Abstract
Previous work from both our lab and others have indicated that exposure to 50 Hz magnetic fields (ELF-MF) was able to modify ion channel functions. However, very few studies have investigated the effects of MF on γ-aminobutyric acid (GABA) type A receptors (GABAARs) channel functioning, which are fundamental to overall neuronal excitability. Here, our major goal is to reveal the potential effects of ELF-MF on GABAARs activity in rat cerebellar granule neurons (CGNs). Our results indicated that exposing CGNs to 1 mT ELF-MF for 60 min. significantly increased GABAAR currents without modifying sensitivity to GABA. However, activation of PKA by db-cAMP failed to do so, but led to a slight decrease instead. On the other hand, PKC activation or inhibition by PMA or Bis and Docosahexaenoic acid (DHA) mimicked or eliminated the field-induced-increase of GABAAR currents. Western blot analysis indicated that the intracellular levels of phosphorylated PKC (pPKC) were significantly elevated after 60 min. of ELF-MF exposure, which was subsequently blocked by application of DHA or EP1 receptor-specific (prostaglandin E receptor 1) antagonist (SC19220), but not by EP2-EP4 receptor-specific antagonists. SC19220 also significantly inhibited the ELF-MF-induced elevation on GABAAR currents. Together, these data obviously demonstrated for the first time that neuronal GABAA currents are significantly increased by ELF-MF exposure, and also suggest that these effects are mediated via an EP1 receptor-mediated PKC pathway. Future work will focus on a more comprehensive analysis of the physiological and/or pathological consequences of these effects.
Keywords: 50 Hz magnetic fields, GABAA currents, EP receptors, PKC pathway, rat cerebellar granule neurons
Introduction
Electromagnetic fields in the extremely low frequency (ELF) range is ubiquitously present in various environments in everyday life. The major sources of 50 Hz magnetic fields (ELF-MF) pertaining to the general public are in-house installations, household appliances and powerlines 1. A number of studies in vitro have noted that exposure to ELF-MF has multiple biological effects, including changes in gene expression, regulation of cell survival and promotion of cell differentiation 2,3. Recent studies have demonstrated that exposure to ELF-MF can produce higher order effects. For example, investigation by Salunke et al. (2014) indicated that long-term exposure to ELF-MF significantly increased anxiety without affecting locomotion, and there was a significant elevation of both GABA and glutamate levels in the hippocampus and hypothalamus of mice exposed 4. Furthermore, ELF-MF exposure can change dendritic spine density and morphology in the entorhinal cortical neurons, and had caused a long-lasting increase in the excitatory state of the neurons in the cortex and hippocampus 5,6. Although the effect of ELF-MFs on the activity of neuronal excitability controlling channels including Ca2+-active potassium channels and Na+ channels have previous investigated, very few studies have investigated the effects of ELF-MF on ligand-gated channels, particularly γ-aminobutyric acid (GABA) type A receptors (GABAARs).
It is well-known that inhibitory neurotransmission is largely mediated by GABA acting through GABAARs. These receptors are heteropentameric, ligand-gated chloride channels that belong to the Cys-loop ligand-gated ion channel superfamily 7. GABAARs are expressed ubiquitously in neurons along the entire neuraxis and their activity is important for animal development and neuronal differentiation 8,9. They are also critical for the structural and functional maturation of neurons 10–12. Moreover, deficits in GABAAR-mediated neurotransmission have been implicated in various pathophysiological disorders, such as anxiety, epilepsy and schizophrenia 13–15. Given the importance of GABAARs, studying the effects of ELF-MFs is required for an understanding of the possible causes of exposure-induced effects on learning and memory. However, there is currently little information as to whether ELF-MF can modulate GABAAR activity.
Cerebellar granule neurons (CGNs) constitute the largest, homogeneous neuronal population within the mammalian brain. Due to their postnatal generation and well-defined developmental pathway, CGNs have been established as an accurate in vitro model for studying neuronal development and maturation 16. Furthermore, in vitro CGNs cultures have also long been a model for studying GABAA receptors 17,18 as well as a model for neuronal cell development and apoptosis 19–21. We have previously shown that exposure of CGNs to 10–60 min. of ELF-MF significantly increased Nav currents (INa) by 30–125% with a significant shift of their steady-state activation curve in both a time- and intensity-dependent manner 22. Since this phenomenon is similar to the effects seen with intracellular application of arachidonic acid (AA) and prostaglandin E2 (PGE2) on the INa in CGNs 23, a later mechanistic study revealed that the ELF-MF increase in neuronal INa occurs via a PKA-dependent pathway (cPLA2 → AA → PGE2 → EP receptors → PKA).
Therefore, the objective of this study was (i) to determine whether exposure to ELF-MF affected GABAA receptor currents and (ii) whether a PKA-dependent mechanism might be involved. Our results demonstrate for the first time that GABAA receptor currents are significantly increased by ELF-MF exposure via an EP receptor-mediated PKC signalling pathway.
Materials and methods
Ethics statement
This study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Fudan University (Permit Number: 20090614-001). All surgeries were performed under sodium pentobarbital anaesthesia and all efforts were made to minimize animal suffering.
Cell culture
Cells were derived from the cerebellum of 7–8-day-old Sprague–Dawley rat pups as previously described 24. Isolated cells were plated onto 35 mm diameter Petri dishes coated with poly-l-lysine (10 μg/ml) at a density of 106 cells/ml. Cultured cells were incubated at 37°C under 5% CO2 in DMEM supplemented with 10% foetal calf serum, glutamine (5 mM), insulin (5 μg/ml), KCl (25 mM) and 1% antibiotic–antimycotic solution (25 μg Streptomycin, 10,000 μg Amphotericin B, 10,000 UI Penicillin). All experiments were carried out using primary CGNs after 5–7 days in culture.
ELF-MF exposure system
We used the same system (I-ONE, Shanghai, China) for magnetic field exposure of cerebellar GCs as has been used in previous studies, with some revisions 25–28. Briefly, a 50 Hz magnetic field was generated by a pair of horizontal Helmholtz coils (20 cm in height, and 20 cm in radius, each plate consists of 150 turns of copper wire) placed parallel to each other. The coils were powered by a generator system, which consists with a signal generator and an amplifier, that produced the input voltage of the pulse, and resulting magnetic flux densities could be regulated within the range 0–1.0 mT. Both the ELF-MF frequency and flux density were monitored by a MF sensor that was connected to a digital multimeter. The geometry of the system assured a uniform field in the area of a central cylinder (10 cm in height and 6 cm in radius) for the exposed cultured cells. The surfaces of the culture plates were perpendicular to the force lines of the alternating magnetic field in the solenoid. Air and culture medium temperatures were continuously monitored for the duration of all experiments 22. The incubator was keep closed all throughout the ELF-MF or non-MF experiments to make sure that the conditions remained stable. Non-MF groups (sham) were incubated in the same incubator in which the conditions were the same as for the exposed groups, but MF exposure system was off.
GABAAR current recordings
Whole-cell currents from granule neurons were recorded with a patch-clamp technique. Prior to GABAAR current recordings, the culture medium was replaced with a bath solution containing the following: NaCl 145 mM, KCl 2.5 mM, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) 10 mM, MgCl2 1 mM and glucose 10 mM (pH adjusted to 7.4 with NaOH). Soft-glass pipettes (BR749321 BRAND® micro haematocrit capillary, Sigma-Aldrich, St. Louis, MO, USA) were filled with an internal solution containing the following: KCl 145 mM, HEPES 10 mM, CaCl2 1 mM, MgCl2 1 mM, ethylene glycol tetraacetic acid (EGTA) 10 mM and ATP 1 mM (pH adjusted to 7.2 with KOH). The pipette resistance was 5–7 MΩ after filling with the internal solution. The recordings were performed at 23–25°C. GABAA currents were recorded while the membrane potential was held at −70 mV. 100 μM GABA was given for 3 sec. using a gravity perfusion system to induce an inward Cl− current. There was a 40 sec. interval between each GABA perfusion 29,30. In the protocol to study the concentration-response relationship of GABAA receptors, we used a 20 sec. interval between GABA applications instead of the 40 sec. interval. All currents were recorded using an Axopatch 700B amplifier (Axon Instruments, Foster City, CA, USA) operated in voltage-clamp mode using a computer connected to the recording equipment via a Digidata 1440A analog-to-digital (A/D) interface. Current was digitally sampled at 100 μsec. (10 kHz). Current signals were filtered by a 1 kHz, three-pole Bessel filter. Data acquisition and analysis were performed with pClamp 10.2 software (Axon Instruments) and/or Origin8.0 (Microcal Analysis Software, Northampton, MA, USA).
Western blotting
The cells were lysed in HEPES-NP40 lysis buffer (20 mM HEPES, 150 mM NaCl, 0.5% NP-40, 10% glycerol, 2 mM ethylenediaminetetraacetic acid, 100 μM Na3VO4, 50 mM NaF, pH 7.5 and 1% proteinase inhibitor cocktail) on ice for 30 min. After centrifugation, the supernatant was mixed with 2× SDS loading buffer and boiled for 5 min. Proteins were separated on a 10% polyacrylamide gel, transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA), blocked with 10% non-fat milk and incubated at 4°C overnight with either a rabbit polyclonal antibody against phosphorylated PKC (pPKC) PAN (#9371; Cell Signaling Technology, Beverly, MA, USA) or a mouse monoclonal antibody against Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (KC-5G4; KangChen Bio-tech, Shanghai, China). After extensive washing with TBST (Tris-Buffered Saline with Tween-20), the membrane was incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (1:10,000; KangChen Bio-Tech) for 2 hrs at room temperature. Chemiluminescent signals were generated using a SuperSignal West Pico trial kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) and detected using a ChemiDoc XRS System (Bio-Rad Laboratories Inc., Hercules, CA, USA). All measured protein bands were normalized to GAPDH and sham/GAPDH was set to 1.0.
