Abstract
BACKGROUND:
Dyspnea is the major source of disability in COPD. In COPD, environmental cues (eg, the prospect of having to climb stairs) become associated with dyspnea and may trigger dyspnea even before physical activity commences. We hypothesized that brain activation relating to such cues would be different between patients with COPD and healthy control subjects, reflecting greater engagement of emotional mechanisms in patients.
METHODS:
Using functional MRI (FMRI), we investigated brain responses to dyspnea-related word cues in 41 patients with COPD and 40 healthy age-matched control subjects. We combined these findings with scores on self-report questionnaires, thus linking the FMRI task with clinically relevant measures. This approach was adapted from studies in pain that enabled identification of brain networks responsible for pain processing despite absence of a physical challenge.
RESULTS:
Patients with COPD demonstrated activation in the medial prefrontal cortex and anterior cingulate cortex, which correlated with the visual analog scale (VAS) response to word cues. This activity independently correlated with patient responses on questionnaires of depression, fatigue, and dyspnea vigilance. Activation in the anterior insula, lateral prefrontal cortex, and precuneus correlated with the VAS dyspnea scale but not with the questionnaires.
CONCLUSIONS:
The findings suggest that engagement of the emotional circuitry of the brain is important for interpretation of dyspnea-related cues in COPD and is influenced by depression, fatigue, and vigilance. A heightened response to salient cues is associated with increased symptom perception in chronic pain and asthma, and the findings suggest that such mechanisms may be relevant in COPD.
Dyspnea causes immense suffering for patients with COPD. Objective measures of lung function, such as spirometry, correlate poorly with dyspnea.1 Despite dyspnea being subjective, it remains the best predictor of mortality.2 There is a clear need to better understand the mechanisms of dyspnea to find new treatments.
Contemporary models emphasize that the experience of dyspnea is strongly influenced by psychologic processes, particularly depression3‐5 and dyspnea-related fear and anxiety.5‐9 Although the physical sensation of dyspnea commonly originates from sensory afferent sources, including the heart, lungs, and muscles, conscious awareness of dyspnea arises in the brain.5‐8 Repeated association between environmental cues and dyspnea may increase the salience of such cues. For example, a ringing telephone could be associated with the need to move quickly to answer it and, thus, may trigger brain anticipatory dyspnea circuitry even before physical activity commences. One way of measuring the activity in these brain circuits is with functional MRI (FMRI).10
In this study, FMRI was used to investigate brain processes associated with responses to dyspnea-related cues in COPD. We adapted methodologies in which salient images or word cues engage pain processing networks in the brain despite the absence of a physical challenge.11,12 Brain state before a stimulus is known to influence subsequent perception.13 We hypothesized that differences in brain activation to dyspnea-related environmental cues between patients with COPD and healthy control subjects may reflect differences in salience of these cues and changes in the cognitive-affective state.
Materials and Methods
Participants
We recruited 41 patients (15 women; mean ± SD age, 68.0 ± 8.2 years) with mild to moderate COPD (according to GOLD [Global Initiative for Chronic Obstructive Lung Disease] criteria) in the week before commencing a course of pulmonary rehabilitation and 40 age- and sex-matched healthy control subjects (16 women; mean age, 69.1 ± 8.1 years). Demographics are shown in Table 1; medical details and recruitment procedures are provided in e-Appendix 1 (859.5KB, pdf) , e-Figure 1 (859.5KB, pdf) , and e-Table 1 (859.5KB, pdf) . All participants gave written informed consent. The study was approved by Oxfordshire Research Ethics Committee A (09/H0604/108).
TABLE 1 ] .
Participant Details and Physiologic Data
| Demographic | Patients | Control Subjects |
| Age, y | 68.0 ± 8.2 | 69.1 ± 8.1 |
| Male (female) sex | 15 (26) | 16 (24) |
| IMD score | 12.1 ± 6.8 | 11.7 ± 9.2 |
| BMI, kg/m2 | 28.4 ± 6.7 | 25.2 ± 3.2a |
| MRC breathlessness score (1-5) | 3 (IQR 2-4) | 1 (IQR 1-1)b,c |
| GOLD stage (0-IV) | 2 (IQR 1-3) | … |
| Resting Borg score (1-10) | 0.8 ± 1.1 | 0.06 ± 0.2b |
| Resting Sao2, % | 94.4 ± 2.6 | 96.4 ± 1.3b |
| Resting heart rate, beats/min | 82.8 ± 13.7 | 72.2 ± 11.0b |
| MSWT distance, m | 320 ± 185 | 804 ± 274b |
| Smoking history, pack-y | 40.3 ± 33.3 | 3.1 ± 6.6b |
| FEV1, % predicted | 58 ± 21 | 99 ± 24b |
Data are presented as mean ± SD, No., or median (IQR). GOLD = Global Initiative for Chronic Obstructive Lung Disease; IMD = English Indices of Deprivation, 2010 data; IQR = interquartile range; MRC = Medical Research Council; MSWT = modified shuttle walk test; Sao2 = oxygen saturation as measured by pulse oximetry.
