Abstract
Atrial fibrillation (AF) that newly occurs during critical illness presents challenges for both short- and long-term management. During critical illness, patients with new-onset AF are clinically evaluated for hemodynamic instability owing to the arrhythmia as well as for potentially reversible arrhythmia triggers. Hemodynamically significant AF that persists during critical illness may be treated with heart rate or rhythm control strategies. Recent evidence suggests that patients in whom AF develops during acute illness (eg, sepsis, postoperatively) have high long-term risks for AF recurrence and for AF-associated complications, such as stroke, heart failure, and death. Therefore, we suggest increased efforts to improve communication of AF events between inpatient and outpatient providers and to reassess patients who had experienced new-onset AF during critical illness after they transition to the post-ICU setting. We describe various strategies for the assessment and long-term management of patients with new-onset AF during critical illness.
Atrial fibrillation (AF) is the most common arrhythmia encountered in the ICU, affecting approximately 6% of critically ill patients.1 AF appears markedly more common among critically ill patients with certain conditions, such as severe sepsis.2 Nearly 33% of critically ill patients with sepsis have AF, and 10% have new-onset AF.3 The high incidence of AF during critical illnesses such as sepsis may be due to the convergence of age and diabetes as shared risk factors as well as the acute inflammation, catecholamine surges, myocardial injury, ischemia, and atrial stretch that occur during critical illness.
New-onset AF that occurs during critical illness appears to be a marker of immediate and future poor prognosis. Fewer than one-half of patients with new-onset AF survive an acute sepsis hospitalization.2 Acutely, supraphysiologic heart rates induced by AF result in loss of cardiac output, which may destabilize already critically ill patients. Subacutely, decreased cardiac output, atrial stasis, and coagulopathy of sepsis4 may converge to produce increased in-hospital stroke rates.2 In addition, AF may be an underrecognized manifestation of acute cardiac dysfunction during sepsis and a marker of critical illness severity.5
Despite the poor outcomes associated with new-onset AF during critical illness, little evidence guides its management. Highlighting the knowledge gaps surrounding treatment of AF during critical illness, systematic reviews have concluded that little direct evidence guides the management of AF during noncardiac critical illness.6,7 Thus, recommendations for the management of AF during critical illness generally are based on observational studies and expert opinion. We suggest that, first, principles in management of AF during critical illness include assessments for new hemodynamic collapse and drivers of arrhythmia. Second, clinicians should seek potentially reversible AF triggers, such as electrolyte disturbances (eg, hypokalemia, hypomagnesemia, acidemia),8‐10 β-agonist medications, acute myocardial injury or stretch, or airway obstruction.11
In addition to the management of the underlying condition, multiple strategies are available to treat AF in the short-term among critically ill patients. Guidelines strongly recommend (but with a low level of supporting evidence) urgent direct current (DC) cardioversion for patients in whom shock or severe cardiogenic pulmonary edema from reduced cardiac output develops due to severe tachycardia (eg, heart rates > 150 beats/min) or loss of atrial systole resulting from AF.12,13 Unfortunately, DC cardioversion of AF during critical illness frequently is unsuccessful, often requires multiple attempts, and is associated with high AF recurrence among patients in whom initial cardioversion is successful. In one surgical ICU, only 37% of patients with new-onset AF responded to multiple attempts at DC cardioversion.14 Failure to convert to sinus rhythm after attempted cardioversion of new-onset AF during septic shock may be an additional marker of poor prognosis.15
In patients with AF during critical illness who do not require emergent cardioversion, clinicians may attempt to improve hemodynamics with antiarrhythmic medications, such as amiodarone, or with agents to slow atrioventricular nodal conduction (β-blockers, nondihydropyridine calcium channel blockers, magnesium; digoxin, and to some extent, amiodarone). Each medication class has theoretical advantages and disadvantages for use among critically ill patients. β-Blockers are available in very short-acting formulations (ie, esmolol) to reduce heart rate and attenuate myocardial excitability; however, β-blockers also have negative inotropic and vasodilatory effects that may produce hypotension and reduce cardiac output. Similarly, nondihydropyridine calcium channel blockers may slow heart rate but also reduce inotropy, produce vasodilation, and cause hypotension. Cardiac glycosides (digoxin) may slow nodal conduction to reduce heart rates without causing hypotension but are longer acting, less effective in high catecholamine states that characterize critical illness,16 and have a low therapeutic index with high potential for toxicity among critically ill patients. Amiodarone may slow nodal conduction and have antiarrhythmic effects resulting in cardioversion but also presents risks for proarrhythmic effects, drug interactions, and organ toxicities.17,18 Amiodarone infusion may also be associated with hypotension and reduced cardiac output, especially among patients with concurrent cardiovascular disease, an effect that may be related to its cosolvents.19,20
Only small, single-center studies have compared the effectiveness of selected AF therapies in critically ill patients. In a randomized trial among 60 critically ill patients with recent-onset AF and rapid ventricular response, IV diltiazem was more successful than amiodarone in achieving heart rate control but with greater incidence of hypotension after administration (30% vs 2.5%).17 In another trial of 60 hospitalized patients with new-onset AF, IV amiodarone was associated with greater conversion to sinus rhythm at 24 h (92%) than IV digoxin (71%) but with a potentially greater number of adverse reactions among patients receiving amiodarone, including bradycardia and death.18
Unfortunately, controlled comparisons among other AF therapies in critically ill patients are lacking. Indirect evidence among noncritically ill patients suggests that β-blockers and calcium channel blockers may similarly reduce heart rate during new-onset AF21 and are superior to digoxin.22 IV magnesium therapy may also provide effective rate control during acute AF, and in one trial, it was superior to amiodarone for rhythm control.23‐25 Interestingly, esmolol infusion decreased mortality among patients with tachycardia and vasopressor-dependent septic shock in a single-center randomized trial.26 Whether esmolol would have similar effects during AF is unclear. However, given the limited and indirect evidence base for choice of initial AF therapy during critical illness, rate control therapy with esmolol for new-onset AF during critical illness may be a reasonable first choice, with consideration for use of magnesium, diltiazem, and amiodarone as second-line therapy and digoxin as third-line therapy in situations where esmolol is ineffective or associated with complications.
New-onset AF during sepsis is also associated with increased short-term risks of ischemic stroke, with threefold greater stroke rates compared with patients without AF during sepsis.2 Risks of stroke appear to be approximately 0.2% per day during sepsis,2 but the risk/benefit ratio of anticoagulation during acute critical illness is unclear. Critically ill patients may have thrombocytopenia, renal failure, liver failure, invasive devices, and unscheduled procedures that may substantially increase risks of severe bleeding. Data currently are lacking regarding rates of severe bleeding or estimates of stroke risk reduction with use of systemic anticoagulation during critical illness. Thus, anticoagulation treatment for new-onset AF among critically ill patients with elevated bleeding risk cannot be recommended currently as a treatment where benefits outweigh risks.
Importantly, poor outcomes associated with new-onset AF during acute illnesses continue long after improvement of the illness. For example, more than one-half of patients with new-onset AF in the setting of a potential AF precipitant (eg, pulmonary embolism, thyrotoxicosis, acute alcohol consumption, sepsis, surgery)27‐29 have a later recurrence of AF. Patients with new-onset AF during acute illness also have elevated long-term risks of stroke,27‐29 heart failure, and death.28,29 Among patients with new-onset AF during sepsis who had an ischemic stroke following a hospitalization for sepsis, nearly one-half were not given another AF diagnosis before the stroke, a finding that highlights the possibility that AF occurring during an acute illness may represent a previous missed opportunity30 to recognize AF31 and potentially reduce long-term cardiovascular events. Given the potential consequences for long-term events, improved documentation of new AF events during a hospitalization and communication between inpatient and outpatient providers are warranted.
Post-ICU Management of New-Onset AF
New-onset AF in the acute setting (eg, sepsis) that reverts to sinus rhythm may still indicate a propensity to develop the arrhythmia again potentially because of associated comorbidities or other predisposing factors. In the post-ICU setting, fully evaluating patients who experience new-onset AF during critical illness would be important because of a high risk of recurrence, associated comorbidities, and the need to proactively manage AF whether paroxysmal or persistent. AF often is asymptomatic, and only one in 12 paroxysms of AF are symptomatic.32 Indeed, asymptomatic AF may carry a poor prognosis,33 and symptoms are not a good guide to whether AF is present.34 Associated comorbidities such as hypertension and heart failure need to be managed as part of a holistic approach to AF management. Most cardiologists would perform echocardiography on patients with AF to glean information on cardiac structure and function. Thyroid function assessment could be useful, especially because subclinical thyroid disease may be prevalent among elderly patients.
The first priority of long-term AF management is stroke prevention. The presence of AF increases the risk of stroke fivefold overall, but this depends on the presence or absence of stroke risk factors35 irrespective of whether a rate or rhythm control strategy is planned. However, in patients recovering from critical illness, consideration of a thromboembolism prophylaxis or an anticoagulation approach should be informed by changes in patient care goals, renal function, bleeding risks, and medications (which may interact with anticoagulants) following the need for intensive care.
In older guidelines, there was much emphasis on targeting patients at high risk for stroke to offer such patients vitamin K antagonists (VKAs), such as warfarin. However, many such patients were undertreated with VKAs, and aspirin was used instead despite it being minimally effective and not safe when used as thromboprophylaxis.36,37 Today, in the era of non-VKA oral anticoagulants (NOACs) the focus of guidelines is to initially identify low-risk patients (CHA2DS2-VASc [congestive heart failure, hypertension, age ≥ 75 years, diabetes, previous stroke/transient ischemic attack, vascular disease, age 65 to 74 years, sex category] score of 0 for men and 1 for women) who do not need antithrombotic therapy (step 1).38 Subsequent to this step, effective stroke prevention can be offered to patients with one or more additional stroke risk factors (step 2), and effective stroke prevention means an oral anticoagulant with either a VKA (with good-quality anticoagulation control as reflected by a time in therapeutic range > 70%39) or an NOAC. In an anticoagulation-naive patient receiving a new diagnosis of AF, decision-making between a VKA or an NOAC can be helped by calculating the SAMe-TT2R2 (sex female, age < 60 years, medical history [more than two comorbidities], treatment [interacting drugs, eg, amiodarone for rhythm control], tobacco use [doubled], race [doubled]) score,40 which allows the prescriber to use simple clinical factors to determine which patients would likely do well taking a VKA (SAMe-TT2R2 score 0-2) or in whom good-quality anticoagulation control as reflected by a high time in therapeutic range is less likely (SAMe-TT2R2 score > 2) and an NOAC would be a better option. The SAMe-TT2R2 score has been validated to predict labile international normalized ratios, thromboembolism, mortality, and bleeding.41‐43
Subsequent management of AF requires a decision on rate or rhythm control, and this is largely patient centered and symptom directed. Rate control is noninferior to rhythm control as a management strategy for mortality, thromboembolism, and so forth,44 but rhythm control (by antiarrhythmic drugs, electrical cardioversion, or ablation) improves symptoms and functional status compared with rate control, at least in the short to medium term.45 In patients for whom rate control is chosen, this is again guided by symptoms because a strategy of lenient rate control is noninferior to strict rate control.
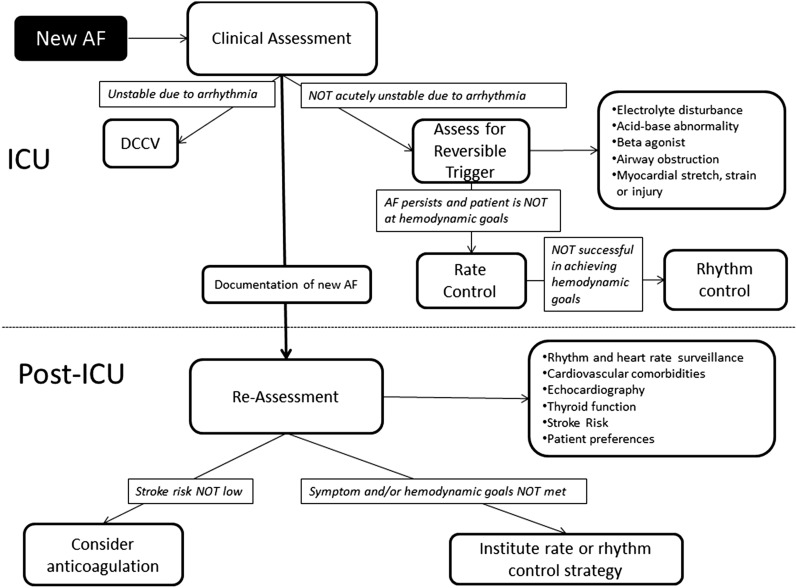
In conclusion, the development of new-onset AF in the ICU requires thorough evaluation and proactive management in the post-ICU setting. The management cascade requires the following aspects to be investigated and managed: (1) stroke prevention; (2) rate vs rhythm control, depending on symptoms; and (3) detection and treatment of comorbidities (eg, hypertension, heart failure, ischemic heart disease). A systematic and holistic approach is needed to improve our management of this common arrhythmia and to reduce its major health-care burden (Fig 1).
Figure 1 –
Approach to the short-term and long-term management of new-onset AF during critical illness. The initial approach involves clinical assessment for hemodynamic and respiratory stability related to AF, with evaluation for reversible triggers and initiation of heart rate or rhythm control treatments to meet hemodynamic goals. After clinical improvement, patients should be systematically reevaluated for stroke risk (eg, CHA2DS2-VASc [congestive heart failure, hypertension, age ≥ 75 y, diabetes, previous stroke/transient ischemic attack, vascular disease, age 65-74 y, sex category] score) and evidence of AF recurrence to guide initiation of thromboembolism prophylaxis, rate control, or rhythm control. AF = atrial fibrillation; DCCV = direct current cardioversion.
Acknowledgments
Conflict of interest: G. Y. H. L. has served as a consultant for Bayer AG; Merck Sharp & Dohme Corp; Sanofi SA; Bristol-Myers Squibb Company/Pfizer Inc; Daiichi Sankyo Company Limited; Biotronik SE & Co KG; Medtronic plc; Portola Pharmaceuticals, Inc; and Boehringer Ingelheim GmbH and has been on the speakers bureau for Bayer AG, Bristol-Myers Squibb Company/Pfizer Inc, Boehringer Ingelheim GmbH, Daiichi Sankyo Company Limited, and Medtronic plc. None declared (A. J. W., D. K. H.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- AF
atrial fibrillation
- DC
direct current
- NOAC
non-vitamin K antagonist oral anticoagulant
- SAMe-TT2R2
sex female, age < 60 years, medical history (more than two comorbidities), treatment (interacting drugs, eg, amiodarone for rhythm control), tobacco use (doubled), race (doubled)
- VKA
vitamin K antagonist
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [K01HL116768 to Dr Walkey].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Annane D, Sébille V, Duboc D, et al. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am J Respir Crit Care Med. 2008;178(1):20-25. [DOI] [PubMed] [Google Scholar]
- 2.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128(4):2864-2875. [DOI] [PubMed] [Google Scholar]
- 5.Walkey AJ, Ambrus D, Benjamin EJ. The role of arrhythmias in defining cardiac dysfunction during sepsis. Am J Respir Crit Care Med. 2013;188(6):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrigo M, Bettex D, Rudiger A. Management of atrial fibrillation in critically ill patients. Crit Care Res Pract. 2014;2014:840615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanji S, Stewart R, Fergusson DA, McIntyre L, Turgeon AF, Hébert PC. Treatment of new-onset atrial fibrillation in noncardiac intensive care unit patients: a systematic review of randomized controlled trials. Crit Care Med. 2008;36(5):1620-1624. [DOI] [PubMed] [Google Scholar]
- 8.Krijthe BP, Heeringa J, Kors JA, et al. Serum potassium levels and the risk of atrial fibrillation: the Rotterdam Study. Int J Cardiol. 2013;168(6):5411-5415. [DOI] [PubMed] [Google Scholar]
- 9.Khan AM, Lubitz SA, Sullivan LM, et al. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2013;127(1):33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ninio DM, Saint DA. The role of stretch-activated channels in atrial fibrillation and the impact of intracellular acidosis. Prog Biophys Mol Biol. 2008;97(2-3):401-416. [DOI] [PubMed] [Google Scholar]
- 11.Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8(9):1436-1443. [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Alpert JS, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. [DOI] [PubMed] [Google Scholar]
- 13.Camm AJ, Lip GY, De Caterina R, et al. ; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719-2747. [DOI] [PubMed] [Google Scholar]
- 14.Mayr A, Ritsch N, Knotzer H, et al. Effectiveness of direct-current cardioversion for treatment of supraventricular tachyarrhythmias, in particular atrial fibrillation, in surgical intensive care patients. Crit Care Med. 2003;31(2):401-405. [DOI] [PubMed] [Google Scholar]
- 15.Meierhenrich R, Steinhilber E, Eggermann C, et al. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care. 2010;14(3):R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang EL, Chan WL, Cleland JG, et al. Placebo controlled trial of xamoterol versus digoxin in chronic atrial fibrillation. Br Heart J. 1990;64(4):256-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delle Karth G, Geppert A, Neunteufl T, et al. Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Crit Care Med. 2001;29(6):1149-1153. [DOI] [PubMed] [Google Scholar]
- 18.Hou ZY, Chang MS, Chen CY, et al. Acute treatment of recent-onset atrial fibrillation and flutter with a tailored dosing regimen of intravenous amiodarone. A randomized, digoxin-controlled study. Eur Heart J. 1995;16(4):521-528. [DOI] [PubMed] [Google Scholar]
- 19.Souney PF, Cooper WD, Cushing DJ. PM101: intravenous amiodarone formulation changes can improve medication safety. Expert Opin Drug Saf. 2010;9(2):319-333. [DOI] [PubMed] [Google Scholar]
- 20.Cushing DJ, Cooper WD, Gralinski MR, Lipicky RJ. The hypotensive effect of intravenous amiodarone is sustained throughout the maintenance infusion period. Clin Exp Pharmacol Physiol. 2010;37(3):358-361. [DOI] [PubMed] [Google Scholar]
- 21.Platia EV, Michelson EL, Porterfield JK, Das G. Esmolol versus verapamil in the acute treatment of atrial fibrillation or atrial flutter. Am J Cardiol. 1989;63(13):925-929. [DOI] [PubMed] [Google Scholar]
- 22.Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. Ann Emerg Med. 1997;29(1):135-140. [DOI] [PubMed] [Google Scholar]
- 23.Onalan O, Crystal E, Daoulah A, Lau C, Crystal A, Lashevsky I. Meta-analysis of magnesium therapy for the acute management of rapid atrial fibrillation. Am J Cardiol. 2007;99(12):1726-1732. [DOI] [PubMed] [Google Scholar]
- 24.Chiladakis JA, Stathopoulos C, Davlouros P, Manolis AS. Intravenous magnesium sulfate versus diltiazem in paroxysmal atrial fibrillation. Int J Cardiol. 2001;79(2-3):287-291. [DOI] [PubMed] [Google Scholar]
- 25.Moran JL, Gallagher J, Peake SL, Cunningham DN, Salagaras M, Leppard P. Parenteral magnesium sulfate versus amiodarone in the therapy of atrial tachyarrhythmias: a prospective, randomized study. Crit Care Med. 1995;23(11):1816-1824. [DOI] [PubMed] [Google Scholar]
- 26.Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683-1691. [DOI] [PubMed] [Google Scholar]
- 27.Lubitz SA, Yin X, Rienstra M, et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131(19):1648-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146(5):1187-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gialdini G, Nearing K, Bhave PD, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312(6):616-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walkey AJ. Preventing cardiovascular complications of acute infection: a missed opportunity? Circulation. 2014;129(13):1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanna T, Diener HC, Passman RS, et al. ; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478-2486. [DOI] [PubMed] [Google Scholar]
- 32.Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89(1):224-227. [DOI] [PubMed] [Google Scholar]
- 33.Martinez C, Katholing A, Freedman SB. Adverse prognosis of incidentally detected ambulatory atrial fibrillation. A cohort study. Thromb Haemost. 2014;112(2):276-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potpara TS, Polovina MM, Marinkovic JM, Lip GY. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation: the Belgrade Atrial Fibrillation Study. Int J Cardiol. 2013;168(5):4744-4749. [DOI] [PubMed] [Google Scholar]
- 35.Pisters R, Lane DA, Marin F, Camm AJ, Lip GY. Stroke and thromboembolism in atrial fibrillation. Circ J. 2012;76(10):2289-2304. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwlaat R, Capucci A, Lip GY, et al. ; Euro Heart Survey Investigators. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27(24):3018-3026. [DOI] [PubMed] [Google Scholar]
- 37.Lip GY. The role of aspirin for stroke prevention in atrial fibrillation. Nat Rev Cardiol. 2011;8(10):602-606. [DOI] [PubMed] [Google Scholar]
- 38.Lip GY, Lane DA. Modern management of atrial fibrillation requires initial identification of “low-risk” patients using the CHA2DS2-VASc score, and not focusing on “high-risk” prediction. Circ J. 2014;78(8):1843-1845. [DOI] [PubMed] [Google Scholar]
- 39.De Caterina R, Husted S, Wallentin L, et al. Vitamin K antagonists in heart disease: current status and perspectives (section III). Position paper of the ESC Working Group on Thrombosis—Task Force on Anticoagulants in Heart Disease. Thromb Haemost. 2013;110(6):1087-1107. [DOI] [PubMed] [Google Scholar]
- 40.Apostolakis S, Sullivan RM, Olshansky B, Lip GYH. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT₂R₂ score. Chest. 2013;144(5):1555-1563. [DOI] [PubMed] [Google Scholar]
- 41.Gallego P, Roldán V, Marin F, et al. SAMe-TT2R2 score, time in therapeutic range, and outcomes in anticoagulated patients with atrial fibrillation. Am J Med. 2014;127(11):1083-1088. [DOI] [PubMed] [Google Scholar]
- 42.Poli D, Antonucci E, Testa S, Lip GY. A prospective validation of the SAME-TT2R 2 score: how to identify atrial fibrillation patients who will have good anticoagulation control on warfarin. Intern Emerg Med. 2014;9(4):443-447. [DOI] [PubMed] [Google Scholar]
- 43.Lip GYH, Haguenoer K, Saint-Etienne C, Fauchier L. Relationship of the SAMe-TT2R2 score to poor-quality anticoagulation, stroke, clinically relevant bleeding, and mortality in patients with atrial fibrillation. Chest. 2014;146(3):719-726. [DOI] [PubMed] [Google Scholar]
- 44.Al-Khatib SM, Allen LaPointe NM, Chatterjee R, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160(11):760-773. [DOI] [PubMed] [Google Scholar]
- 45.Chung MK, Shemanski L, Sherman DG, et al. ; AFFIRM Investigators. Functional status in rate- versus rhythm-control strategies for atrial fibrillation: results of the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Functional Status Substudy. J Am Coll Cardiol. 2005;46(10):1891-1899. [DOI] [PubMed] [Google Scholar]