Chemicals
AH23848 hemicalcium salt (A8227), AH6809 (A1221), bisindolylmaleimide (Bis, B3931), cis-4,7,10,13,16,19-Docosahexaenoic acid (DHA, D2534), dibutyryl cyclic AMP (db-cAMP, D0627), Ethylene glycol-bis(2-aminoethylether)-N,N,N?,N?-tetraacetic acid (EGTA, E4378), 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES, H3375), Insulin (I4011), Phorbol 12-myristate 13-acetate (PMA, P8139), poly-l-lysine (P2636), Prostaglandin E2 (PGE2, P5640) and SC 19220 (S3065) were all purchased from Sigma-Aldrich (St. Louis, MO, USA). L-798, 106 (cat. no. 3342) was from Tocris Bioscience (Bristol, UK). DMEM (12100-046), Foetal calf serum (10099-141), and the antibiotic–antimycotic (15240-062) solution were all purchased from Gibco Life Technologies (Grand Island, NY, USA).
Statistical analysis
Statistical analysis was performed using a Student’s t-test with either a non-paired or paired comparison, as relevant. Values are given as the means ± SEM, with n representing the number of cells tested. A value of P < 0.05 was considered a statistically significant difference between groups. When multiple comparisons were made, the data were analysed using a one-way anova followed by post hoc analysis with Tukey and Fisher LSD tests for samples of more than two. All analyses were performed using Origin Pro software (OriginLab Corporation, Northampton, MA, USA).
Results
ELF-MF exposure increased GABAA receptor currents without modifying their sensitivity to GABA
To investigate whether ELF-MF exposure modified GABAA receptor current amplitudes in CGNs, CGNs were exposed to ELF-MF (1 mT) for 60 min., the amplitude of the GABAA currents increased by approximately 21.5% ± 8.4% as compared to cells that had received no ELF-MF exposure (Fig. 1A, n = 10, P < 0.05). When ELF-MF exposure was shorter than 60 min., GABAAR currents were not significantly increased (Fig. 1B).
Figure 1.
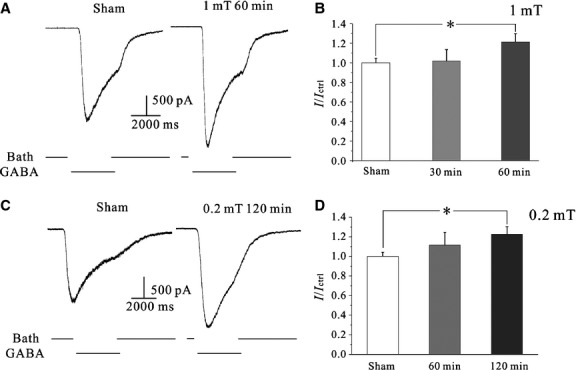
Effects of 50 Hz ELF-MF on CGN GABAA receptor currents. (A) Current traces for the effects of ELF-MF (1 mT for 1 hr) on GABAA receptor currents. (B) Statistical analysis of the time-dependent effects of ELF-MF exposure on GABAA receptor current amplitudes. Data are means ± SEM. *P < 0.05 by one-way anova for two groups connected by a straight line. (C) Representative current traces for the effects of MF exposure (0.2 mT for 2 hr) on GABAA receptor currents. (D) Statistical analysis of the time-dependent effects of ELF-MF exposure on GABAA currents. Data are means ± SEM. *P < 0.05 by one-way anova for two groups connected by a straight line.
We also tested the effects of low-intensity ELF-MF (0.2 mT) on GABAA currents. As shown in Figure 1C, when CGNs were exposed to 0.2 mT ELF-MF, a longer exposure time was needed to induce an increase in GABAAR currents. Moreover, the amplitude of GABAAR currents only increased by 11.5% ± 12.9% (n = 7, P > 0.05) when CGNs were exposed to ELF-MF (0.2 mT) for 1 hr. When the ELF-MF (0.2 mT) exposure time was increased to 2 hrs, the GABAAR current amplitude was increased by 22.8% ± 7.6% (Fig. 1D, n = 14, P < 0.05). However, the mean capacitance of the recorded cells in the sham group (8.85 ± 0.55 pF, n = 15) and for the ELF-MF treatment group (9.47 ± 0.41 pF, n = 14) showed no significant difference (P > 0.05). This similarity in capacitance indicates that the ELF-MF-induced increase in current amplitude was not due to differences or abnormalities in cell morphology.
Previous work has indicated that long-term cellular ELF-MF exposure may alter levels of protein expression 31, leading to potential difficulties in identifying the primary factor involved in our observed ELF-MF-induced increases in GABAAR currents. Thus, we chose to focus on the mechanism by which a relatively short-term exposure to a 1 mT ELF-MF (60 min.) induces increases in GABAAR currents.
The increase in GABAA receptor current amplitude could also be due to an increase in the sensitivity of receptors to GABA. To test this hypothesis, we applied the GABA concentration-dependent experiment for CGNs with or without ELF-MF exposure. Currents were recorded while the membrane potential was held at −70 mV. Different concentrations of GABA (100 nM to 1 mM, beginning with the lower range) were given to induce GABA receptor current in CGNs with 20 sec. intervals between each concentration (Fig. 2A). The data were then fitted with the Hill equation: I = Imax/(1 + (EC50/[GABA])^nH), where the GABA-induced current I is a function of the GABA concentration, EC50 is the GABA concentration required for inducing a half-maximal current, nH is the Hill coefficient, and Imax is the maximum current. The maximum current was then used to normalize the concentration-response curve for each individual trace. The average of the normalized currents for each GABA concentration was used to plot the data. Our results showed that the GABAA receptor current was significantly increased with ELF-MF exposure (Fig. 2B). However, the GABAA receptor concentration-response relationship was not significantly changed by ELF-MF exposure when compared to the non-ELF-MF exposed controls (Fig. 2C). Fitting the normalized current amplitude as a function of GABA concentration using the Hill formula indicates that the Hill regression parameters of sham or ELF-MF exposure have no significant difference (Table 1). Collectively, these results indicate that the ELF-MF-induced increases of GABAA receptor currents is not due to an increase in receptor sensitivity to GABA.
Figure 2.

Effects of ELF-MF on GABA sensitivity of GABAA receptor. (A) GABAA currents elicited by different concentrations of GABA applied to the cells with a gravity perfusion system in either sham or MF exposure groups (1 mT for 1 hr). (B) Curve of GABAA receptor current amplitude as a function of GABA concentration with control (sham exposure) or ELF-MF exposure. (C) Plot of the normalized current amplitude as a function of GABA concentration with the treatment of sham or ELF-MF exposure; Data points were processed by Hill regressions in Origin 8.0. Data are means ± SEM. *P < 0.05 by two-sample t-test for two groups connected by a straight line.
Table 1.
Hill regression parameters of Concentration- response relationship of GABAA current with sham or ELF-MF exposure (n = 6–7)
| Sham | ELF-MF | |
|---|---|---|
| Normalized Imax | 1.00 ± 0.01 | 1.02 ± 0.05 |
| EC50 | 11.80 ± 2.04 | 15.73 ± 3.76 |
| nH | 1.04 ± 0.04 | 0.82 ± 0.13 |
| Kd = EC50^nH | 13.02 | 9.67 |
ELF-MF exposure increased GABAA receptor currents by activation of a PKC-dependent pathway
Previous studies have indicated that GABAA receptor activity can be modulated by the activation of protein kinase A 32. As such, db-cAMP (a cAMP analogue) was added to the bath solution and CGNs were then incubated for 1 hr to elucidate whether a PKA-dependent pathway was involved in effects of ELF-MF exposure. However, 20 μM db-cAMP was unable to mimic ELF-MF exposure-induced increases in GABAAR currents. Interestingly, it reduced the current amplitude by 28.6 ± 6.1% (n = 8; Fig. 3A), suggesting that a PKA pathway was not involved in the effects of ELF-MF on GABAA-R currents increase. Since a previous study had reported that GABAAR currents were modified in a PKC-dependent manner 33,34, we thus identified whether a PKC pathway was involved in the ELF-MF exposure-induced increases of GABAAR currents. Incubating CGNs with 100 nM PMA (a PKC activator) in the bath solution for 1 hr was able to partly mimic the enhancement effects of ELF-MF exposure on GABAAR currents (Fig. 3B), increasing GABAAR current amplitude by 20.4 ± 7.2% (n = 28, P < 0.05).
Figure 3.
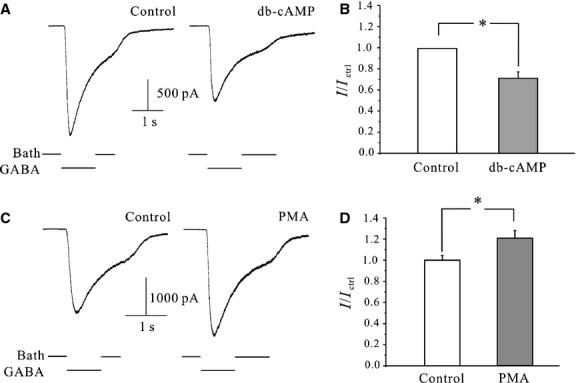
Effects of PKA or PKC pathway on CGNs GABAA receptor currents. (A) Control currents and those following the application of 20 μM db-cAMP for 1 hr. (B) Statistical analysis of the effects of 1-hr application of db-cAMP on GABAA currents. (C) Control currents and currents following the application of 100 nM PMA for 1 hr. (D) Statistical analysis of the effects of PKC activation on GABAA currents. Data are means ± SEM. *P < 0.05 by two-sample t-test for two groups connected by a straight line.
Moreover, the effects of ELF-MF exposure-induced increase of GABAAR currents were attenuated upon application of both Bis and DHA, two PKC inhibitors (Fig. 4A and B). ELF-MF exposure slightly decreased GABAA receptor currents by 9.6 ± 7.3% in the present of Bis (P > 0.05, when compared to Bis with sham, n = 15). In the present of DHA, ELF-MF exposure did not result in an increase of GABAA receptor currents, but rather a slight decrease in current amplitude by 15.0 ± 11.2% (P > 0.05, when compared to DHA with sham). Taken together, these data suggest that a PKC pathway may be involved in the increases in current amplitude seen upon ELF-MF exposure.
Figure 4.

Effects of activation of PKC pathway on ELF-MF-induced increases of GABAA currents. (A) Current traces in the presence of 10 μM Bisindolylmaleimide (Bis) in sham or 1 mT 1 hr MF exposure-treated groups (upper panel), and statistical analysis of the effects of Bis in ELF-MF-induced increases of GABAA currents (lower panel). (B) Current traces in the present of 10 μM DHA in sham or ELF-MF exposure-treated groups (upper panel), and statistical analysis of the effects of DHA on ELF-MF-induced increases of GABAA currents (lower panel). (C) Western blot and statistical analysis of the effects of DHA on pPKC levels in sham or ELF-MF exposure-treated groups. Data are means ± SEM. *P < 0.05 by two-sample t-test for two groups connected by a straight line.
To confirm the involvement of a PKC-dependent pathway in the ELF-MF exposed increases of GABAA receptor currents, we used Western blotting in conjunction with a phospho-specific antibody to measure levels of pPKC in response to ELF-MF exposure. As shown in Figure 4C, there was a significant increase in pPKC levels (30.8 ± 14.9%, P < 0.05) after a 1 hr exposure of CGNs to ELF-MF. Moreover, the inhibition of PKC activity by DHA effectively eliminated the ELF-MF exposure-induced increases of pPKC (Fig. 4C).
EP1 receptor activation was associated with ELF-MF exposure increases in GABAA receptor currents
Our previous study indicated that there was an increase in intracellular AA and PGE2 levels following ELF-MF exposure in CGNs. Furthermore, that ELF-MF exposure significantly increased neuronal INa through PGE2 receptor activation 22. We therefore used Prostanoid EP receptor antagonists to examine whether EP receptors mediated the observed increases in GABAA current and pPKC levels as a result of ELF-MF exposure. In the presence of 20 μM SC19220 (an EP1 receptor-specific antagonist) the effects of ELF-MF increase of GABAA receptor currents were reduced to 3.7 ± 13.2% (Fig. 5A, n = 17, P > 0.05 when compared to SC19220 with sham). Similarly, SC19220 also eliminated the effects of ELF-MF on pPKC levels. In the presence of SC19220, ELF-MF mildly increased pPKC levels by 0.4 ± 9.7% (Fig. 5D, n = 5, P > 0.05 when compared to sham). Importantly, AH23848 (an EP2 receptor specific-antagonist), L-798, 106 (an EP3 receptor specific-antagonist), and AH6809 (an EP4 specific-antagonist) were all unable to reduce or ablate the effects of MF-induced increases in pPKC levels (Fig. 5B and C, n = 10). Taken together, results suggest that a PKC pathway activated by EP1 receptors mediates the MF-induced increases in GABAAR current (Fig. 6).
Figure 5.

Effects of EP1 antagonists on ELF-MF-induced increases of GABAA currents. (A) Current traces in the present of 20 μM EP1 receptor antagonist SC19220 with or without 1 mT MF exposure for 1 hr (upper panel), and statistical analysis of the effects of SC19220 on ELF-MF-induced increases of GABAA currents by two-sample t-test (lower panel). (B–D) Western blot and statistical analysis of the effects of AH6809 (AH68), AH23848 (AH23), L-798,106, and SC19220 (SC19) on pPKC levels of CGNs with or without ELF-MF exposure. Data are means ±SEM. *P < 0.05 by one-way anova for two groups connected by a straight line.
Figure 6.
A proposed model depicting the mechanisms that are likely to be involved in the modulation of GABAA receptors currents by ELF-MF exposure in CGNs. ELF-EMF active cPLA2 and up-regulated AA and PGE2, which can act in an autocrine or paracrine manner to activate EP receptors. Ligand binding of EP1 is associated with PKC activation and consequently modulates GABAA receptors currents.
Discussion
Although ELF-MF exposure has been previously reported to modulate the activity of voltage-gated ion channels, including sodium and calcium channels 22,35, few studies to date measured the effects of MF exposure on neuronal, ligand-gated ion channels. For the first time, we report here that ELF-MF exposure enhances GABAA receptor currents in cerebellar GCs. In particular, neuronal exposure to ELF-MF influences EP receptor-mediated activity of a PKC-dependent pathway and accounts for the induction of GABAA receptor currents.
Our previous study revealed that intracellular AA and PGE2 levels were increased following ELF-MF exposure by enhancing cPLA2 activity in cerebellar GCs. Increased PGE2 activated a PKA pathway via EP receptors, which then enhanced neuronal INa.22 In this study, our results indicated that ELF-MF-induced increases of PGE2 not only modified neuronal INa, but also modified neuronal GABAA receptor currents. Interestingly, we noted that although this ELF-MF exposure-induced response is modulated by induction of PGE2, the PKA signalling pathway that induced enhancement of INa did not associate with ELF-MF-induced increase of GABAA currents. Although PKA has been previously shown to be a modulator of GABAA receptors, thereby enhancing or reducing the function of neuronal GABAA receptors by acute reduction in channel opening frequency or chlorine increases in gene expression 32,36. However, the activation of a PKC signalling pathway was shown to underlie the effects seen in our study. This might result from the differential activation of PGE2 receptor. A higher level of PGE2 might be produced by ELF-MF exposure since our previous study identified that all of four types EP receptors are expressed in CGNs 23.
Numerous studies have indicated that PGE2 is mediated by the family of EP receptors, which consists of four isoforms: EP1-EP4 37,38. When activated, EP2 and EP4 receptors increase the concentration of intracellular cAMP 39,40, but EP3 activation resulted in a decrease of intracellular cAMP levels 41. However, EP1 receptor activation coupled to increase phospholipase C (PLC), which mediates activation of protein kinase (PKC) and subsequent elevation of cytosolic free calcium 42. Although our previous study identified that all four types of EP receptors were expressed in CGNs 23, pharmacological experiments with both EP receptor-specific antagonists and agonists indicated that ELF-MF exposure and PGE2-induced increases of GABAA currents was mediated solely by activation of the EP1 receptor. This explains why it is the PKC rather than the PKA pathway that is associated with ELF-MF exposure-induced increases in GABAA receptor currents.
Although our previous study suggested that ELF-MF exposure increased PKA activity, our study here showed that activating PKA alone should induce an inhibitory effect on GABAA receptor currents. This is because incubation of CGNs with db-cAMP reduced the amplitude of GABAA receptor currents. However, ELF-MF exposure-induced PKA activity did not seem to offset the PKC-driven effects induced by ELF-MF exposure on GABAA currents since both the ELF-MF driven increases in percentage of current amplitude and PMA stimulation were similar. It is likely that PKA activity induced by ELF-MF exposure occurred prior to PKC activation. This is because immunoblot assays of intracellular levels of pPKA indicated a significant increase in ELF-MF exposure-induced levels of phospho-PKA (10–30 min.) 22. The time difference among PKA, PKC or other kinase signal pathway activation raises the issue of the complexity of the biological effects induced by ELF-MF exposure. This, along with differences in experimental conditions and/or intensity and duration of MF exposure, partly explains why the reported results involved in the neuronal effects of ELF-MF in various organisms are variable and/or contradictory.
Phosphorylation of the intracellular domains between transmembrane domain 3 and 4 of the GABAA receptor β and γ subunits by serine/threonine and tyrosine kinases has been shown to alter receptor function. This is either by a direct effect on receptor properties, such as the probability of channel opening or desensitization, or by regulating trafficking of the receptor to and from the cell surface 43,44. Previous immunocytochemical work have shown the expression of all three βsubunit types (β1, β2 and β3) in the granule cell layer 45, thus PKC might modify GABAA receptor by phosphorylation of this subunit. The PGE2/EP1/PKC-mediated increases of GABAA receptor currents occurred after a 60 min. ELF-MF exposure. However, ELF-MF exposure failed to induce changes in receptor sensitivity to GABA, thus the mechanism of upregulation might be independent of the transcription or translation of GABAA receptor proteins. An additional possibility is that it is involved in the regulation of GABAA receptor trafficking to the membrane.
Previous studies have shown that the modified effects of PKC activation on GABAA receptors is diverse and appears to be dependent on subunit composition 34,46. Currently, there is evidence suggesting that PKC upregulates both GABAA receptor cell-surface expression and their resulting surface stability 47. However, in cultured cortical neurons, PKC activity leads to a decrease in cell-surface GABAA receptors and associated currents by phosphorylation of other proteins within the endocytic cascade 33,48. Furthermore, cell surface expression and postsynaptic accumulation of GABAA receptors is a complex process, including subunit gene expression, assembly of subunits into receptors, as well as exocytosis, endocytic recycling, diffusion dynamics and the degradation of GABAA receptors 49. As we have no evidence here to indicate whether ELF-MF exposure-induced and PGE2/EP1/PKC-mediated upregulation of GABAA receptors are associated with its trafficking to the membrane, further research will be required to test this possibility and its underlying mechanism.
The neuronal effects of ELF-MF have been extensively studied in various organisms 50,51. Although the reported results are variable and/or contradictory, this is due in part to differences in experimental conditions and in the flux density and/or duration of MF exposure. However, MF has recently been reported to modulate neuronal excitability and neurogenesis 51–53. Coincidently, GABAA receptor activity not only serves to regulate the excitability of neural circuits, but also plays a role in neuronal development and differentiation 8,9. Thus, our in vitro findings on the effects of ELF-MF exposure on GABAA receptors may provide evidence and mechanistic insight as to the effects of MF on neuronal excitation and neurogenesis in the central nervous system (CNS). Moreover, deficits in GABAAR-mediated neurotransmission are known to associate with pathophysiological disorders, including anxiety disorders, epilepsy and schizophrenia 13–15. Therefore, further exploration is required to comprehensively analyse the physiological and/or pathological effects of ELF-MF exposure on GABAA receptors. Moreover, whether ELF-MF-induced enhancement of GABAA receptors may be relevant for (i) the treatment of brain disorders associated with deficits in GABAA receptor functioning or (ii) as a potential therapeutic approach for disorders associated with neurogenesis. In addition, we did not address the mechanism by which ELF-MF triggered the bio-effect pathway (cPLA2 → AA → PGE2 → EP receptors) in the present study. Combining our current study with our previous data, we speculate that magnetic field may induce an electrophoretic effect of cell surface protein molecules and change the charge distribution of the cell membrane surface, which then enhances cPLA2 activity and increased intracellular AA and PGE2 levels in CGNs. Finally, since 1 mT of exposure is not encountered in the daily lives of the general public and seldom in occupational settings, the significance of our work is more applicable to biological mechanisms than population health risk assessments.
Acknowledgments
The study was supported by a grant from the National Basic Research Program of China (2011CB503703) and the Shanghai Leading Academic Discipline Project (B111).
Conflicts of interest
The authors confirm that there are no conflicts of interest.
References
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks) Potential health effects of exposure to electromagnetic fields (EMF) 2015.
- Martinez-Samano J, Torres-Duran PV, Juarez-Oropeza MA, et al. Effect of acute extremely low frequency electromagnetic field exposure on the antioxidant status and lipid levels in rat brain. Arch Med Res. 2012;43:183–9. doi: 10.1016/j.arcmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Bai WF, Xu WC, Feng Y, et al. Fifty-Hertz electromagnetic fields facilitate the induction of rat bone mesenchymal stromal cells to differentiate into functional neurons. Cytotherapy. 2013;15:961–70. doi: 10.1016/j.jcyt.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Salunke BP, Umathe SN, Chavan JG. Involvement of NMDA receptor in low-frequency magnetic field-induced anxiety in mice. Electromagn Biol Med. 2014;33:312–26. doi: 10.3109/15368378.2013.839453. [DOI] [PubMed] [Google Scholar]
- Xiong J, He C, Li C, et al. Changes of dendritic spine density and morphology in the superficial layers of the medial entorhinal cortex induced by extremely low-frequency magnetic field exposure. PLoS ONE. 2013;8:e83561. doi: 10.1371/journal.pone.0083561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balassa T, Varro P, Elek S, et al. Changes in synaptic efficacy in rat brain slices following extremely low-frequency magnetic field exposure at embryonic and early postnatal age. Int J Dev Neurosci. 2013;31:724–30. doi: 10.1016/j.ijdevneu.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Andang M, Lendahl U. Ion fluxes and neurotransmitters signaling in neural development. Curr Opin Neurobiol. 2008;18:232–6. doi: 10.1016/j.conb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol. 2007;17:81–6. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, et al. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27:5224–35. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, et al. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–15. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Charych EI, Liu F, Moss SJ, et al. GABA(A) receptors and their associated proteins: implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology. 2009;57:481–95. doi: 10.1016/j.neuropharm.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM. Epilepsy, E/I balance and GABA(A) receptor plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiatry. 2003;64:21–7. [PubMed] [Google Scholar]
- D’Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci. 2009;32:30–40. doi: 10.1016/j.tins.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Gallo V, Wise BC, Vaccarino F, et al. gamma-Aminobutyric acid- and benzodiazepine-induced modulation of [35S]-t-butylbicyclophosphorothionate binding to cerebellar granule cells. J Neurosci. 1985;5:2432–8. doi: 10.1523/JNEUROSCI.05-09-02432.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Fritschy JM. Cerebellar granule cells in vitro recapitulate the in vivo pattern of GABAA-receptor subunit expression. Brain Res Dev Brain Res. 1995;88:1–16. doi: 10.1016/0165-3806(95)00062-i. [DOI] [PubMed] [Google Scholar]
- D’Mello SR, Galli C, Ciotti T, et al. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci USA. 1993;90:10989–93. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CL, Zeng XM, Zhou MH, et al. Kv 1.1 is associated with neuronal apoptosis and modulated by protein kinase C in the rat cerebellar granule cell. J Neurochem. 2008;106:1125–37. doi: 10.1111/j.1471-4159.2008.05449.x. [DOI] [PubMed] [Google Scholar]
- Jiao S, Liu Z, Ren WH, et al. cAMP/protein kinase A signalling pathway protects against neuronal apoptosis and is associated with modulation of Kv2.1 in cerebellar granule cells. J Neurochem. 2007;100:979–91. doi: 10.1111/j.1471-4159.2006.04261.x. [DOI] [PubMed] [Google Scholar]
- He YL, Liu DD, Fang YJ, et al. Exposure to extremely low-frequency electromagnetic fields modulates Na+ currents in rat cerebellar granule cells through increase of AA/PGE2 and EP receptor-mediated cAMP/PKA pathway. PLoS ONE. 2013;8:e54376. doi: 10.1371/journal.pone.0054376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YJ, Zhou MH, Gao XF, et al. Arachidonic acid modulates Na+ currents by non-metabolic and metabolic pathways in rat cerebellar granule cells. Biochem J. 2011;438:203–15. doi: 10.1042/BJ20110569. [DOI] [PubMed] [Google Scholar]
- Mei YA, Wu MM, Huan CL, et al. 4-aminopyridine, a specific blocker of K(+) channels, inhibited inward Na(+) current in rat cerebellar granule cells. Brain Res. 2000;873:46–53. doi: 10.1016/s0006-8993(00)02469-0. [DOI] [PubMed] [Google Scholar]
- De Mattei M, Varani K, Masieri FF, et al. Adenosine analogs and electromagnetic fields inhibit prostaglandin E2 release in bovine synovial fibroblasts. Osteoarthritis Cartilage. 2009;17:252–62. doi: 10.1016/j.joca.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Ongaro A, Varani K, Masieri FF, et al. Electromagnetic fields (EMFs) and adenosine receptors modulate prostaglandin E(2) and cytokine release in human osteoarthritic synovial fibroblasts. J Cell Physiol. 2012;227:2461–9. doi: 10.1002/jcp.22981. [DOI] [PubMed] [Google Scholar]
- Varani K, Vincenzi F, Targa M, et al. Effect of pulsed electromagnetic field exposure on adenosine receptors in rat brain. Bioelectromagnetics. 2012;33:279–87. doi: 10.1002/bem.20704. [DOI] [PubMed] [Google Scholar]
- Wu X, Cao MP, Shen YY, et al. Weak power frequency magnetic field acting similarly to EGF stimulation, induces acute activations of the EGFR sensitive actin cytoskeleton motility in human amniotic cells. PLoS ONE. 2014;9:e87626. doi: 10.1371/journal.pone.0087626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YC, Cao LH, Yang XL. Modulation by brain natriuretic peptide of GABA receptors on rat retinal ON-type bipolar cells. J Neurosci. 2006;26:696–707. doi: 10.1523/JNEUROSCI.3653-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan XQ, Yao JJ, Liu DD, et al. Abeta40 modulates GABA(A) receptor alpha6 subunit expression and rat cerebellar granule neuron maturation through the ERK/mTOR pathway. J Neurochem. 2014;128:350–62. doi: 10.1111/jnc.12471. [DOI] [PubMed] [Google Scholar]
- Dibirdik I, Kristupaitis D, Kurosaki T, et al. Stimulation of Src family protein-tyrosine kinases as a proximal and mandatory step for SYK kinase-dependent phospholipase Cgamma2 activation in lymphoma B cells exposed to low energy electromagnetic fields. J Biol Chem. 1998;273:4035–9. doi: 10.1074/jbc.273.7.4035. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, et al. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–8. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, et al. PKC modulation of GABAA receptor endocytosis and function is inhibited by mutation of a dileucine motif within the receptor beta 2 subunit. Neuropharmacology. 2005;48:181–94. doi: 10.1016/j.neuropharm.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Song M, Messing RO. Protein kinase C regulation of GABAA receptors. Cell Mol Life Sci. 2005;62:119–27. doi: 10.1007/s00018-004-4339-x. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liu X, Yang T, et al. Exposure to extremely low-frequency electromagnetic fields inhibits T-type calcium channels via AA/LTE4 signaling pathway. Cell Calcium. 2014;55:48–58. doi: 10.1016/j.ceca.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Porter NM, Twyman RE, Uhler MD, et al. Cyclic AMP-dependent protein kinase decreases GABAA receptor current in mouse spinal neurons. Neuron. 1990;5:789–96. doi: 10.1016/0896-6273(90)90338-g. [DOI] [PubMed] [Google Scholar]
- Narumiya S. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med. 2009;87:1015–22. doi: 10.1007/s00109-009-0500-1. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Bastien L, Sawyer N, Grygorczyk R, et al. Cloning, functional expression, and characterization of the human prostaglandin E2 receptor EP2 subtype. J Biol Chem. 1994;269:11873–7. [PubMed] [Google Scholar]
- Regan JW, Bailey TJ, Pepperl DJ, et al. Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Mol Pharmacol. 1994;46:213–20. [PubMed] [Google Scholar]
- Fulton AM, Ma X, Kundu N. Targeting prostaglandin E EP receptors to inhibit metastasis. Cancer Res. 2006;66:9794–7. doi: 10.1158/0008-5472.CAN-06-2067. [DOI] [PubMed] [Google Scholar]
- Tang CH, Yang RS, Fu WM. Prostaglandin E2 stimulates fibronectin expression through EP1 receptor, phospholipase C, protein kinase Calpha, and c-Src pathway in primary cultured rat osteoblasts. J Biol Chem. 2005;280:22907–16. doi: 10.1074/jbc.M500130200. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–43. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–50. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, et al. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–50. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91:1009–22. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramian AM, Comenencia-Ortiz E, Vithlani M, et al. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi R, Cupello A, Robello M. Modulation of the expression of GABA(A) receptors in rat cerebellar granule cells by protein tyrosine kinases and protein kinase C. Biochim Biophys Acta. 2002;1564:263–70. doi: 10.1016/s0005-2736(02)00460-1. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi A, Ciotti MT, Ledda M, et al. Exposure to 50 Hz electromagnetic radiation promote early maturation and differentiation in newborn rat cerebellar granule neurons. J Cell Physiol. 2005;204:532–8. doi: 10.1002/jcp.20322. [DOI] [PubMed] [Google Scholar]
- Marchionni I, Paffi A, Pellegrino M, et al. Comparison between low-level 50 Hz and 900 MHz electromagnetic stimulation on single channel ionic currents and on firing frequency in dorsal root ganglion isolated neurons. Biochim Biophys Acta. 2006;1758:597–605. doi: 10.1016/j.bbamem.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Aldinucci C, Carretta A, Maiorca SM, et al. Effects of 50 Hz electromagnetic fields on rat cortical synaptosomes. Toxicol Ind Health. 2009;25:249–52. doi: 10.1177/0748233709103031. [DOI] [PubMed] [Google Scholar]
- Cuccurazzu B, Leone L, Podda MV, et al. Exposure to extremely low-frequency (50 Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Exp Neurol. 2010;226:173–82. doi: 10.1016/j.expneurol.2010.08.022. [DOI] [PubMed] [Google Scholar]