P < .01.
P < .001.
Mann-Whitney-Wilcoxon test.
Functional MRI
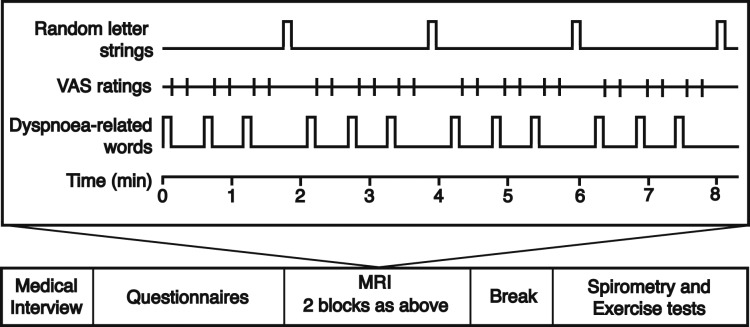
Imaging was performed with a 3T MAGNETOM Trio, A Tim System (Siemens Healthcare GmbH) using a 12-channel head coil. Participants underwent two FMRI scans (each 8 min, 20 s, with a 30-s break between) and one structural scan (Fig 1).
Figure 1 –
Functional MRI task and protocol. Participants were presented with and asked to rate dyspnea-related word cues. Dyspnea-related word cues and ratings were each displayed for 7 s. Every block was separated by a fixation cross (12 s), and every third block was followed by random letter strings (7 s). VAS = visual analog scale.
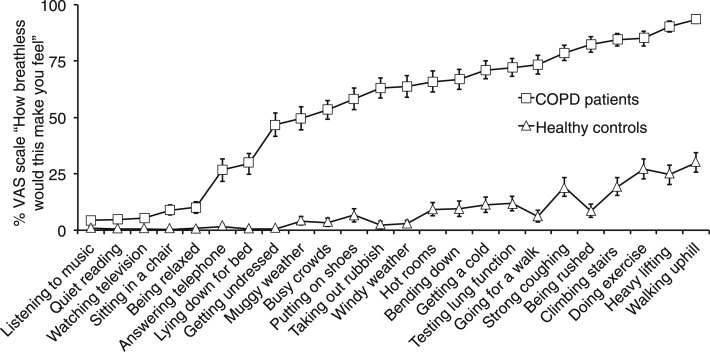
During scanning, participants were shown a randomized set of dyspnea-related word cues. These cues were designed to induce recall of everyday situations that may be associated with dyspnea in patients with COPD, ranging from low to high valence (Fig 2). Participants viewed each word and rated it using a button box according to how breathless and how anxious it would make them feel on a visual analog scale (VAS) of 0 to 10 (anchors not at all and very much) presented on-screen. To familiarize themselves with the protocol before scanning, participants completed a set of eight test words.
Figure 2 –
Mean VAS responses to the question, “How breathless would this make you feel,” in 41 patients with COPD and 40 healthy age- and sex-matched control subjects. Anchors were not at all and very much. Error bars represent SEM. See Figure 1 legend for expansion of abbreviation.
Heart rate, oxygen saturation as measured by pulse oximetry (Sao2) as measured by a MultiGas Monitor 9500 (MR Equipment), respiration (chest respiratory bellows), and end-tidal Po2 and Pco2 (Normocap 200 Capnometer [Datex Division Instrumentation Corp]; nasal cannula [Salter Labs]) were continuously measured throughout the scan. All physiologic data were sampled at 50 Hz and recorded along with scan volume triggers through PowerLab 8 using Chart 5 (ADInstruments).
Psychologic Measurements
Before scanning, a comprehensive assessment of respiratory perception and impact was obtained using the following self-report questionnaires: St. George’s Respiratory Questionnaire,14 Medical Research Council dyspnea scale,15 Dyspnea-12 questionnaire,16 and Catastrophizing about Asthma Scale17 and Pain Vigilance and Awareness Questionnaire18 (modified by substituting the word “breathlessness” for the words “asthma” and “pain,” respectively). Depression, anxiety, fatigue, and demotivation are important coexisting psychologic processes in COPD and were measured with the Center for Epidemiologic Studies Depression Scale,19 State-Trait Anxiety Inventory,20 Fatigue Severity Scale,21 and Behavioral Inhibition System/Behavioral Activation System scale.22
Spirometry and Exercise Testing
Participants underwent spirometry performed by a trained respiratory nurse using Association for Respiratory Technology and Physiology standards23 and a modified shuttle walk test (MSWT) performed twice. Before, during, and after the MSWT, heart rate and Sao2 were measured every minute using a fingertip pulse oximeter (Go2; Nonin Medical Inc). Participants rated their dyspnea on a modified Borg scale immediately before and after the MSWT (e-Table 2 (859.5KB, pdf) ).
Data Analysis
All FMRI data processing was carried out with FEAT (FMRI Expert Analysis Tool) version 5.98 (FMRIB Software Library [www.fmrib.ox.ac.uk/fsl]) using a whole-brain approach with standard parameters. First-level analyses used a general linear model with multiple explanatory variables, which were presentation of word cues, trial-by-trial dyspnea and anxiety ratings of word cues, random letter strings, and periods when subjects were rating using the VAS. Physiologic noise correction was performed using RETROICOR (retrospective image correction).24,25
A multiple regression analysis using SPSS Statistics software (IBM Corporation) was performed for all questionnaire scores to identify the major psychologic factors contributing to the Dyspnea-12 scores (dependent variable). The following scores were identified and included as additional regressors in the higher-level analysis: state anxiety, fatigue, depression, and awareness and vigilance.
A higher (group)-level analysis was performed. Because fatigue, depression, and vigilance are known to be major factors in dyspnea, these were considered regressors of interest. Because state anxiety may have been confounded by experimental factors, this was considered a regressor of no interest. Because we had no prior expectation of any link between these factors and breathlessness in control subjects, these regressors were not interrogated further in the control group. To correct for multiple comparisons, z-statistic image thresholds were set using clusters determined by z > 2.3 and a (corrected) cluster significance threshold of P = .05 across the whole brain.26
We performed conjunction analyses (conjunction null) to determine common areas of mean brain activation in each group and among the questionnaire regressors in patients only. Unpaired t tests determined between-group differences. F tests were performed to test for shared variance between the different variables.
Questionnaires were scored according to their respective manuals. For spirometry, the measurement associated with the highest FEV1 value was used. For MSWT, the measurement associated with the farthest distance walked was used. Data were compared using Student t test. Correlations among FEV1, MSWT, and Dyspnea-12 scores were assessed with MATLAB software (MathWorks, Inc), and P < .017 (Bonferroni corrected) were considered significant. A detailed description of study methods can be found in e-Appendix 1 (859.5KB, pdf) .
Results
Participants
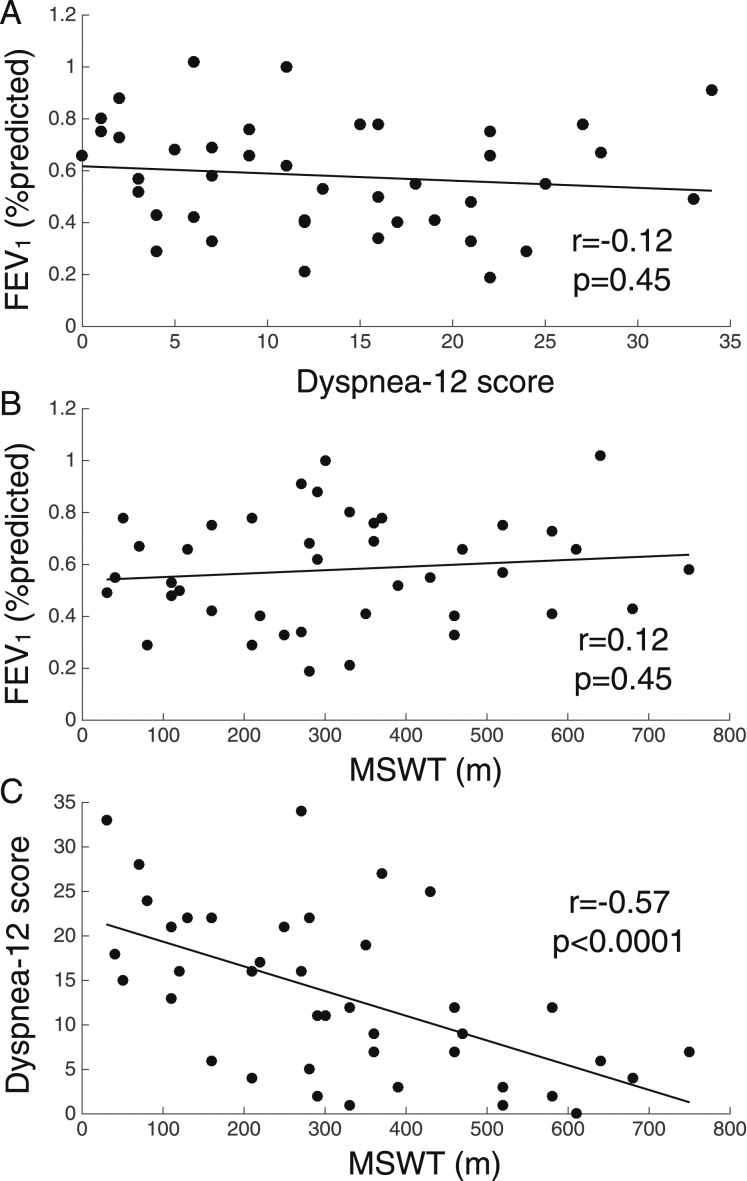
Table 1 presents participant demographics and spirometry and MSWT values. FEV1 did not correlate with Dyspnea-12 score or MSWT distance, but a negative correlation was observed between Dyspnea-12 score and MSWT distance (Fig 3). In patients, dyspnea and anxiety VAS scores correlated with Dyspnea-12 scores (dyspnea, r = 0.51, P = .0007; anxiety, r = 0.56, P < .0001).
Figure 3 –
A-C, FEV1, exercise performance, and dyspnea in patients with COPD. Percent predicted FEV1 plotted against Dyspnea-12 score (A) and distance walked on MSWT for patient group (B), and Dyspnea-12 score plotted against distance walked on MSWT for the patient group (C). MSWT = modified shuttle walk test.
Questionnaires and VAS Ratings
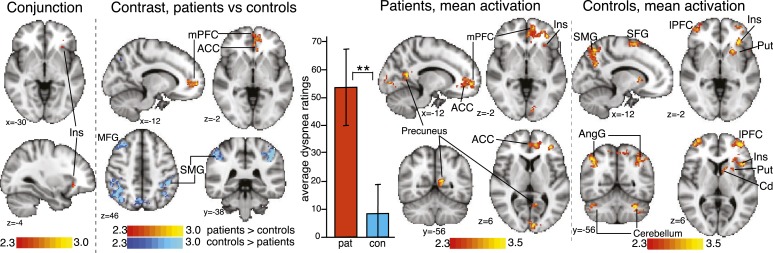
Patients scored significantly higher than control subjects on the Dyspnea-12, St. George’s Respiratory Questionnaire, Catastrophic Thinking Scale, Awareness and Vigilance Scale, Fatigue Severity Scale, Center for Epidemiologic Studies Depression Scale, and State-Trait Anxiety Inventory Scale but not on the Behavioral Inhibition System/Behavioral Activation System scale (Table 2). During FMRI, patients rated word cues higher for both dyspnea (mean ± SD: patients, 53.6 ± 13.5; control subjects, 8.4 ± 10.4; P < .001) and dyspnea-anxiety (patients, 43.1 ± 18.6; control subjects, 5.8 ± 10.8; P < .001) (Fig 4).
TABLE 2 ] .
Questionnaire Scores
| Questionnaire | Patients (n = 41) | Control Subjects (n = 40) |
| Dyspnea-12 | 13.2 ± 9.2 | 0.0 ± 0.0a |
| Physical | 8.1 ± 5.2 | 0.0 ± 0.0a |
| Affective | 5.1 ± 4.3 | 0.0 ± 0.0a |
| St. George’s Respiratory Questionnaire | 52.0 ± 17.0 | 6.9 ± 5.1a |
| Symptom | 61.8 ± 18.0 | 2.7 ± 2.1a |
| Activity | 69.7 ± 22.4 | 3.1 ± 3.1a |
| Impact | 39.0 ± 18.0 | 0.6 ± 1.4a |
| Catastrophic Thinking Scaleb | 14.5 ± 12.0 | 0.0 ± 0.2a |
| Helplessness | 5.6 ± 5.7 | 0.0 ± 0.2a |
| Magnification | 4.1 ± 3.1 | 0.0 ± 0.0a |
| Rumination | 4.9 ± 4.4 | 0.0 ± 0.0a |
| Awareness and Vigilance Scalec | 41.7 ± 14.6 | 12.9 ± 11.4a |
| Fatigue Severity Scale | 42.9 ± 11.0 | 22.3 ± 12.0a |
| BIS/BAS | 53.6 ± 8.6 | 54.7 ± 8.5 |
| BAS: drive | 10.0 ± 2.8 | 10.5 ± 2.7 |
| BAS: fun-seeking | 9.2 ± 2.5 | 9.4 ± 2.4 |
| BAS: reward responsiveness | 9.5 ± 2.9 | 9.5 ± 3.1 |
| BIS: inhibition | 16.4 ± 3.8 | 16.5 ± 3.7 |
| Center for Epidemiologic Studies Depression Scale | 14.8 ± 9.3 | 7.2 ± 6.6a |
| State anxiety | 35.1 ± 9.9 | 25.6 ± 7.5a |
| Trait anxiety | 37.6 ± 11.0 | 29.1 ± 6.8a |
Data are presented as mean ± SD. Component scores are included where appropriate. BAS = Behavioral Approach System; BIS = Behavioral Inhibition System.
P < .001.
Modified from use in asthma.
Modified from use in pain.
Figure 4 –
Activation (contrasts and conjunction) correlating with VAS ratings to dyspnea word cues. Maps are cluster level corrected for multiple comparisons at P < .05 across the whole brain. Maps represent conjunction analysis (activations common to both groups), comparisons between groups (patients > control subjects in red-yellow, control subjects > patients in blue-light blue), and mean activations in patients and control subjects. Bar graph is mean ± SD dyspnea ratings for each group. **P < .001. Cerebellum is crus I and VI. ACC = anterior cingulate cortex; AngG = angular gyrus; Cd = caudate; con = control subjects; Ins = insula; lPFC = lateral prefrontal cortex; MFG = middle frontal gyrus; mPFC = medial prefrontal cortex; pat = patients; Put = putamen; SMG = supramarginal gyrus, SFG = superior frontal gyrus. See Figure 1 legend for expansion of other abbreviation.
Functional MRI
A full list of additional activations, their coordinates, and their z scores are presented in e-Tables 3 (859.5KB, pdf) , 4 (859.5KB, pdf) , and 5 (859.5KB, pdf) .
Correlation of FMRI Signal With Dyspnea VAS Ratings, Patients:
Activations were observed in the medial prefrontal cortex (mPFC), anterior insula, lateral prefrontal cortex (lPFC), anterior cingulate cortex (ACC), and precuneus (Fig 4).
Correlation of FMRI Signal With Dyspnea VAS Ratings, Control Subjects:
Activations were observed in the lPFC, anterior insula, putamen, caudate, angular gyrus, supramarginal gyrus, and superior frontal gyrus (Fig 4).
Correlation of FMRI Signal With VAS Ratings, Comparison of Patients and Control Subjects:
Common activation was found in the left-side anterior insula (Fig 4). A direct group comparison revealed stronger activation in the left-side mPFC and the ACC in patients; the reverse contrast showed stronger activation in the supramarginal gyrus, angular gyrus, precuneus, and middle frontal gyrus regions in control subjects.
Influence of Depression, Fatigue, and Vigilance on Correlation of FMRI Signal With Dyspnea VAS Ratings, Patients Only:
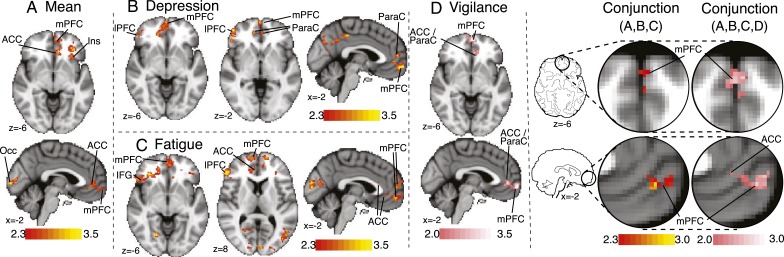
Significant negative correlations were observed between both depression and fatigue and activations in the mPFC, lPFC, and ACC. We observed clusters of subthreshold-positive activation at z = 2.0 for vigilance scores (Fig 5) in the mPFC and ACC. F tests revealed no shared variance between the contrasts. Conjunction analysis between all questionnaires and the mean activation revealed significant overlap in the mPFC and ACC (Fig 5).
Figure 5 –
Activations correlating with VAS ratings of dyspnea word cues for patients only. A, Mean positive activations in patients in the two slices common to B, C, and D. B-D, Activations correlating negatively with depression (B), negatively with fatigue (C), and positively with vigilance (D), respectively. Conjunction analysis represents brain areas where significant overlap exists among these activations. Maps for mean, depression, and fatigue are whole-brain analysis, cluster level corrected for multiple comparisons at P < .05, with a cluster threshold of z < 2.3, and activations are presented using standard red-yellow scaling. Activation in the vigilance contrast is presented with a cluster threshold of z < 2.0, and activations are presented with a pink scale to highlight this different statistical threshold. Maps represent mean correlation with respective questionnaire scores in patients only. IFG = inferior frontal gyrus; Occ = occipital cortex; ParaC = paracingulate cortex. See Figure 1 and 4 legends for expansion of other abbreviations.
Correlation of FMRI Signal With Anxiety VAS Ratings:
No significant mean effects were observed in either patients or control subjects. No group differences were identified.
Discussion
In patients, we observed activation of the mPFC, lPFC, ACC, anterior insula, and precuneus that correlated with the subjective dyspnea (VAS) response to the word cues. Furthermore, some of the variability in the brain response to these word cues is explained by measures of depression, fatigue, and vigilance.
The findings in the mPFC and ACC are of particular interest because FMRI activation in these areas was stronger than in healthy control subjects (Fig 4). The mPFC has been linked with fear-related memory processes and emotional learning and is a key structure engaged in chronic but not in acute pain.27,28 For example, in patients with chronic back pain, acute thermal pain engages the insula, whereas spontaneous chronic pain is associated with activity in the mPFC.27 The mPFC is considered a key component of a chronic pain suffering model that includes the lPFC and ACC.29 The mPFC has not been identified in any previous FMRI studies of dyspnea; however, none examined the chronic state. Activation in the ACC has been identified in two FMRI studies of experimental dyspnea,30,31 but activations were not correlated with a particular psychologic measure.
Depression is a well-established major influence in dyspnea. Taking parallels from the study of pain where depression enhances pain unpleasantness through the mPFC,32 the present data suggest that similar mechanisms may be in play in dyspnea. Despite strong clinical associations with COPD, the brain mechanisms of fatigue on sensory processes remain poorly understood. The present data begin to elucidate this by showing that level of fatigue in patients with COPD correlates with prefrontal activation in response to dyspnea-related cues, which might indicate that fatigue influences emotional processing. Hypervigilance is similarly well described in chronic dyspnea6,9 and an important component of fear avoidance.33 Hypervigilance may amplify dyspnea perception by altering the way the mPFC and ACC respond to dyspnea-specific situations.
Activation was also observed in the lPFC, which is known for its role in cognitive decisions regarding reacting to potentially harmful stimuli.29 The present data suggest that fatigue and depression influence the brain’s processing of dyspnea cues by acting in this structure. This may represent the patients’ altered evaluation of the word cues in the context of real-life experiences. The lPFC has been identified in experimentally induced acute dyspnea,30 but this is the first time in our knowledge that it has been linked to specific behavioral measures.
Dyspnea ratings were correlated with activation in the left-side anterior insula, a structure associated with interoception, the conscious awareness of bodily sensations.34,35 The insula has been identified in all FMRI studies of acutely induced dyspnea.7 Although the overall insular activation to the word cue task was stronger in patients than in control subjects (e-Fig 2 (859.5KB, pdf) ), the correlation between dyspnea VAS ratings and FMRI activations was the same (Fig 4). Taken together, these findings raise the question about whether activity in the insula is dyspnea specific or relates to more universal interoceptive processes and whether emotional engagement in dyspnea is downstream of or separate from interoception.
The findings in healthy control subjects (Fig 4) identify broadly the same brain areas as in other healthy volunteer FMRI studies of dyspnea and respiratory sensation.7,36,37 In the mPFC and ACC, patients with COPD demonstrate FMRI activation to these cues that is not observed in healthy control subjects. We propose that the findings relate to the different psychologic processing of these environmental cues, with greater salience and stronger negative meaning in COPD. In the everyday life of patients with COPD, these cues may be learned associations of normally innocuous scenarios of dyspnea or simply anticipation of physical activities, which may prime the brain for readiness. It is known that cues can exacerbate symptom perception in pain38,39 and asthma40 by enhancing brain activity in relevant areas before stimulus onset.41 The present data suggest that similar mechanisms of heightened responses to dyspnea-related environmental cues could increase the threat value or amplify the sensations of dyspnea, making dyspnea more unpleasant or more frightening.
We further speculate that engagement of these frontal areas may either lead to reorganization of the brain’s emotional circuitry (as has been proposed in chronic pain29) or alternatively, that these circuits are simply recruited more readily. However, further research is necessary.
The interpretation of the present imaging findings is supported by clinical evidence suggesting that emotional learning processes contribute to dyspnea. To characterize the mechanisms of emotional learning and determine the effect of chronicity, longitudinal FMRI studies are necessary. These could examine either natural change in dyspnea over time or brain changes in response to an intervention. For example, dyspnea-related fear measured before pulmonary rehabilitation correlates with improvements in dyspnea,9 strongly suggesting the importance of emotional learning.
Limitations
This study used word cues to engage brain networks responsible for dyspnea processing. The approach may be more suited to interrogating the emotional-cognitive aspects that modulate dyspnea rather than those brain activations related to direct sensory input.42,43 It is worth noting that the word cue responses correlate with Dyspnea-12 scores, so they are likely to represent a meaningful aspect of clinical dyspnea. Healthy control subjects may interpret word cues differently, reflecting a differing response to real-life situations. Targeting such variation was the aim of the study. However, to compare absolute dyspnea between patients and control subjects, a different approach might be taken, for example, adopting the paradigm of O’Donnell et al44 or comparing word cues with similar dyspnea valence.
The activations relating to the vigilance contrast did not survive cluster thresholding at the standard threshold of z > 2.3. Although we have less confidence in this particular finding, it would be misleading to ignore it completely. More work is needed to determine the role of vigilance in COPD.
Because smoking history was strongly associated with group (ie, patients, control subjects), we did not include it as a regressor in the FMRI analysis. Smoking has known effects upon the brain, but it remains unclear whether it has specific effects on the FMRI signal. We, therefore, included a control task (random letter strings) that demonstrated no difference between the groups. We suggest that future work might compare brain responses in groups matched for smoking history.
Conclusions
The findings suggest that emotional processes such as depression, fatigue, and vigilance play an important role in shaping the brain mechanisms associated with interpreting dyspnea-related cues in COPD. Heightened responses to salient cues are associated with increased symptom perception in other disorders, and the findings suggest that similar mechanisms may also be relevant in COPD. Engagement of these emotion-regulating areas may contribute to the poor correlation between lung function and dyspnea severity.
Future work would look in more detail at these structures and how interventions may affect dyspnea processing. Understanding the neural processing of dyspnea in a clinical population is crucial for advances to be made in its treatment, such as the development of neuroimaging biomarkers that allow patient stratification, leading to individualized treatments.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: M. H. and K. T. S. P. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. M. H., A. H., F. M. H., R. J. D., K. W., and K. T. S. P. contributed to the study concept and design; M. H., A. H., E. E., K. W., and K. T. S. P. contributed to the data acquisition and analysis; M. H., A. H., E. E., F. M. H., K. W., and K. T. S. P. contributed to drafting the manuscript and reviewing it for important intellectual content; and M. H., A. H., F. M. H., K. W., and K. T. S. P. contributed to the final approval of the manuscript.
Conflict of interest: None declared.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. This work is solely the responsibility of the authors.
Other contributions: The authors thank Steve Knight, BSc; Bethan Hughes, BSc; Isabel Chabata, MSc; Debby Nicoll, PhD; Tara Harris, RCN; Fran Sinfield, BSc; Emma Tucker, MSc; Rachel Lardner, MSc; Julie Young, MSc; and the Oxfordshire Pulmonary Rehabilitation Team for continued support of the study; and Richard Wise, PhD; Andrea Reinecke, PhD; Vishvarani Wanigasekera, DPhil; Annabel Nickol, PhD; and Najib Rahman, DPhil for generous academic input and support. The research materials supporting this publication can be accessed by contacting kyle.pattinson@nda.ox.ac.uk.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- ACC
anterior cingulate cortex
- FMRI
functional MRI
- lPFC
lateral prefrontal cortex
- mPFC
medial prefrontal cortex
- MSWT
modified shuttle walk test
- Sao2
oxygen saturation as measured by pulse oximetry
- VAS
visual analog scale
Footnotes
†Deceased.
Part of this article has been presented at the British Thoracic Society Winter Meeting, December 4-6, 2013, London, England.
FUNDING/SUPPORT: The study was funded by the Medical Research Council (United Kingdom) as part of a Clinician Scientist Fellowship awarded to Dr Pattinson [G0802826] and by the National Institute for Health Research Biomedical Research Centre, Oxford based at Oxford University Hospitals NHS Trust and the University of Oxford. Dr Wiech is supported by the Medical Research Council (United Kingdom).
References
- 1.Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56(11):880-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440. [DOI] [PubMed] [Google Scholar]
- 3.Borges-Santos E, Wada JT, da Silva CM, et al. Anxiety and depression are related to dyspnea and clinical control but not with thoracoabdominal mechanics in patients with COPD. Respir Physiol Neurobiol. 2015;210:1-6. [DOI] [PubMed] [Google Scholar]
- 4.Neuman A, Gunnbjörnsdottir M, Tunsäter A, et al. Dyspnea in relation to symptoms of anxiety and depression: a prospective population study. Respir Med. 2006;100(10):1843-1849. [DOI] [PubMed] [Google Scholar]
- 5.von Leupoldt A, Kenn K. The psychology of chronic obstructive pulmonary disease. Curr Opin Psychiatry. 2013;26(5):458-463. [DOI] [PubMed] [Google Scholar]
- 6.Hayen A, Herigstad M, Pattinson KT. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45-50. [DOI] [PubMed] [Google Scholar]
- 7.Herigstad M, Hayen A, Wiech K, Pattinson KT. Dyspnoea and the brain. Respir Med. 2011;105(6):809-817. [DOI] [PubMed] [Google Scholar]
- 8.Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssens T, De Peuter S, Stans L, et al. Dyspnea perception in COPD: association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program. Chest. 2011;140(3):618-625. [DOI] [PubMed] [Google Scholar]
- 10.Pattinson K. Functional brain imaging in respiratory medicine. Thorax. 2015;70(6):598-600. [DOI] [PubMed] [Google Scholar]
- 11.Ogino Y, Nemoto H, Inui K, Saito S, Kakigi R, Goto F. Inner experience of pain: imagination of pain while viewing images showing painful events forms subjective pain representation in human brain. Cereb Cortex. 2007;17(5):1139-1146. [DOI] [PubMed] [Google Scholar]
- 12.Corradi-Dell’Acqua C, Hofstetter C, Vuilleumier P. Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. J Neurosci. 2011;31(49):17996-18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex. 2011;21(3):719-726. [DOI] [PubMed] [Google Scholar]
- 14.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321-1327. [DOI] [PubMed] [Google Scholar]
- 15.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax. 2010;65(1):21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Peuter S, Lemaigre V, Van Diest I, Van den Bergh O. Illness-specific catastrophic thinking and overperception in asthma. Health Psychol. 2008;27(1):93-99. [DOI] [PubMed] [Google Scholar]
- 18.McCracken LM. “Attention” to pain in persons with chronic pain: a behavioral approach. Behav Ther. 1997;28(2):271-284. [Google Scholar]
- 19.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. [Google Scholar]
- 20.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 21.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121-1123. [DOI] [PubMed] [Google Scholar]
- 22.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS Scales. J Pers Soc Psychol. 1994;67(2):319-333. [Google Scholar]
- 23.Levy ML, Quanjer PH, Booker R, Cooper BG, Holmes S, Small I; General Practice Airways Group. Diagnostic spirometry in primary care: proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations: a General Practice Airways Group (GPIAG) document, in association with the Association for Respiratory Technology & Physiology (ARTP) and Education for Health. Prim Care Respir J. 2009;18(3):130-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks JC, Faull OK, Pattinson KT, Jenkinson M. Physiological noise in brainstem FMRI. Front Hum Neurosci. 2013;7:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey AK, Pattinson KT, Brooks JC, Mayhew SD, Jenkinson M, Wise RG. Brainstem functional magnetic resonance imaging: disentangling signal from physiological noise. J Magn Reson Imaging. 2008;28(6):1337-1344. [DOI] [PubMed] [Google Scholar]
- 26.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12(6):900-918. [DOI] [PubMed] [Google Scholar]
- 27.Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26(47):12165-12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apkarian VA, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152(3):S49-S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88(3):1500-1511. [DOI] [PubMed] [Google Scholar]
- 31.von Leupoldt A, Sommer T, Kegat S, et al. Dyspnea and pain share emotion-related brain network. Neuroimage. 2009;48(1):200-206. [DOI] [PubMed] [Google Scholar]
- 32.Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010;67(11):1083-1090. [DOI] [PubMed] [Google Scholar]
- 33.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30(1):77-94. [DOI] [PubMed] [Google Scholar]
- 34.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655-666. [DOI] [PubMed] [Google Scholar]
- 35.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59-70. [DOI] [PubMed] [Google Scholar]
- 36.Pattinson KT, Governo RJ, MacIntosh BJ, et al. Opioids depress cortical centers responsible for the volitional control of respiration. J Neurosci. 2009;29(25):8177-8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faull OK, Jenkinson M, Clare S, Pattinson KT. Functional subdivision of the human periaqueductal grey in respiratory control using 7tesla fMRI. Neuroimage. 2015;113:356-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30(39):12964-12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvie DS, Broecker M, Smith RT, Meulders A, Madden VJ, Moseley GL. Bogus visual feedback alters onset of movement-evoked pain in people with neck pain. Psychol Sci. 2015;26(4):385-392. [DOI] [PubMed] [Google Scholar]
- 40.De Peuter S, Van Diest I, Lemaigre V, et al. Can subjective asthma symptoms be learned? Psychosom Med. 2005;67(3):454-461. [DOI] [PubMed] [Google Scholar]
- 41.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A. 2010;107(1):355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fairhurst M, Fairhurst K, Berna C, Tracey I. An fMRI study exploring the overlap and differences between neural representations of physical and recalled pain. PLoS One. 2012;7(10):e48711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayen A, Herigstad M, Kelly M, et al. The effects of altered intrathoracic pressure on resting cerebral blood flow and its response to visual stimulation. Neuroimage. 2013;66:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Donnell CR, Schwartzstein RM, Lansing RW, Guilfoyle T, Elkin D, Banzett RB. Dyspnea affective response: comparing COPD patients with healthy volunteers and laboratory model with activities of daily living. BMC Pulm Med. 2013;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement