP. In the event of a disagreement between the two pathologists, a third member of the central pathologist review committee served as a tiebreaker after an independent review. The diagnosis and eligibility for any of the studies was then determined using a combination of HRCT scan and SLB findings (Tables 1-3).
TABLE 1 ] .
Determining Eligibility by HRCT Scan Pattern
| Definite UIP | Consistent With UIP | Inconsistent With UIP |
| Presence of subpleural basilar predominant reticular opacities, and honeycombing | Presence of subpleural basilar predominant reticular opacities, without honeycombing | Presence of upper lobe or peribronchovascular changes, extensive ground-glass opacities, micronodules, discrete cysts, significant mosaic attenuation/air trapping, or consolidation |
HRCT = high-resolution CT; UIP = usual interstitial pneumonia.
TABLE 3 ] .
Combining HRCT Scan and Pathology Patterns to Determine Eligibility
| HRCT Scan Pattern | Pathology Pattern | Eligible? |
| Definite UIP | Definite UIP | Yes |
| Definite UIP | Probable UIP | Yes |
| Definite UIP | Possible UIP | Yes |
| Definite UIP | Not UIP | No |
| Definite UIP | Biopsy specimen not available | Yes |
| Consistent with UIP | Definite UIP | Yes |
| Consistent with UIP | Probable UIP | Yes |
| Consistent with UIP | Possible UIP | No |
| Consistent with UIP | Not UIP | No |
| Consistent with UIP | Biopsy specimen not available | No |
| Inconsistent with UIP/other diagnosis | Any pathology | No |
See Table 1 legend for expansion of abbreviations.
TABLE 2 ] .
Determining Eligibility by SLB Pattern
| Definite UIP | Probable UIP | Possible UIP |
| (1) Clear evidence of chronic scarring and architectural destruction, often seen as honeycombing; (2) the presence of fibroblast foci typically at the interface between older scarred lung tissue and preserved lung tissue; (3) a patchy, subpleural, or paraseptal pattern of disease, with intervening architecturally normal tissue; and (4) absence of features suggesting an alternative diagnosis, such as hyaline membranes, extensive organizing pneumonia, granulomatous inflammation, prominent bronchiolocentricity of the inflammation/fibrosis, marked interstitial inflammation, and lymphoid hyperplasia | (1) Clear evidence of chronic scarring and architectural destruction, presence of honeycombing; and (2) absence of features suggesting an alternative diagnosis, such as hyaline membranes, extensive organizing pneumonia, granulomatous inflammation, prominent bronchiolocentricity of the inflammation/fibrosis, marked interstitial inflammation, and lymphoid hyperplasia | (1) Clear evidence of chronic scarring and architectural destruction, without honeycombing; and (2) absence of features suggesting an alternative diagnosis, such as hyaline membranes, extensive organizing pneumonia, granulomatous inflammation, prominent bronchiolocentricity of the inflammation/fibrosis, marked interstitial inflammation, and lymphoid hyperplasia |
SLB = surgical lung biopsy. See Table 1 legend for expansion of other abbreviation.
Adjudication Process for Diagnosis of IPF
If a patient was considered ineligible, the clinical site PI could request an AC review. Prior to the AC review, the subject’s clinical information was forwarded to the AC members along with digital HRCT scan images and, when available, SLB pathology images. For each case, one member was assigned to be the primary reviewer. A conference call was held for the determination of eligibility. The radiologist and pathologist and at least two clinicians were required. The group reviewed the historical data with particular attention to features that could suggest the possibility of an alternative diagnosis.
All AC members had access to the HRCT scan and pathology images during the conference calls. The radiologist reviewed and discussed the CT images; the pathologist reviewed the histologic features when a surgical biopsy was available. Discussion continued until a consensus was reached. If no consensus could be reached, a majority vote was used.
Outcome Measures
The AC reviewed the following events:
• Each death and respiratory serious adverse event was reviewed to determine if the cause was an AEx or progressive disease or if it was attributable to a cardiovascular event or other cause.
• In the ACE-IPF and PANTHER-IPF trials, nonelective hospitalizations were reviewed to determine if the hospitalization was attributable to a bleeding event (for ACE-IPF only) or an AEx or was respiratory related. The AC would classify a hospitalization as respiratory related if a review of the available clinical records indicated that the reason for hospitalization was primarily worsening respiratory symptoms.
• In ACE-IPF, the AC reviewed bleeding events requiring medical attention to determine if they met the protocol definition of major bleeding.6
• Suspected AExs.
AEx Definition for IPFnet Protocols
The following three criteria defined AEx in subjects with acute respiratory worsening7:
Clinical (All of the Following Required):
(1) Unexplained worsening of dyspnea or cough within 30 days, triggering unscheduled medical care; (2) no clinical suspicion or overt evidence of cardiac event, pulmonary embolism, or DVT; (3) no pneumothorax.
Radiologic/Physiologic (1 and 2 Required):
(1) New superimposed ground-glass opacities or consolidation on CT scan, or new alveolar opacities on chest radiograph; (2) decline of ≥ 5% in resting room air oxygen saturation by pulse oximetry from last recorded level or decline of ≥ 8 mm Hg in resting room air Pao2 from last recorded level.
Microbiologic (All of the Following Required):
(1) No clinical evidence for infection (ie, absence of grossly purulent sputum, fever > 39°C orally); (2) lack of positive microbiologic results (if done) from lower respiratory tract defined as clinically significant bacterial growth on sputum or endotracheal aspirate cultures, quantitative culture by protected brush specimen ≥ 103 colony-forming units/mL or BAL ≥ 104 colony-forming units/mL, and the presence of specific pathogens on stains of any of the previously mentioned; (3) lack of positive pathogen in blood cultures (if done).
When subjects were identified to meet the criterion of unexplained worsening of dyspnea or cough within 30 days, triggering unscheduled medical care, this triggered the collection of all the available clinical records. All potential cases of AEx were reviewed by the clinical center PI first. If an AEx was suspected, the case was referred to the AC. If there was disagreement among committee members after review and discussion, the majority opinion was recorded. Events were classified as “definite acute exacerbation,” “unclassifiable acute worsening,” or “not acute exacerbation” (Table 4).
TABLE 4 ] .
Evaluation of Suspected Acute Exacerbations
| Decision | Reason |
| Definite acute exacerbation | All criteria met, no alternative etiology |
| Unclassifiable acute worsening | Insufficient data to evaluate all criteria, no alternative etiology |
| Not acute exacerbation | Alternative etiology identified that explains acute worsening |
Bleeding Events for the ACE-IPF Trial
Bleeding events were categorized as either major or minor. They were evaluated by previously published criteria6 for both severity and consideration of discontinuation of study drug.
Results
The AC held an average of eight telephone conferences per clinical trial. During each conference, up to three to four cases were discussed for diagnostic adjudication alone. The AC would review up to 10 cases for diagnosis, outcome measures, or bleeding events in a given conference call. For each case, the data coordinating center allocated a coordinator who dedicated approximately 2 to 4 h to the task of data gathering from the clinical centers, posting the information in the study website, and organizing the conference call.
Diagnosis of IPF
The IPFnet screened a total of 1,015 subjects. The majority (n = 83) of the 327 screening failures were related to lack of IPF diagnosis. Based on the IPFnet diagnostic algorithm for IPF, 93 subjects with a surgical lung biopsy in the STEP-IPF trial were reviewed at the clinical center level, 51 at the central level, and three at the tie-breaker level, where a third pathologist decided the diagnosis if there was disagreement between the local and central pathologists. In the ACE-IPF trial, 78 subjects with a surgical lung biopsy were reviewed at the clinical center level, 75 at the central level, and five at the tie-breaker level. For the PANTHER-IPF study, 175 subjects with a surgical lung biopsy were reviewed at the clinical center level, 167 at the central level, and 14 at the tie-breaker level. The AC subsequently reviewed 23 cases for diagnostic confirmation, and only nine were ultimately deemed eligible (1.3% of the 668 enrolled subjects). Adjudication of study eligibility based on the diagnosis of IPF was requested in nine cases in the PANTHER-IPF study, three cases in the ACE-IPF trial, and 11 cases in the STEP-IPF trial. In the PANTHER-IPF study, six cases were judged to be IPF and three were not. In the ACE-IPF study, one case was judged to be IPF and two were not. In the STEP-IPF study, two cases were judged to be IPF and nine were not (Table 5). Unanimity was achieved in 22 out of the 23 cases adjudicated for diagnosis, and one case was adjudicated based on a majority vote. In 14 cases, pathology material was available for review by the AC. The most common reason for exclusion due to IPF diagnosis was suspected chronic hypersensitivity pneumonitis (Table 6). An illustrative case adjudicated by the committee is included in e-Appendix 1 (8MB, pdf) and e-Figures 1-7 (8MB, pdf) . It is important to emphasize that the AC did not review all the ineligible cases, only those for which the local PI made a formal request.
TABLE 5 ] .
Adjudication of Eligibility
| Case | Study | Discussion Points | Eligible |
| 1 | PANTHER-IPF | HRCT scan was compatible with UIP, and biopsy specimen was probable UIP. | Yes |
| 2 | PANTHER-IPF | Biopsy specimen had smoking-related changes in upper lobes, but predominant pathology was UIP. | Yes |
| 3 | PANTHER-IPF | No air trapping noted on HRCT scan; biopsy specimen had definite UIP. | Yes |
| 4 | PANTHER-IPF | HRCT scan showed significant air trapping and lacked honeycombing. Biopsy specimen lacked honeycombing, and the abnormalities were bronchiolocentric. | No |
| 5 | PANTHER-IPF | Biopsy specimen had mixed NSIP/UIP patterns. HRCT scan was typical of UIP. No evidence of alternative diagnosis. | Yes |
| 6 | PANTHER-IPF | HRCT scan and biopsy specimen most consistent with chronic HP. | No |
| 7 | PANTHER-IPF | HRCT scan had only minimal mosaic attenuation and lacked other evidence for chronic HP. | Yes |
| 8 | PANTHER-IPF | Data reviewed, all consistent with definite IPF diagnosis. | Yes |
| 9 | PANTHER-IPF | HRCT scan had widespread air trapping, and biopsy specimen showed airway-centered fibrosis. | No |
| 10 | ACE-IPF | Air trapping seen on HRCT scan and bronchiolocentric scarring in biopsy specimen | No |
| 11 | ACE-IPF | HRCT scan was not diagnostic. Biopsy specimen was suspicious for Ehlers-Danlos. | No |
| 12 | ACE-IPF | HRCT scan was adjudicated as “consistent with UIP.” Biopsy specimen was definite UIP. | Yes |
| 13 | STEP-IPF | HRCT scan showed honeycombing, traction bronchiectasis, and subpleural distribution of disease. The atypical features were presence of minimal ground-glass opacities and the upper lobe predominance of disease. The unanimous opinion of the committee was that IPF was highly likely and that there was no suggestion of another diagnosis to explain the findings. | Yes |
| 14 | STEP-IPF | Chronic HP suggested by clinical history and HRCT scan | No |
| 15 | STEP-IPF | HRCT scan, biopsy specimen, and clinical data were consistent with chronic HP. | No |
| 16 | STEP-IPF | HRCT scan was inconsistent with UIP. No biopsy specimen available. Most likely diagnosis was idiopathic NSIP. | No |
| 17 | STEP-IPF | HRCT scan had significant air trapping. Biopsy specimen was believed to be definite UIP. | No |
| 18 | STEP-IPF | HRCT scan was not diagnostic of UIP. No biopsy specimen was available. | No |
| 19 | STEP-IPF | Data reviewed and discussed. No definite diagnosis of IPF could be established. | No |
| 20 | STEP-IPF | Data reviewed and discussed. No definite diagnosis of IPF could be established. | No |
| 21 | STEP-IPF | History of bird exposure. HRCT scan had air trapping. | No |
| 22 | STEP-IPF | Short exposure to amiodarone believed not to be the cause for HRCT scan findings of definite UIP and the prolonged clinical course | Yes |
| 23 | STEP-IPF | Chronic HP suggested by HRCT scan pattern and clinical data | No |
ACE-IPF = Anticoagulant Effectiveness in Idiopathic Pulmonary Fibrosis; HP = hypersensitivity pneumonitis; IPF = idiopathic pulmonary fibrosis; NSIP = nonspecific interstitial pneumonia; PANTHER-IPF = Evaluating the Effectiveness of Prednisone, Azathioprine, and N-acetylcysteine in Patients With Idiopathic Pulmonary Fibrosis; STEP-IPF = Sildenafil Trial of Exercise Performance in Idiopathic Pulmonary Fibrosis. See Table 1 legend for expansion of other abbreviations.
TABLE 6 ] .
Adjudication Results
| Characteristic | STEP-IPF | ACE-IPF | PANTHER-IPF |
| Eligibility | 11 | 3 | 9 |
| Eligible | 2 | 1 | 6 |
| Not eligible | |||
| Chronic HP | 5 | 1 | 3 |
| Ehlers-Danlos | … | 1 | … |
| NSIP | 1 | … | … |
| SLB not available | 3 | … | … |
| Hospitalizations | Not reviewed | 36 | 57 |
| Respiratory | … | 28 | 28 |
| Other | … | 8 | 29 |
| AExs | 28 | 28 | 32 |
| Definite | 11 | 9 | 9 |
| Not AEx | 8 | 8 | 9 |
| Unclassifiable | 9 | 11 | 11 |
| Other | … | … | 3 |
| Bleeding events | … | 16 | … |
| Major | … | 3 | … |
| Minor | … | 13 | … |
At the completion of its final clinical trial, the IPFnet was composed of 25 centers, and 10 centers requested diagnostic review by the AC at some point. Only one of the newer participating centers requested diagnostic adjudication (n = 1). The STEP-IPF trial was conducted in 14 centers, eight of which requested diagnostic review by the AC. The ACE-IPF study was conducted in 20 centers, two of which requested diagnostic review by the AC. The PANTHER-IPF study was conducted in 25 centers, five of which requested diagnostic review by the AC. The three centers with the highest volume of patient enrollment were responsible for approximately one-half of the requests (11 of 23) for diagnostic review by the AC.
Clinical End Point Classification
The AC adjudicated 88 suspected AEx events. Twenty-nine events were judged to support a confident diagnosis of AEx, whereas 31 events were adjudicated as unclassifiable acute worsening. In cases of unclassifiable acute worsening, 75% were missing a CT scan, 10% were missing infection workup, and in 15% of cases, even with the available data, there was sufficient ambiguity that a definite diagnosis for the acute worsening could not be established. The majority of the hospitalizations reviewed from the ACE-IPF and PANTHER-IPF studies were deemed to be “respiratory” based on review of available clinical records (Table 6). The AC did not review hospitalizations for the STEP-IPF trial. The AC reviewed a total of 16 bleeding events in the ACE-IPF study, and only three were considered major.
Discussion
The diagnostic process established by the IPFnet in 2005 followed a multidisciplinary stepwise approach that was later incorporated into the 2011 American Thoracic Society/European Respiratory Society guidelines.1 It relied primarily on local expertise for the review of clinical data and classification of HRCT scans and, when applicable, central review of biopsy materials.
The process itself was highly efficient, as only a minority of cases required review by the adjudication committee. It is important to note that the IPFnet was composed of centers with recognized multidisciplinary expertise. It is possible that the same level of proficiency may not be seen if less experienced centers are involved in the recruitment of subjects for IPF clinical trials. The majority of cases brought up for diagnosis adjudication in the STEP-IPF trial were ultimately deemed not to have IPF. This was a somewhat surprising finding, as one might think that the diagnosis should be more straightforward in patients with more advanced disease. This was in part due to the clinical challenge of distinguishing advanced chronic hypersensitivity pneumonitis from IPF, as six cases had data that supported a diagnosis of chronic hypersensitivity pneumonitis. Alternatively, it could be related to a learning curve for the local teams, as we consider that the STEP-IPF trial was the first large multicenter clinical trial that used the interactive analysis of well-defined HRCT scan and histology patterns for the diagnosis of IPF.
The IPFnet clinical trials screened 1,015 patients, and 327 (32.2%) were deemed ineligible. This is comparable to the INPULSIS trials (nintedanib vs placebo),8 which reported 29.4% screening failure, but much lower than the Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis (ASCEND) study (pirfenidone vs placebo),9 which had a screening failure rate of 64.4%. INPULSIS and ASCEND used a centralized diagnostic process, whereas the IPFnet studies relied largely on local expertise. Twenty-five percent of screening failures in the IPFnet studies were deemed to be related to lack of IPF diagnosis, whereas in the ASCEND study that was the reason in 44.5%. Although these data do not allow one to conclude that a central diagnostic process leads to a higher rate of screening failures, the possibility needs to be considered as future IPF clinical trials are planned.
The IPFnet data coordinating center used a coordinator who dedicated part of her time to the AC, and each committee member donated approximately 30 h in effort for the duration of the network; the chairman’s effort was about 90 h. Therefore, clinical trials that choose to have central multidisciplinary adjudication of diagnosis and outcome measures of efficacy and safety could have additional complexity and cost.
In the population recruited for the IPFnet clinical trials, suspected chronic hypersensitivity pneumonitis was the most common confounder diagnosis, underscoring the need for a very detailed history of exposures and the careful review of appropriately obtained HRCT scans. Chronic hypersensitivity pneumonitis could be suspected on either HRCT scan or pathologic features. Findings often present included upper lobe fibrotic changes and mosaic attenuation with air trapping on expiratory HRCT scan views. Pathologic findings included bronchocentric disease distribution, noncaseating granulomas, or both.
AExs have significant morbidity and mortality and are considered an important outcome measure in IPF.10 As the committee applied the data, often collected from community hospitals, to the strict set of criteria we established for the diagnosis of AEx, a more definitive adjudication was often not possible, mostly for lack of imaging demonstrating new ground-glass opacities. Future IPF clinical trials that incorporate AEx as an outcome measure will have to emphasize the importance of prompt communication between the treating physician and the trial center in the event of a hospitalization so that the clinical investigation can be completed whenever feasible. On the other hand, it was considerably easier to define the primary reason for each hospitalization as well as the magnitude of bleeding events. Clinical trials with recruiting centers outside the United States will have to consider the differences in admitting thresholds and practices for IPF if “respiratory hospitalizations” are used as an outcome measure. In our experience, adjudication of acute exacerbations is essential to an accurate categorization of serious adverse events.
In summary, the IPFnet diagnostic process was generally efficient, and the adjudication committee contributed to ensure that a uniform phenotypic cohort was enrolled into the clinical trials. The adjudication committee played a key role in adjudicating all adverse outcomes in the two IPFnet studies that were terminated early because of safety issues, facilitating analysis of the trial outcomes and rapid publication of the study results. We hope that our experience will assist investigators in the design and conduct of future IPF clinical trials.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: All authors assume responsibility for the overall content and integrity of the article. J. d. A., M. S., H. R. C., T. G.-B., T. C., D. L., and R. J. K. contributed to study design and approved the final manuscript; T. G.-B. contributed to data management and organization; J. d. A., M. S., H. R. C., T. C., D. L., and R. J. K. contributed to adjudication decisions and analyzed data; J. d. A. and R. J. K. contributed to manuscript writing; and M. S., H. R. C., T. C., and D. L. contributed to editing the manuscript for important intellectual content.
Conflict of interest: J. d. A. has received grant support from the National Institutes of Health/National Heart, Lung, and Blood Institute; InterMune, Inc; Boehringer Ingelheim GmbH; Genentech, Inc; and Gilead, and has served as consultant for ImmuneWorks; InterMune, Inc; Genentech, Inc; and Boehringer Ingelheim GmbH. H. R. C. is a consultant to the following companies: AstraZeneca/MedImmune LLC; Bayer AG; Biogen Idec; FibroGen, Inc; Genentech, Inc; Genoa Pharmaceuticals; Gilead; GlaxoSmithKline; Mesoblast Ltd; Moerae Matrix; PatientsLikeMe; Pfizer Inc; Promedior, Inc; and ProMetic Life Sciences Inc. H. R. C. has a research contract with Boehringer Ingelheim GmbH. D. L.’s institution and laboratory receives research support from the National Heart, Lung, and Blood Institute; Siemens Corporation; Veracyte, Inc; and PAREXEL. D. L. is a consultant to PAREXEL; Boehringer Ingelheim GmbH; Genentech, Inc; Gilead; and InterMune, Inc. R. J. K. is a consultant to the following companies: AstraZeneca/Medimmune LLC, Boehringer Ingelheim GmbH, Genentech, Inc, Gilead, and Janssen. None declared (M. S., T. G.-B., T. C.).
Role of sponsors: The funders of this study had no role in the design and conduct of the study; not in the collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Collaborators: The following IPFnet members participated in the STEP-IPF study: Protocol Chairs—M. Schwarz, D. A. Zisman. IPFnet Steering Committee Chair—G. Hunninghake. Clinical Centers—J. Chapman, M. Olman, S. Lubell, L. D. Morrison, M. P. Steele, T. Haram, J. Roman, R. Perez, T. Perez, J. H. Ryu, J. P. Utz, A. H. Limper, C. E. Daniels, K. Meiras, S. Walsh, K. K. Brown, M. Schwarz, MD, C. Bair, D. Kervitsky, J. A. Lasky, S. Ditta, J. deAndrade, V. J. Thannickal, M. Stewart, D. A. Zisman, J. Lynch, E. Calahan, P. Lopez, T. E. King Jr, H. R. Collard, J. A. Golden, P. J. Wolters, R. Jeffrey, I. Noth, D. K. Hogarth, N. Sandbo, M. E. Strek, S. R. White, C. Brown, I. Garic, S. Maleckar, F. J. Martinez, K. R. Flaherty, M. Han, B. Moore, G. B. Toews, D. Dahlgren, G. Raghu, J. Hayes, M. Snyder, J. E. Loyd, L. Lancaster, W. Lawson, R. Greer, W. Mason, R. J. Kaner, V. Monroy, M. Wang. Core Lab Chairs—Radiology: D. A. Lynch; Pathology: T. Colby. Data Coordinating Center—K. J. Anstrom, R. C. Becker, E. L. Eisenstein, N. R. MacIntyre, L. D Morrison, J. Rochon, M. P. Steele, J. S. Sundy, L. Davidson-Ray, P. Dignacco, R. Edwards, R. Anderson, R. Beci, S. Calvert, K. Cain, T. Gentry-Bumpass, D. Hill, M. Ingham, E. Kagan, J. Kaur, C. Matti, J. McClelland, A. Meredith, T. Nguyen, J. Pesarchick, R. S. Roberts, W. Tate, T. Thomas, J. Walker, D. Whelan, J. Winsor, Q. Yang, E. Yow. NHLBI—H. Y. Reynolds, X. Tian, J. Kiley. The following IPFnet members participated in the ACE-IPF study: Protocol Chairs—I. Noth, M. Olman. IPFnet Steering Committee Chairs—M. Schwarz, G. B. Toews, G. Hunninghake. Clinical Centers—D. A. Culver, J. Chapman, M. Olman, S. Lubell, R. Wehrmann, L. D. Morrison, M. P. Steele, T. Haram, R. Kidd, M. Kallay, E. Lyda, J. H. Ryu, J. P. Utz, A. H. Limper, C. E. Daniels, K. Meiras, S. Walsh, S. Sahn, N. O’Banner, F. Stokes, K. K. Brown, C. Bair, D. Kervitsky, N. A. Ettinger, S. Merli, J. de Andrade, V. J. Thannickal, M. Stewart, J. Belperio, J. P. Lynch III, E. Calahan, P. Lopez, T. E. King Jr, H. R. Collard, J. Golden, P. Wolters, A. Eller, I. Noth, D. K. Hogarth, N. Sandbo, M. E. Strek, S. Maleckar, G. Rahimova, L. Sardin, J. Roman, R. Perez, T. Perez, M. Glassberg, E. Simonet, F. J. Martinez, K. Baumann, K. Chan, A. Chughtai, B. Gross, K. R. Flaherty, M. L. Han, R. Hyzy, E. Kazerooni, B. Moore, J. Myers, G. B. Toews, E. White, D. Dahlgren, M. Rossman, M. Kreider, K. Le, J. Fitzgerald, C. Glazer, M. B. Scholand, L. Brewster, A. Johnson, G. Raghu, P. Berry-Bell, A. Snydsman, J. E. Loyd, L. Lancaster, W. Lawson, R. Greer, K. Kinser, R. Richardson, W. Mason, R. J. Kaner, K. Bandong, D. Antin-Ozerkis, C. Holm, J. Estrom. Core Lab Chairs—D. A. Lynch; Pathology: T. Colby. Data Coordinating Center—K. J. Anstrom, E. L. Eisenstein, J. S. Sundy, L. Davidson-Ray, P. Dignacco, R. Edwards, R. Beci, S. Calvert, T. Gentry-Bumpass, D. Hill, P. V. Hofmann, K. Hwang, J. Kaur, C. Matti, A. Meredith, J. Pesarchick, S. Ramey, R. S. Roberts, A. Sharlow, J. Winsor, Q. Yang, E. Yow. NHLBI—G. G. Weinmann, H. Reynolds, B. Schmetter, X. Tian, J. Kiley. The following IPFnet members participated in the PANTHER-IPF study: Protocol Committee Chairs—F. J. Martinez, G. Raghu. IPFnet Steering Committee Chairs—M. Schwarz, G. B. Toews. Clinical Centers—J. Zibrak, A. Demersky, M. Vey, I. O. Rosas, P. Debrosse, D. A. Culver, J. Chapman, M. Olman, S. Lubell, R. Wehrmann, L. D. Morrison, M. P. Steele, T. Haram, R. Kidd, M. Kallay, E. Lyda, J. H. Ryu, J. P. Utz, A. H. Limper, C. E. Daniels, K. Meiras, S. Walsh, S. Sahn, N. O’Banner, F. Stokes, M. Padilla, G. Berhanu, K. K. Brown, C. Bair, D. Kervitsky, N. A. Ettinger, S. Merli, G. J. Criner, I. Q. Swift, A. Satti, F. Cordova, N. Patel, K. West, G. Jones, J. A. Lasky, S. Ditta, J. de Andrade, V. J. Thannickal, M. Stewart, J. Belperio, J. P. Lynch III, E. Calahan, P. Lopez, T. E. King Jr, H. R. Collard, J. Golden, P. Wolters, A. Eller, I. Noth, D. K. Hogarth, N. Sandbo, M. E. Strek, S. Maleckar, G. Rahimova, L. Sardin, J. Roman, R. Perez, T. Perez, M. Glassberg, E. Simonet, F. J. Martinez, K. Baumann, K. Chan, A. Chughtai, B. Gross, K. R. Flaherty, M. L. Han, R. Hyzy, E. Kazerooni, B. Moore, J. Myers, G. B. Toews, E. White, D. Dahlgren, M. Rossman, M. Kreider, K. Le, J. Fitzgerald, C. Glazer, M. B. Scholand, L. Brewster, A. Johnson, G. Raghu, P. Berry-Bell, A. Snydsman, J. E. Loyd, L. Lancaster, W. Lawson, R. Greer, W. Mason, R. J. Kaner, K. Bandong, D. Antin-Ozerkis, C. Holm, J. Estrom. Core Lab Chairs—D. A. Lynch, T. Colby. Data Coordinating Center—K. J. Anstrom, R. C. Becker, E. L. Eisenstein, J. S. Sundy, L. Davidson-Ray, P. Dignacco, R. Edwards, R. Beci, S. Calvert, K. Cain, T. Gentry-Bumpass, D. Hill, K. Huang, J. Kaur, C. Matti, A. Meredith, J. Pesarchick, S. Ramey, R. S. Roberts, A. Sharlow, J. Winsor, E. Yow. NHLBI—G. G. Weinmann, H. Reynolds, B. Schmetter, X. Tian, J. Kiley.
Additional information: The e-Appendix and e-Figures can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- AC
adjudication committee
- ACE-IPF
Anticoagulant Effectiveness in Idiopathic Pulmonary Fibrosis
- AEx
acute exacerbation
- ASCEND
Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis
- HRCT
high-resolution CT
- IPF
idiopathic pulmonary fibrosis
- IPFnet
IPF Clinical Research Network
- NAC
N-acetylcysteine
- PANTHER-IPF
Evaluating the Effectiveness of Prednisone, Azathioprine, and N-acetylcysteine in Patients With Idiopathic Pulmonary Fibrosis
- PI
principle investigator
- SLB
surgical lung biopsy
- STEP-IPF
Sildenafil Trial of Exercise Performance in Idiopathic Pulmonary Fibrosis
- UIP
usual interstitial pneumonia
Footnotes
FUNDING/SUPPORT: The IPFnet was funded by the National Heart, Lung, and Blood Institute [Grants U10HL80513 (data coordinating center), U10HL80413, U10HL80274, U10HL80370, U10HL80371, U10HL80383, U10HL80411, U10HL80509, U10HL80510, U10HL80543, U10HL80571, and U10HL80685 (clinical centers)] and The Cowlin Family Fund at the Chicago Community Trust.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Contributor Information
for the IPFnet Investigators:
M. Schwarz, D. A. Zisman, G. Hunninghake, J. Chapman, M. Olman, S. Lubell, L. D. Morrison, M. P. Steele, T. Haram, J. Roman, R. Perez, T. Perez, J. H. Ryu, J. P. Utz, A. H. Limper, C. E. Daniels, K. Meiras, S. Walsh, K. K. Brown, M. Schwarz, C. Bair, D. Kervitsky, J. A. Lasky, S. Ditta, J. deAndrade, V. J. Thannickal, M. Stewart, D. A. Zisman, J. Lynch, E. Calahan, P. Lopez, T. E. King, Jr, H. R. Collard, J. A. Golden, P. J. Wolters, R. Jeffrey, I. Noth, D. K. Hogarth, N. Sandbo, M. E. Strek, S. R. White, C. Brown, I. Garic, S. Maleckar, F. J. Martinez, K. R. Flaherty, M. Han, B. Moore, G. B. Toews, D. Dahlgren, G. Raghu, J. Hayes, M. Snyder, J. E. Loyd, L. Lancaster, W. Lawson, R. Greer, W. Mason, R. J. Kaner, V. Monroy, M. Wang, D. A. Lynch, T. Colby, K. J. Anstrom, R. C. Becker, E. L. Eisenstein, N. R. MacIntyre, L. D Morrison, J. Rochon, M. P. Steele, J. S. Sundy, L. Davidson-Ray, P. Dignacco, R. Edwards, R. Anderson, R. Beci, S. Calvert, K. Cain, T. Gentry-Bumpass, D. Hill, M. Ingham, E. Kagan, J. Kaur, C. Matti, J. McClelland, A. Meredith, T. Nguyen, J. Pesarchick, R. S. Roberts, W. Tate, T. Thomas, J. Walker, D. Whelan, J. Winsor, Q. Yang, E. Yow, H. Y. Reynolds, X. Tian, J. Kiley, I. Noth, M. Olman, M. Schwarz, G. B. Toews, G. Hunninghake, D. A. Culver, J. Chapman, M. Olman, S. Lubell, R. Wehrmann, L. D. Morrison, M. P. Steele, T. Haram, R. Kidd, M. Kallay, E. Lyda, J. H. Ryu, J. P. Utz, A. H. Limper, C. E. Daniels, K. Meiras, S. Walsh, S. Sahn, N. O’Banner, F. Stokes, K. K. Brown, C. Bair, D. Kervitsky, N. A. Ettinger, S. Merli, J. de Andrade, V. J. Thannickal, M. Stewart, J. Belperio, J. P. Lynch, III, E. Calahan, P. Lopez, T. E. King, Jr, H. R. Collard, J. Golden, P. Wolters, A. Eller, I. Noth, D. K. Hogarth, N. Sandbo, M. E. Strek, S. Maleckar, G. Rahimova, L. Sardin, J. Roman, R. Perez, T. Perez, M. Glassberg, E. Simonet, F. J. Martinez, K. Baumann, K. Chan, A. Chughtai, B. Gross, K. R. Flaherty, M. L. Han, R. Hyzy, E. Kazerooni, B. Moore, J. Myers, G. B. Toews, E. White, D. Dahlgren, M. Rossman, M. Kreider, K. Le, J. Fitzgerald, C. Glazer, M. B. Scholand, L. Brewster, A. Johnson, G. Raghu, P. Berry-Bell, A. Snydsman, J. E. Loyd, L. Lancaster, W. Lawson, R. Greer, K. Kinser, R. Richardson, W. Mason, R. J. Kaner, K. Bandong, D. Antin-Ozerkis, C. Holm, J. Estrom, D. A. Lynch, T. Colby, K. J. Anstrom, E. L. Eisenstein, J. S. Sundy, L. Davidson-Ray, P. Dignacco, R. Edwards, R. Beci, S. Calvert, T. Gentry-Bumpass, D. Hill, P. V. Hofmann, K. Hwang, J. Kaur, C. Matti, A. Meredith, J. Pesarchick, S. Ramey, R. S. Roberts, A. Sharlow, J. Winsor, Q. Yang, E. Yow, G. G. Weinmann, H. Reynolds, B. Schmetter, X. Tian, J. Kiley, F. J. Martinez, G. Raghu, M. Schwarz, G. B. Toews, J. Zibrak, A. Demersky, M. Vey, I. O. Rosas, P. Debrosse, D. A. Culver, J. Chapman, M. Olman, S. Lubell, R. Wehrmann, L. D. Morrison, M. P. Steele, T. Haram, R. Kidd, M. Kallay, E. Lyda, J. H. Ryu, J. P. Utz, A. H. Limper, C. E. Daniels, K. Meiras, S. Walsh, S. Sahn, N. O’Banner, F. Stokes, M. Padilla, G. Berhanu, K. K. Brown, C. Bair, D. Kervitsky, N. A. Ettinger, S. Merli, G. J. Criner, I. Q. Swift, A. Satti, F. Cordova, N. Patel, K. West, G. Jones, J. A. Lasky, S. Ditta, J. de Andrade, V. J. Thannickal, M. Stewart, J. Belperio, J. P. Lynch, III, E. Calahan, P. Lopez, T. E. King, Jr, H. R. Collard, J. Golden, P. Wolters, A. Eller, I. Noth, D. K. Hogarth, N. Sandbo, M. E. Strek, S. Maleckar, G. Rahimova, L. Sardin, J. Roman, R. Perez, T. Perez, M. Glassberg, E. Simonet, F. J. Martinez, K. Baumann, K. Chan, A. Chughtai, B. Gross, K. R. Flaherty, M. L. Han, R. Hyzy, E. Kazerooni, B. Moore, J. Myers, G. B. Toews, E. White, D. Dahlgren, M. Rossman, M. Kreider, K. Le, J. Fitzgerald, C. Glazer, M. B. Scholand, L. Brewster, A. Johnson, G. Raghu, P. Berry-Bell, A. Snydsman, J. E. Loyd, L. Lancaster, W. Lawson, R. Greer, W. Mason, R. J. Kaner, K. Bandong, D. Antin-Ozerkis, C. Holm, J. Estrom, D. A. Lynch, T. Colby, K. J. Anstrom, R. C. Becker, E. L. Eisenstein, J. S. Sundy, L. Davidson-Ray, P. Dignacco, R. Edwards, R. Beci, S. Calvert, K. Cain, T. Gentry-Bumpass, D. Hill, K. Huang, J. Kaur, C. Matti, A. Meredith, J. Pesarchick, S. Ramey, R. S. Roberts, A. Sharlow, J. Winsor, E. Yow, G. G. Weinmann, H. Reynolds, B. Schmetter, X. Tian, and J. Kiley
References
- 1.Raghu G, Collard HR, Egan JJ, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaherty KR, King TE, Jr, Raghu G, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170(8):904-910. [DOI] [PubMed] [Google Scholar]
- 3.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW; Idiopathic Pulmonary Fibrosis Clinical Research Network. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ; Idiopathic Pulmonary Fibrosis Clinical Research Network. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr, Raghu G; Idiopathic Pulmonary Fibrosis Clinical Research Network. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noth I, Anstrom KJ, Calvert SB, et al. ; Idiopathic Pulmonary Fibrosis Clinical Research Network (IPFnet). A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186(1):88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collard HR, Moore BB, Flaherty KR, et al. ; Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richeldi L, du Bois RM, Raghu G, et al. ; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-2082. [DOI] [PubMed] [Google Scholar]
- 9.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. ; ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083-2092. [DOI] [PubMed] [Google Scholar]
- 10.Collard HR, Yow E, Richeldi L, Anstrom KJ, Glazer C; IPFnet investigators. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res. 2013;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement