Abstract
Aims
The aims of this study were (i) to develop a population pharmacokinetic (PK) model of tacrolimus in a Mexican renal transplant paediatric population (n = 53) and (ii) to test the influence of different covariates on its PK properties to facilitate dose individualization.
Methods
Population PK and variability parameters were estimated from whole blood drug concentration profiles obtained at steady-state using the non-linear mixed effect modelling software NONMEM® Version 7.2.
Results
Tacrolimus PK profiles exhibited high inter-patient variability (IPV). A two compartment model with first order input and elimination described the tacrolimus PK profiles in the studied population. The relationship between CYP3A5 genotype and tacrolimus CL/F was included in the final model. CL/F in CYP3A5*1/*1 and *1/*3 carriers was approximately 2- and 1.5-fold higher than in CYP3A5*3/*3 carriers (non-expressers), respectively, and explained almost the entire IPV in CL/F. Other covariates retained in the final model were the tacrolimus dose and formulation type. Limustin® showed markedly lower concentrations than the rest of the formulations.
Conclusions
Population PK modelling of tacrolimus in paediatric renal transplant recipients identified the tacrolimus formulation type as a significant covariate affecting the blood concentrations and confirmed the previously reported significant effect of CYP3A5 genotype on CL/F. It allowed the design of a proposed dosage based on the final model that is expected to help to improve tacrolimus dosing.
Keywords: CYP3A5, formulation, paediatric, population pharmacokinetics, renal transplant, tacrolimus
What is Already Known about this Subject
Tacrolimus is a highly effective and widely used to prevent organ rejection in transplant patients.
Several elements contribute to the high inter-patient variability on tacrolimus pharmacokinetics (PK) including demographics and biological factors as well as certain polymorphisms.
The influence of tacrolimus formulation (generic or innovator) on its PK has not been tested.
What this Study Adds
This study presents a population PK model of tacrolimus that found significant effect of CYP3A5*3, formulation and dose of tacrolimus on some of its PK parameters.
An estimator of the tacrolimus dose was developed based on the individual estimates of the PK parameters obtained from the final population PK model in which differences between formulations were important.
Introduction
One fundamental goal in organ transplantation is suppression of allograft rejection. Thus development of immunosuppressive drugs was the key to successful allograft function. Immunosuppressive agents are used for induction (intensive immunosuppression in the initial days after transplantation), maintenance and reversion of rejection 1.
Tacrolimus is a highly effective and widely used drug in preventing organ rejection 2,3. It is characterized by a poor oral bioavailability, high inter-patient pharmacokinetic (PK) variability (IPV) 4 and a narrow therapeutic index between 5 and 10 ng ml–1 5. These factors cause that small variations in its exposure may result in reduced immunosuppression or drug toxicity with potential serious adverse effects, such as nephrotoxicity 6.
Sources of variability in tacrolimus PK include patient age 7–9, race 10–12, food intake 13, hepatic dysfunction 14, haematocrit (Hct) 15–17, aspartate aminotransferase (AST) 18, time after transplant 4,18 and concomitant corticosteroid administration 4,15. Furthermore tacrolimus is metabolized by the 3A4 and 3A5 isoforms of cytochrome P450 (CYP) and is also a substrate of the P-glycoprotein (P-gp) transporter. Therefore genetic factors like single nucleotide polymorphisms (SNPs) in CYP3A4, CYP3A5 and ABCB1 genes have been reported as another important potential source of variability in tacrolimus PK 19.
Individuals who are homozygous carriers of the CYP3A5*3 variant allele are associated with lower clearance (CL) and smaller dose requirements than CYP3A5*1 carriers 20. The three SNPs of ABCB1 that have been reported to be implicated on the PK of tacrolimus are ABCB1 1236 T, 2677 T/A and 3435 T 3. Patients with the combination of *1/*1 or *1/*3 for CYP3A5*3 polymorphisms and CC-GG-CC for the ABCB1 exons 12-21-26 showed up to a three-fold higher tacrolimus apparent CL (CL/F). Indeed, the inclusion of these two covariates explained 24% of IPV in CL/F 21. On the other hand other studies have reported a lack of effect of ABCB1 polymorphisms on the PK of tacrolimus 22.
The PK properties of tacrolimus have been extensively studied. Most studies were focused on the adult population and only a few of them dealt with dose individualization based on population pharmacokinetic models including significant covariates like Hct, CYP3A5 genotype or post-operative days 23. To the best of our knowledge the effect of drug formulation on the PK blood profiles of tacrolimus has not been yet addressed.
On the basis of the above considerations the aims of the current work were to develop a population PK model of tacrolimus with data from Mexican paediatric renal transplant patients and to test the influence of different covariates, including CYP3A5 and ABCB1 genotypes, and formulation type on its PK properties to facilitate dose individualization.
Methods
The current PK study was based on the concentration–time profiles obtained from 53 paediatric renal transplant recipients treated in the department of nephrology at the Federico Gómez Children’s Hospital of Mexico. The study was conducted according to the principles of the revised World Medical Association’s Declaration of Helsinki 2008 and was approved by the Institutional Internal Review Boards and Ethics Committees from the hospital. Written informed consent/assent was obtained from all parents of our patients.
Drug administration and blood collection
Most patients received a standardized immunosuppressive regimen including tacrolimus, mycophenolate mofetil and prednisone (n = 44), whereas nine received tacrolimus and mycophenolate mofetil only. The twice daily tacrolimus dose (every 12 h) adjustment was performed in accordance with local practice, typically based on monitoring trough concentrations trying to maintain blood concentrations between 5 and 10 ng ml–1.
Patients received tacrolimus formulations authorized by their social security provider. Patients received one of the following tacrolimus formulations: (i) the innovator tacrolimus Prograf® (n = 29), (ii) and the generics Framebin® (n = 5), Limustin® (n = 9) or Tenacrine® (n = 3). For seven patients formulation type was not available.
One full pharmacokinetic profile per patient was obtained at steady-state. Blood samples were collected in Vacutainer® tubes with 7.2 mg of K2EDTA at times 0 (pre-dose), 0.5, 1, 2, 3, 4, 6, 8 and 12 h after the morning dose. Blood samples were stored at –70 °C until analysis.
Assay of tacrolimus
Concentrations of tacrolimus in whole blood were assessed using the chemiluminescent microparticle immunoassay (CMIA) in an ARCHITECT system (Abbott-Laboratories, Abbott Park, IL, USA) according to the manufacturer’s information as it was used previously by our research group 24.
Genotyping
We designed PCR primers in order to flank the CYP3A5/*1/*3 (dbSNP: rs776746) and ABCB1 1236C > T, 2677G > T/A and 3435C > T (dbSNP: exon 12 rs1128503, exon 21 rs2032582 and exon 26 rs1045642; http://www.ncbi.nlm.nih.gov/projects/SNP/) polymorphisms, using the CYP3A5 ENSG00000106258 and ABCB1 (MDR1) ENSG0000085563 sequences as reference (Ensembl release 65-Dec 2011© WTSI/EBI http://www.ensembl.org/index.html), and the Primer3 software (http://frodo.wi.mit.edu/). DNA was obtained from peripheral blood cells using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the recommendations of the manufacturer.
CYP3A5/*1/*3 and ABCB1 (MDR1) genotyping was performed by amplification through polymerase chain reaction (PCR) and subsequent DNA sequencing. The DNA concentration was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Sequencing reaction was performed using BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies Corporation) and after was purified using Centri-Sep columns (Princeton, Separations, Inc., Foster City, CA, USA), according to supplier specifications. The purified samples were mixed with 15 ml of Hi-Di Formamide (Life Technologies Corporation), incubated at 96 °C for 2.5 min and chilled at 4 °C. The analysis was performed on an ABI PRISM 310 Genetic Analyzer (Life Technologies Corporation). Obtained sequences were compared with the reference in order to characterize the polymorphisms present in each patient 24.
Data analysis
A total of 405 tacrolimus blood concentrations obtained from 53 patients were used to develop the population PK model for tacrolimus. The population analysis was performed with the software NONMEM version 7.2, (ICON Development Solutions) with the first order conditional estimation (FOCE) and the INTERACTION option. Concentrations of tacrolimus were obtained at steady-state conditions after an unknown number of previous oral administrations every 12 h. That study characteristic was taken into account during the analysis using the SS = 1 option provided by NONMEM 25,26. R software version 3.0.1 was used for the generation of diagnostic and evaluation plots 27.
Data were logarithmically transformed for the analysis. IPV was modelled exponentially and residual error was described using an additive error model on the logarithmic scale. Data were collected during one occasion and therefore inter-occasion variability was not tested. Only two samples were reported to be below the limit of quantification and they were not considered for the analysis.
Model selection
Selection between models was based mainly on the (i) visual inspection of the goodness of fit plots, (ii) parameter precision evaluated using the standard errors provided by NONMEM, and (iii) minimum value of the objective function provided by NONMEM approximately equal to −2 × log(likelihood) (–2LL). A decrease in –2LL of 6.63 or 10.83 points between two nested models was considered significant at the 1 and 0.1% levels, respectively. For the case of non-nested models the Akaike Information Criteria (AIC) was used instead of –2LL 28.
Model development
First the base population model was selected, followed by the covariate selection process, and finally by the evaluation of the selected population PK model.
Base population model
The first order input rate model with or without an absorption lag time (tlag), and the transit compartments model 29 were considered to describe drug absorption. With respect to drug disposition one and two compartment models were fitted to the data. Dose levels administered ranged from 0.5 to 6 mg. Dose dependent absorption, distribution and elimination were also taken into account during the analysis. The effect of dose was explored as a continuous covariate (see below). Due to the short sampling period, time dependent kinetics were not considered. The significance of the off-diagonal elements of the Ω variance-covariance matrix was also explored at this stage.
Covariate selection
The set of patient characteristics tested for significant covariate effects were age, gender, body weight (WT), body surface area (BSA), serum creatinine (CRs), haemoglobin (Hb), Hct, alanine aminotransferase (ALT), AST, serum total protein (STP), serum albumin (Alb), post-operative days, prednisone and verapamil co-administration, tacrolimus dose (DTOT), tacrolimus formulation (FOR), and CYP3A5 and ABCB1 genotype. Table1 provides a summary of the individual characteristics of the paediatric kidney transplant recipients studied.
Table 1.
Characteristics of 53 Mexican pediatric kidney transplant recipients
| Mean | SD | Median | Range | |
|---|---|---|---|---|
| Gender [male/female* (n)] | 34/19 | |||
| Age (years) | 14.6 | 3.2 | 16 | 2–19 |
| Body weight (kg) | 48.2 | 15.2 | 48 | 11.2–75.5 |
| Height (cm) | 149 | 17.8 | 153 | 81–170 |
| BSA (m2) | 1.4 | 0.3 | 1.4 | 0.5–1.9 |
| Crs (mg dl–1) | 1.1 | 0.4 | 1.1 | 0.4–2.6 |
| Hb (g dl–1) | 12.9 | 1.7 | 13.3 | 8.5–17 |
| Hct (%) | 38.4 | 5 | 39.3 | 24.2–49.1 |
| AST (U l–1) | 21.4 | 8.1 | 20 | 7–46 |
| ALT (U l–1) | 38 | 13.4 | 35 | 22–103 |
| STP (g dl–1) | 7.2 | 1.2 | 7.3 | 0.17–8.7 |
| Alb (g dl–1) | 4.2 | 0.7 | 4.3 | 0.49–5.1 |
| Post-operative days (days) | 391.6 | 327.2 | 244 | 50–1230 |
| Tacrolimus total dose (mg) | 2.3 | 1.3 | 2 | 0.5–6 |
| Tacrolimus weighted dose (mg kg–1) | 0.054 | 0.043 | 0.047 | 0.009–0.268 |
| Prednisone dose† (mg day–1) | 6.7 | 2.2 | 7.5 | 2–12.5 |
| Verapamil dose† (mg day–1) | 184.5 | 94.8 | 170 | 37.5–360 |
| CYP3A5 genotype* (n) | ||||
| *1/*1 | 3 | |||
| *1/*3 | 21 | |||
| *3/*3 | 29 | |||
| ABCB1 genotype* | ||||
| 1236C > T (n) | ||||
| C/C | 16 | |||
| C/T | 18 | |||
| T/T | 19 | |||
| 2677G > T/A (n) | ||||
| G/G | 18 | |||
| G/T† | 19 | |||
| T/T§ | 16 | |||
| 3435C > T (n) | ||||
| C/C | 13 | |||
| C/T | 22 | |||
| T/T | 18 |
For non-continuous covariates, values were the number of patients for each category (n).
Only some patients were receiving this drug.
Includes patients with genotype G/A.
Includes patients with genotype T/A.
Alb, serum albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSA, body surface area; Crs, serum creatinine; Hb, haemoglobin; Hct, haematocrit; STP, serum total protein.
The stepwise covariate model building (SCM) approach that implements the forward inclusion and backward elimination procedures was carried out using Perl Speaks NONMEM (PsN) software version 3.4.2 30. The 1 and 0.1% levels of significance were used during the forward inclusion and backward exclusion to incorporate or to keep a covariate in the model, respectively. For the case of continuous covariates the tested relationships with model parameters were not constrained only to linear models.
Model evaluation
In addition to the visual inspection of the goodness of fit plots, model performance was further evaluated by predictive checks. Prediction-corrected visual predictive checks (pcVPC) 31 were generated from a one thousand simulated dataset. The 95% prediction intervals of the 2.5th, 50th and 97.5th percentiles of the simulated data were represented together with the corresponding percentiles calculated from the raw data. The simulated data were also used to calculate per simulated study the median of the minimum and maximum blood concentration of tacrolimus within the 12 h dosing intervals (Cmax and Cmin, respectively), and the area under the blood concentration vs. time curve within the dosing interval (AUC(0,12 h)). Then the median and the 95th percentile range were compared with the corresponding values obtained from the original data. Values of Cmax, Cmin and AUC(0,12 h) defined from raw data were obtained, using a non-compartmental method with Phoenix 64 Winnonlin software version 6.3 (Pharsight, St Louis, Missouri, USA). AUC(0,12 h) was calculated using the linear trapezoidal/log interpolation method.
Precision of parameter estimates was assessed from the analysis of two thousand bootstrap datasets. Bootstrap analysis and pcVPCs were performed using both PsN and the Xpose program 27,30,32.
To select the best, median and worst fitted individuals, the mean absolute performance error was computed for each individual 33. Performance errors were computed as 100 × (Cpred – Cobs)/Cobs, where Cpred and Cobs refer to the model predicted and observed tacrolimus concentrations, respectively.
Statistical analysis
Statistical comparisons were performed using GraphPad Prism version 5.01 (GraphPad Software, San Diego, California, USA). Differences in pharmacokinetic parameters across different covariate groups (i.e. CYP3A5 polymorphism) were evaluated using Student’s t-test. When the data did not pass the D’Agostino & Pearson omnibus normality test, the Mann–Whitney test was used instead.
Results
Figure1A shows the PK profiles used in the current analysis. Data exhibited wide IPV as was observed by the high values of the coefficient of variation (CV) of the dose-normalized (i) area under the whole blood concentration–time curve from 0 to 12 h (AUC(0,12 h)/D), (ii) maximum whole blood concentration (Cmax/D) and (iii) minimum or ’trough’ whole blood concentration (Cmin/D), 67.5%, 63.7% and 74.4%, respectively. Blood concentrations of tacrolimus ranged from 1.5 to 59.8 ng ml–1. In Figure1B the mean dose-normalized PK profiles in blood are shown in which differences between formulation type are highlighted. It can be observed that one of generics, Limustin®, showed markedly lower dose-normalized concentrations that the rest of formulations. These differences in concentrations have vanished by the end of the dosing interval.
Figure 1.
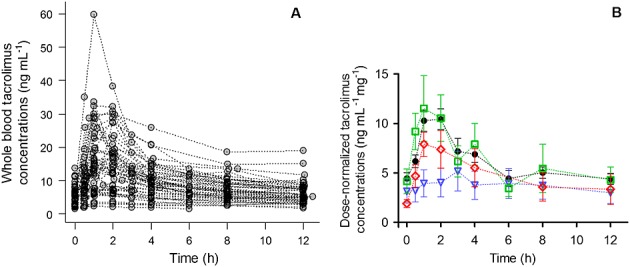
Time course of whole blood tacrolimus concentrations. A) concentration–time curves of 53 Mexican paediatric renal transplant recipients and B) mean and SE of dose-normalized concentration-time curves.  = Prograf®,
= Prograf®,  = Limustin®,
= Limustin®,  = Framebin®,
= Framebin®,  = Tenacrine®. In panel B data from patients with unknown tacrolimus formulations were omitted
= Tenacrine®. In panel B data from patients with unknown tacrolimus formulations were omitted
Model development
Base population model
The transit compartment model did not provide a significantly better fit over the first order rate model (P > 0.05). Bioavailability (F) was fixed to 1 due to absence of data after intravenous administration. The estimation of a lag time (tlag) resulted in a significant difference (P < 0.001). Disposition of tacrolimus in blood was better described with a two compartment model compared with the one compartment model (P < 0.001). The use of a three compartment model did not fit the data better (P > 0.05). IPV was supported by the data on Ka, the first order rate constant of absorption, CL/F, F and residual error. The typical estimates of Ka, tlag, CL/F, Q/F (intercompartmental distribution clearance), V/F and VT/F (apparent volumes of distribution of the central and peripheral compartments, respectively) were 0.4 h–1, 0.4 h, 20 l h–1, 47 l h–1, 36 l, and 362 l, respectively. Estimates of IPV expressed as CV (%) were 79% (Ka), 30% (CL/F), 72% (F) and 32% (residual error). The non-diagonal elements of the Ω variance-covariance matrix were not significant (P > 0.05).
Covariate selection
The full model obtained from the forward inclusion step selected the following covariate effects: (i) FOR on Ka, (ii) DTOT and FOR in the case of F and (iii) CYP3A5 and ABCB1 genotypes on CL/F. The value of –2LL decreased by 121 points with respect to the base population model. The covariate effects of ABCB1 genotype were found to be non-significant during the backward deletion step.
The inclusion of the effect of CYP3A5 genotype on CL/F made the estimate of the IPV on CL/F negligible. The final selected population PK model for tacrolimus included the following covariate relationships: (i) FOR effects on Ka and F (FOR effects were combined for Prograf®, Framebin® and Tenacrine®) and (ii) DTOT effects on F and CYP3A5 genotype on CL/F. IPV was estimated on Ka, V/F, F and residual variability. Table2 lists the estimates of the PK parameters corresponding to the selected PK model and Figure2 shows the goodness of fit plots. Model parameters were estimated with precision and the selected model showed good descriptive performance. Parameter precision evaluated through the bootstrap analysis indicated that in any case the 95% confidence intervals included the zero value. In Figure3 the individual observed and model predicted profiles for the best, median and worst fit patients carrying the CYP3A5 genotype *1/*1, *1/*3 or *3/*3 are shown.
Table 2.
Population PK parameters of final model and bootstrap validation
| PK parameter | Population mean | RSE (%) | Shrinkage (%) | IPV (%) | RSE (%) | Bootstrap (n = 2000) | ||
|---|---|---|---|---|---|---|---|---|
| Median | 5th | 95th | ||||||
| Ka (h–1) = θ1 × Ka_FOR | ||||||||
| θ1 | 0.52 | 27 | 17.5 | 37 | 38 | 0.52 | 0.39 | 0.7 |
| Limustin, Ka_FOR = 1 + θ13 | ||||||||
| Unknown formulation, Ka_FOR = 1 + θ14 | ||||||||
| θ13 | –0.76 | 6 | - | - | - | –0.76 | –0.83 | –0.69 |
| θ14 | –0.51 | 23 | - | - | - | –0.52 | –0.68 | –0.2 |
| V/F (l) | 24.16 | 39 | 14.7 | 66 | 46- | 23.71 | 14.44 | 37.5 |
| CL/F (l h–1) = θ3 × INFCYP3A5 | ||||||||
| θ3 | 11.98 | 8 | - | - | - | 12.03 | 10.67 | 13.91 |
| If CYP3A5*1/*3, INFCYP3A5 = 1 + θ8 | ||||||||
| If CYP3A5*1/*1, INFCYP3A5 = 1 + θ9 | ||||||||
| θ8 | 0.5 | 38 | - | - | - | 0.5 | 0.13 | 0.77 |
| θ9 | 0.93 | 33 | - | - | - | 0.95 | 0.16 | 2.21 |
| Q/F (l h–1) | 32.49 | 20 | - | - | - | 32.65 | 24.3 | 39.71 |
| VT/F (l) | 383.5 | 34 | - | - | - | 373.79 | 251.71 | 708.49 |
| tlag (h) | 0.39 | 6 | - | - | - | 0.39 | 0.35 | 0.43 |
| F (%) = 100 × FDTOT × FFOR | 100* | - | –0.47 | 38 | 22 | - | - | - |
| FDTOT = e[(θ10 × (Dose–2)]) | ||||||||
| θ10 | –0.3 | 19 | - | - | - | –0.3 | –0.4 | –0.22 |
| FFOR | ||||||||
| Limustin, FFOR = 1 + θ11 | ||||||||
| Unknown formulation, FFOR = 1 + θ12 | ||||||||
| θ11 | –0.53 | 22 | - | - | - | –0.53 | –0.67 | –0.3 |
| θ12 | –0.53 | 16 | - | - | - | –0.51 | –0.66 | –0.34 |
| Residual error [ln (ng ml–1)] | 0.12 | 8 | –0.33 | 35 | 49 | 0.12 | 0.11 | 0.14 |
assumed as 100%, was not estimated. CL/F, apparent blood clearance; CYP3A5, genotype of gene coding cytochrome P450-5; F, relative bioavailabilty; FDTOT, effect of the tacrolimus total dose on F; FFOR, effect of FOR on F; FOR, tacrolimus formulation that could be Prograf®, Limustin®, Framebin®, Tenacrine® or unknown; IPV, inter-patient variability; INFCYP3A5, influence of CYP3A5 genotype; KA, absorption first order rate constant; KA_FOR, effect of FOR on KA; Q/F, apparent intercompartmental clearance; RSE, relative standard error; tlag, lag time in absorption; V/F and VT/F, apparent volume of distribution volume of the central and peripheral compartments, respectively.
Figure 2.
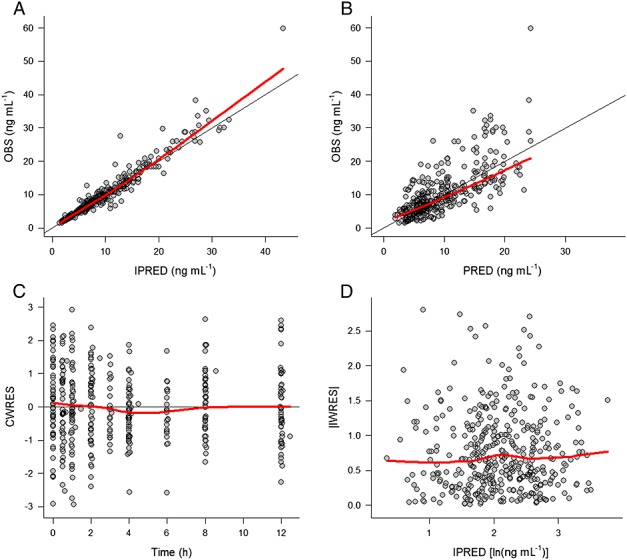
Goodness of fit plots corresponding to the final population PK model. PRED and IPRED, typical and individual model predictions, respectively; OBS, observation; CWRES, conditional weighted residuals, IWRES, individual weighted residuals. Black solid lines indicate perfect fit, red solid lines show a smooth (loess) through the data
Figure 3.
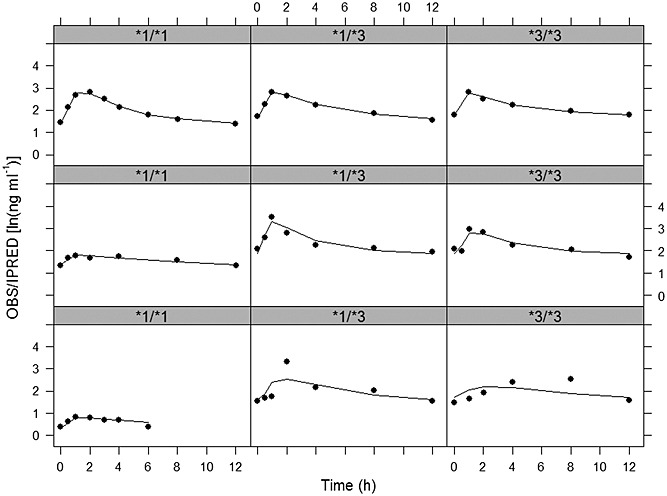
Individual observed (circles) and model predicted (solid lines) tacrolimus concentrations vs. time profiles for the best (upper panels), median (middle panels) and worst (lower panels) fitted patients in the studied population for the CYP3A5 *1/*1 (first column), *1/*3 (second column) and *3/*3 (third column) genotype
The selected model predicts values of CL/F of 23.1, 17.97, and 11.98 l h–1 for patients carrying CYP3A5*1/*1, CYP3A5*1/*3, and CYP3A5*3/*3, respectively. Including the covariate effects of FOR on Ka and FOR and DTOT on F reduced the magnitude of IPV almost by a half, from 79 to 37% (Ka) and from 72 to 38% (F). It was found that one of the generic formulations (Limustin®) was associated to a 50% lower relative bioavailability with respect the innovator Prograf®.
Figure4 shows the effect of the selected covariates on the blood PK profiles of tacrolimus: formulation type on Ka and F (A), CYP3A5 genotype on CL/F (B), and dose effects on F explored on the dose-normalized blood concentration profiles (C).
Figure 4.
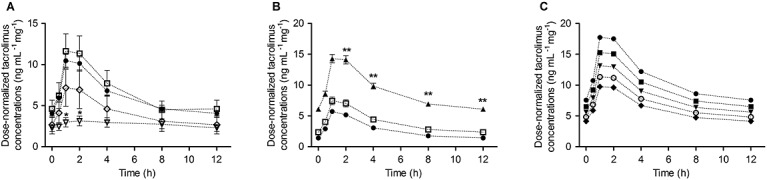
Effect of covariates on typical PK profiles of tacrolimus: A) formulation type, B) CYP3A5 genotype assuming the administration of Prograf® and C) total dose of tacrolimus (DTOT) assuming the administration of Prograf® (dose normalized blood concentration profiles)  Prograf®,
Prograf®,  Limustin®,
Limustin®,  Framebin®,
Framebin®,  Tenacrine®,
Tenacrine®,  *1/*1,
*1/*1,  *1/*3,
*1/*3,  *3/*3,
*3/*3,  0.5 mg,
0.5 mg,  1 mg,
1 mg,  1.5 mg,
1.5 mg,  2 mg,
2 mg,  2.5 mg
2.5 mg
Model evaluation Figure5 shows the pc-VPC corresponding to the complete studied population (A) or stratified by CYP3A5 genotype (B) where it can be observed that the median tendency and the dispersion of the data appear to be very well captured by the model. Proper model performance is also confirmed by the results listed in the online supporting information (online supporting information 2, Table S1, and online supporting information 3, Table S2) where the median of Cmin, Cmax, AUC(0,12 h) and AUC(0,12 h)/D calculated from the raw data are well represented by the corresponding values obtained after simulations.
Figure 5.
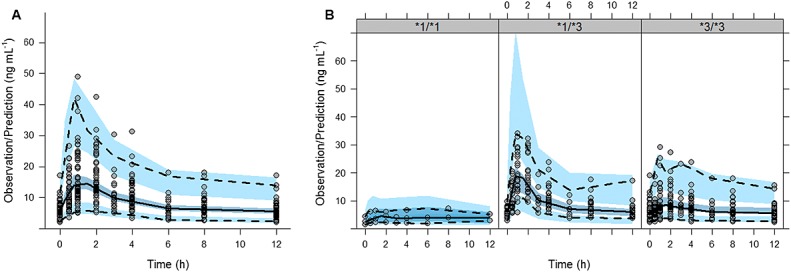
Evaluation of the final model using a prediction-corrected visual predictive check. A) entire population, B) stratified by genotype. Comparison between the observed whole blood tacrolimus concentrations (circles) and the 2.5th (bottom dotted line) 50th (solid line) and 97.5th (top dotted line) percentiles of the raw data, with the 2.5th, 50th, and 97.5th prediction intervals (blue areas) of the corresponding percentiles obtained from 1000 simulated datasets
To explore the role that the inclusion of generic formulations might have on the modelling results, the selected population pharmacokinetic model was fitted only to data from the patients who received the innovator formulation of tacrolimus. The covariate effects of the formulation type were removed from the model for this last analysis. Parameter estimates are shown in the online supporting information (online supporting information 4, Table S3). In general the results were quite comparable between the two analyses (with or without the patients receiving the generic formulations), and the only discrepancies were seen in Ka and V/F, despite the fact that the total apparent volumes of distribution [(V + VT)/F] were similar, 407.7 vs. 353.8 l. It should be noted that the latter analysis was performed using data from 29 patients, instead of 53, which might explain part of the differences found.
Dose individualization
Based on the individual estimates of the PK parameters obtained from the selected model an individualization guide was proposed for tacrolimus dosing using the following expression and considering the selected covariates, CYP3A5 genotype, DTOT, FOR:
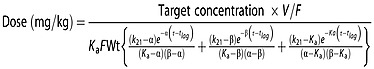 |
where α and β are disposition macro-constants, k21 is the distribution micro-constant governing the drug transfer from the peripheral to the central compartment, τ is the dosing interval (12 h) and Wt is the mean population body weight (kg). The relationship between macro and microconstants was established using standard formulae 34.
The dose of tacrolimus required to get a trough target concentration of 6 ng ml–1 was predicted for one thousand virtual patients in each of the combination between CYP3A5 genotype and formulation type (Prograf® and Limustin®). The medians of the doses predicted for individuals taking Prograf® with CYP3A5 genotype *1/*1, *1/*3 and *3/*3 were 0.18, 0.14 and 0.1 mg kg–1 day–1, respectively. For patients taking Limustin® in all cases the predicted doses were significantly higher (P < 0.001) with values of 0.26, 0.22 and 0.18 mg kg–1 day–1 for the CYP3A5 *1/*1, *1/*3 and *3/*3 genotypes, respectively. Figure6 shows the distribution of the predicted dose across the different CYP3A5 genotypes assuming the patients received the innovator Prograf® or the generic formulation Limustin®.
Figure 6.
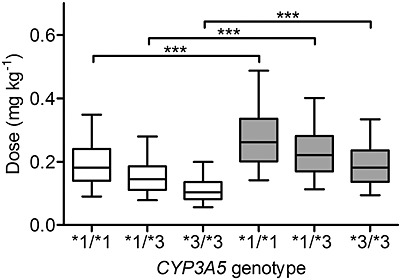
Prediction of tacrolimus doses required to reach a trough target whole blood concentration of 6 ng ml–1 for individuals receiving the innovator tacrolimus Prograf® (white boxes) or the generic formulation Limustin® (grey boxes) by CYP3A5 genotype. The plot shows the results from 1000 simulations performed for each condition (formulation and genotype). The boxes represent the median, 25th and 75th percentiles of the simulated data and the whiskers represent 5th and 95th percentiles. *** P < 0.001
Discussion
Our study aimed to describe for the first time the PK profiles of tacrolimus in a population of Mexican paediatric renal transplant patients evaluating different potential covariates that might have a significant effect on its PK characteristics, such as demographics, clinical data and genetic polymorphisms of ABCB1 and CYP3A5 as well as differences in tacrolimus formulation.
A recent pharmacogenetic study from our research group reported a statistically significant difference in the frequency of the functional (expresser) and non-functional (non-expressers) phenotypes of CYP3A5 in Mexican renal transplant recipients in comparison with those reported for Blacks and Caucasians. On the other hand no differences were found between the Mexican and South Asian populations 24. It was also found that patients who were homozygous and heterozygous expressers (genotypes CYP3A5*1/*1 and *1/*3, respectively) showed a median requirement of 0.16 and 0.13 mg kg–1 day–1 respectively, which was significantly higher than homozygous non-expresser patients (CYP3A5*3/*3) with a median requirement of 0.07 mg kg–1 day–1. No significant differences in dose requirements were observed when comparing children vs. adults 24.
In the current study our population were 53 paediatric renal transplant recipients who had tacrolimus dose requirements with medians of 0.18 and 0.13 mg kg–1 day–1 for CYP3A5*1/*1 and CYP3A5*1/*3 genotypes, respectively, and 0.06 mg kg–1 day–1 for the CYP3A5 *3/*3 carriers. The ultimate aim of the current analysis was to provide a tool to individualize better the dose of tacrolimus in a paediatric population of renal transplant patients.
The PK properties of tacrolimus have been studied mainly in adults 35. The few studies addressing the PK of tacrolimus in the paediatric population found that in general CL/F and V/F in children were about two-fold greater with respect to adult patients 36,37. Our results are in agreement with these observations. Whereas some studies reported that a one compartment model sufficed to describe the data 4,18,23,38, in general, as in our case, the two compartment model was selected 17,21,22,35,39,40. With regard to the typical PK estimates, the values obtained in the current evaluation were similar to those published elsewhere: tlag = 0.39 h 35,41, V/F = 24.16 l 35,39, VT/F =383.5 l 22,41 and Q/F = 32.49 l h–1 21,22,41,42. Regarding CL/F, its population estimate value was 11.98 l h–1 which is in accordance with previous studies 22,23,35,40 as well as the estimate of Ka (0.52 h-1) 18,35.
Our analysis detected significant effects of the CYP3A5 genotypes on CL/F, as other studies have done in the past 21–23,35,38–40,43. CL/F in patients with CYP3A5*1/*3 and *1/*1 genotypes was 50% and 92.9%, respectively, higher than patients with the CYP3A5*3/*3 genotype. Moreover and as other previous reports 22,23,39,44, our study did not find enough evidence of ABCB1 genotype effects on CL/F to be included in the selected model. Therefore the influence of ABCB1 remains controversial 21,45.
On the other hand some studies have found significant covariate effects such as Hct, post-operative days, AST or concomitant administration of corticosteroids as the dose of prednisone on CL/F 4,17,18,23,35,38,39, but this was was not the case in the current evaluation. We further explored the potential significant effect of the above mentioned covariates adding them to the selected population PK model using the same reported covariate relationships 23,38, but no significant effects were detected (P > 0.05). Abnormally low Hct values (<33%) have been related to a reduced fraction of tacrolimus bound to red blood cells and an increased plasma fraction, which is more readily metabolized by the liver resulting in a higher tacrolimus CL/F 17,23,38. AST concentrations were identified as a marker of liver injury 46 and when they are abnormally high (≥200 U l–1), the hepatic tacrolimus elimination can be reduced. In our study population the values of Hct and AST were within normal values of 38.4 ± 5% and 21.4 ± 8.1 U l–1 respectively. Similarly, very high prednisone doses (>25 mg day–1) have been reported to increase tacrolimus CL/F up to 1.6-fold 4, but in our study the mean co-administered prednisone dose was 6.7 ± 2.2 mg day–1. Furthermore some studies in both adult 4,47,48 and paediatric patients 49 have identified that the increase in post-operative days is related to an increase in tacrolimus CL/F probably because, immediately after surgery, changes in gastrointestinal motility occur and are associated with alterations in drug metabolism resulting in low CL/F. Gastrointestinal motility recovers to baseline conditions 2 months after transplantation 4. In the current evaluation the patients were in a stable condition with a mean post-operative day value of 391.6 ± 327.2 days.
In recent years the use of generic formulations of tacrolimus and other immunosuppressive drugs has increased. Given the clinical importance of a drug such as tacrolimus with a narrow therapeutic index, it is essential that generic formulations be identical or bioequivalent to the branded formulation. In 2008 Petan et al 50 carried out a study analyzing the physicochemical properties of five generic formulations against innovator tacrolimus Prograf®, and concluded that the generics tested were not bioequivalent, with the subsequent risk for transplant patients. Other studies have also reported that switching the treatment from the innovator to a generic formulation resulted in significant decreases in blood concentrations of tacrolimus, indicating that when this type of switch in therapy with tacrolimus or other narrow therapeutic index drugs is performed, meticulous clinical care and therapeutic monitoring is required 51–53. We have found similar findings in the current evaluation, quantifying the magnitude in AUC(0,12 h). A recent study reported impaired dissolution and lower content of tacrolimus in a generic formulation with respect to the innovator Prograf® 54. In our analysis the covariate relationship obtained in the final model between formulation type and Ka and F is likely representing these limitations (Table2). The effects of CYP3A5 genotype on CL/F, and tacrolimus dose on F, respectively, were confirmed in an analysis of the subset of patients receiving the innovator formulation Prograf®, indicating that the pooled analysis of drug concentration data from different formulations, some of them reported as non-optimal formulations, did not hamper in our case, the proper characterization of tacrolimus in renal transplant paediatric patients.
In conclusion a population PK model was built and validated to describe the time course of tacrolimus blood concentrations from 53 Mexican paediatric transplant recipients receiving different tacrolimus formulations including the branded product or generic formulations. The CYP3A5 genotype and tacrolimus dose showed a significant influence on CL/F and F, respectively. In addition significant formulation effects were found on Ka and F. An estimation of the tacrolimus dose was calculated based on the final population PK model which is expected to improve the dosing in the routine clinical practice.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work. GCH had personal fees from Pfizer, Roche, Laboratorios Sophia, Grünenthal, Novartis, Abbvie, Amgen, MSD, outside the submitted work.
This study was supported by CONACYT, grant 181368, FOSISS/CONACYT 2008/COI/8727, and Hospital Infantil de México protocols HIM 2007/019 and HIM 2011/026. C.O. Jacobo-Cabral received a CONACYT fellowship 47866. M. Medeiros received a CONACYT grant 205627.
The authors wish to thank María de Lourdes González Flores for technical assistance in sample processing.
Supporting Information
Table S1 Predictive checks
Table S2 Predictive checks stratified according to CYP3A5 genotype
Table S3 Population PK parameters of the final model without the formulation influence (only data of patients receiving Prograf® were modelled)
Supporting info item
References
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–29. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- Duncan MD, Wilkes DS. Transplant-related immunosuppression: a review of immunosuppression and pulmonary infections. Proc Am Thorac Soc. 2005;2:449–55. doi: 10.1513/pats.200507-073JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–53. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- Antignac M, Barrou B, Farinotti R, Lechat P, Urien S. Population pharmacokinetics and bioavailability of tacrolimus in kidney transplant patients. Br J Clin Pharmacol. 2007;64:750–7. doi: 10.1111/j.1365-2125.2007.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, McMichael J, Lever J, Burckart G, Starzl T. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404–30. doi: 10.2165/00003088-199529060-00003. [DOI] [PubMed] [Google Scholar]
- Johnston A. Equivalence and interchangeability of narrow therapeutic index drugs in organ transplantation. Eur J Hosp Pharm: Science and Practice. 2013;20:302–07. doi: 10.1136/ejhpharm-2012-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido S, Asanuma H, Tajima E, Honda M, Nakai H. Pharmacokinetics of tacrolimus in pediatric renal transplant recipients. Transplant Proc. 2001;33:1066–8. doi: 10.1016/s0041-1345(00)02418-0. [DOI] [PubMed] [Google Scholar]
- McDiarmid SV, Colonna JO, 2nd, Shaked A, Vargas J, Ament ME, Busuttil RW. Differences in oral FK506 dose requirements between adult and pediatric liver transplant patients. Transplantation. 1993;55:1328–32. doi: 10.1097/00007890-199306000-00022. [DOI] [PubMed] [Google Scholar]
- Przepiorka D, Blamble D, Hilsenbeck S, Danielson M, Krance R, Chan KW. Tacrolimus clearance is age-dependent within the pediatric population. Bone Marrow Transplant. 2000;26:601–5. doi: 10.1038/sj.bmt.1702588. [DOI] [PubMed] [Google Scholar]
- Mancinelli LM, Frassetto L, Floren LC, Dressler D, Carrier S, Bekersky I, Benet LZ, Christians U. The pharmacokinetics and metabolic disposition of tacrolimus: a comparison across ethnic groups. Clin Pharmacol Ther. 2001;69:24–31. doi: 10.1067/mcp.2001.113183. [DOI] [PubMed] [Google Scholar]
- Felipe CR, Silva HT, Machado PG, Garcia R, da Silva Moreira SR, Pestana JO. The impact of ethnic miscegenation on tacrolimus clinical pharmacokinetics and therapeutic drug monitoring. Clin Transplant. 2002;16:262–72. doi: 10.1034/j.1399-0012.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–23. doi: 10.1038/clpt.2008.141. [DOI] [PubMed] [Google Scholar]
- Bekersky I, Dressler D, Mekki QA. Effect of low- and high-fat meals on tacrolimus absorption following 5 mg single oral doses to healthy human subjects. J Clin Pharmacol. 2001;41:176–82. doi: 10.1177/00912700122009999. [DOI] [PubMed] [Google Scholar]
- Jain AB, Venkataramanan R, Cadoff E, Fung JJ, Todo S, Krajack A, Starzl TE. Effect of hepatic dysfunction and T tube clamping on FK 506 pharmacokinetics and trough concentrations. Transplant Proc. 1990;22:57–9. [PMC free article] [PubMed] [Google Scholar]
- Undre NA, Schafer A. Factors affecting the pharmacokinetics of tacrolimus in the first year after renal transplantation. European Tacrolimus Multicentre Renal Study Group. Transplant Proc. 1998;30:1261–3. doi: 10.1016/s0041-1345(98)00234-6. [DOI] [PubMed] [Google Scholar]
- Minematsu T, Sugiyama E, Kusama M, Hori S, Yamada Y, Ohtani H, Sawada Y, Sato H, Takayama T, Sugawara Y, Makuuchi M, Iga T. Effect of hematocrit on pharmacokinetics of tacrolimus in adult living donor liver transplant recipients. Transplant Proc. 2004;36:1506–11. doi: 10.1016/j.transproceed.2004.04.097. [DOI] [PubMed] [Google Scholar]
- Benkali K, Premaud A, Picard N, Rerolle JP, Toupance O, Hoizey G, Turcant A, Villemain F, Le Meur Y, Marquet P, Rousseau A. Tacrolimus population pharmacokinetic-pharmacogenetic analysis and Bayesian estimation in renal transplant recipients. Clin Pharmacokinet. 2009;48:805–16. doi: 10.2165/11318080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Staatz CE, Willis C, Taylor PJ, Tett SE. Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin Pharmacol Ther. 2002;72:660–9. doi: 10.1067/mcp.2002.129304. [DOI] [PubMed] [Google Scholar]
- Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. 2010;49:141–75. doi: 10.2165/11317350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- de Jonge H, Naesens M, Kuypers DR. New insights into the pharmacokinetics and pharmacodynamics of the calcineurin inhibitors and mycophenolic acid: possible consequences for therapeutic drug monitoring in solid organ transplantation. Ther Drug Monit. 2009;31:416–35. doi: 10.1097/FTD.0b013e3181aa36cd. [DOI] [PubMed] [Google Scholar]
- Musuamba FT, Mourad M, Haufroid V, Delattre IK, Verbeeck RK, Wallemacq P. Time of drug administration, CYP3A5 and ABCB1 genotypes, and analytical method influence tacrolimus pharmacokinetics: a population pharmacokinetic study. Ther Drug Monit. 2009;31:734–42. doi: 10.1097/FTD.0b013e3181bf8623. [DOI] [PubMed] [Google Scholar]
- Shi XJ, Geng F, Jiao Z, Cui XY, Qiu XY, Zhong MK. Association of ABCB1, CYP3A4*18B and CYP3A5*3 genotypes with the pharmacokinetics of tacrolimus in healthy Chinese subjects: a population pharmacokinetic analysis. J Clin Pharmacol Ther. 2011;36:614–24. doi: 10.1111/j.1365-2710.2010.01206.x. [DOI] [PubMed] [Google Scholar]
- Han N, Yun HY, Hong JY, Kim IW, Ji E, Hong SH, Kim YS, Ha J, Shin WG, Oh JM. Prediction of the tacrolimus population pharmacokinetic parameters according to CYP3A5 genotype and clinical factors using NONMEM in adult kidney transplant recipients. Eur J Clin Pharmacol. 2013;69:53–63. doi: 10.1007/s00228-012-1296-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Roca P, Medeiros M, Reyes H, Rodriguez-Espino BA, Alberu J, Ortiz L, Vasquez-Perdomo M, Elizondo G, Morales-Buenrostro LE, Mancilla Urrea E, Castaneda-Hernandez G. CYP3A5 polymorphism in Mexican renal transplant recipients and its association with tacrolimus dosing. Arch Med Res. 2012;43:283–7. doi: 10.1016/j.arcmed.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Beal S, Sheiner L, Boeckmann A. NONMEM Users Guide (1989–2006) Ellicott City, MD, USA: Icon Development Solutions; 2006. [Google Scholar]
- Bauer R. NONMEM Users Guide Introduction to NONMEM 7.2. 0. Ellicott City, MD: ICON Development Solutions; 2011. [Google Scholar]
- Team RC. R: A Language and Environment for Statistical Computing. Austria: Vienna: R Foundation for Statistical Computing. In; 2013. [Google Scholar]
- Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm. 1994;22:431–45. doi: 10.1007/BF02353864. [DOI] [PubMed] [Google Scholar]
- Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit--a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- Troconiz IF, Armenteros S, Planelles MV, Benitez J, Calvo R, Dominguez R. Pharmacokinetic-pharmacodynamic modelling of the antipyretic effect of two oral formulations of ibuprofen. Clin Pharmacokinet. 2000;38:505–18. doi: 10.2165/00003088-200038060-00004. [DOI] [PubMed] [Google Scholar]
- Rowland M, Tozer TN. Clinical pharmacokinetics. Concepts and applications 3rd edn. Baltimore: Williams and Wilkins; 1995. [Google Scholar]
- Zhao W, Elie V, Roussey G, Brochard K, Niaudet P, Leroy V, Loirat C, Cochat P, Cloarec S, Andre JL, Garaix F, Bensman A, Fakhoury M, Jacqz-Aigrain E. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;86:609–18. doi: 10.1038/clpt.2009.210. [DOI] [PubMed] [Google Scholar]
- Wallemacq PE, Furlan V, Moller A, Schafer A, Stadler P, Firdaous I, Taburet AM, Reding R, Clement De Clety S, De Ville De Goyet J, Sokal E, Lykavieris L, Van Leeuw V, Bernard O, Otte JB, Undre NA. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur J Drug Metab Pharmacokinet. 1998;23:367–70. doi: 10.1007/BF03192295. [DOI] [PubMed] [Google Scholar]
- Wallemacq PE, Verbeeck RK. Comparative clinical pharmacokinetics of tacrolimus in paediatric and adult patients. Clin Pharmacokinet. 2001;40:283–95. doi: 10.2165/00003088-200140040-00004. [DOI] [PubMed] [Google Scholar]
- Zuo XC, Ng CM, Barrett JS, Luo AJ, Zhang BK, Deng CH, Xi LY, Cheng K, Ming YZ, Yang GP, Pei Q, Zhu LJ, Yuan H, Liao HQ, Ding JJ, Wu D, Zhou YN, Jing NN, Huang ZJ. Effects of CYP3A4 and CYP3A5 polymorphisms on tacrolimus pharmacokinetics in Chinese adult renal transplant recipients: a population pharmacokinetic analysis. Pharmacogenet Genomics. 2013;23:251–61. doi: 10.1097/FPC.0b013e32835fcbb6. [DOI] [PubMed] [Google Scholar]
- Press RR, Ploeger BA, den Hartigh J, van der Straaten T, van Pelt J, Danhof M, de Fijter JW, Guchelaar HJ. Explaining variability in tacrolimus pharmacokinetics to optimize early exposure in adult kidney transplant recipients. Ther Drug Monit. 2009;31:187–97. doi: 10.1097/FTD.0b013e31819c3d6d. [DOI] [PubMed] [Google Scholar]
- Benkali K, Rostaing L, Premaud A, Woillard JB, Saint-Marcoux F, Urien S, Kamar N, Marquet P, Rousseau A. Population pharmacokinetics and Bayesian estimation of tacrolimus exposure in renal transplant recipients on a new once-daily formulation. Clin Pharmacokinet. 2010;49:683–92. doi: 10.2165/11535950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Grover A, Frassetto LA, Benet LZ, Chakkera HA. Pharmacokinetic differences corroborate observed low tacrolimus dosage in native American renal transplant patients. Drug Metab Dispos. 2011;39:2017–9. doi: 10.1124/dmd.111.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaïes E, Mongi Bacha M, Woillard J-B, Eljebari H, Helal I, Abderrahim E, Jebabli N, Saint-Marcoux F, Marquet P, Abdallah TB, Kheder A, Gorji Y, Lakhal M, Klouz A. Tacrolimus population pharmacokinetics and Bayesian estimation in Tunisian renal transplant recipients. Int J Pharm Pharmaceut Sci. 2013;5(3):108–15. [Google Scholar]
- Zhao W, Fakhoury M, Baudouin V, Storme T, Maisin A, Deschenes G, Jacqz-Aigrain E. Population pharmacokinetics and pharmacogenetics of once daily prolonged-release formulation of tacrolimus in pediatric and adolescent kidney transplant recipients. Eur J Clin Pharmacol. 2013;69:189–95. doi: 10.1007/s00228-012-1330-6. [DOI] [PubMed] [Google Scholar]
- Rong G, Jing L, Deng-Qing L, Hong-Shan Z, Shai-Hong Z, Xin-Min N. Influence of CYP3A5 and MDR1(ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in Chinese renal transplant recipients. Transplant Proc. 2010;42:3455–8. doi: 10.1016/j.transproceed.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Wang J, Zeevi A, McCurry K, Schuetz E, Zheng H, Iacono A, McDade K, Zaldonis D, Webber S, Watanabe RM, Burckart GJ. Impact of ABCB1 (MDR1) haplotypes on tacrolimus dosing in adult lung transplant patients who are CYP3A5 *3/*3 non-expressors. Transpl Immunol. 2006;15:235–40. doi: 10.1016/j.trim.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- Antignac M, Hulot JS, Boleslawski E, Hannoun L, Touitou Y, Farinotti R, Lechat P, Urien S. Population pharmacokinetics of tacrolimus in full liver transplant patients: modelling of the post-operative clearance. Eur J Clin Pharmacol. 2005;61:409–16. doi: 10.1007/s00228-005-0933-6. [DOI] [PubMed] [Google Scholar]
- Staatz CE, Willis C, Taylor PJ, Lynch SV, Tett SE. Toward better outcomes with tacrolimus therapy: population pharmacokinetics and individualized dosage prediction in adult liver transplantation. Liver Transplantation. 2003;9:130–7. doi: 10.1053/jlts.2003.50023. [DOI] [PubMed] [Google Scholar]
- Yasuhara M, Hashida T, Toraguchi M, Hashimoto Y, Kimura M, Inui K, Hori R, Inomata Y, Tanaka K, Yamaoka Y. Pharmacokinetics and pharmacodynamics of FK 506 in pediatric patients receiving living-related donor liver transplantations. Transplant Proc. 1995;27:1108–10. [PubMed] [Google Scholar]
- Petan JA, Undre N, First MR, Saito K, Ohara T, Iwabe O, Mimura H, Suzuki M, Kitamura S. Physiochemical properties of generic formulations of tacrolimus in Mexico. Transplant Proc. 2008;40:1439–42. doi: 10.1016/j.transproceed.2008.03.091. [DOI] [PubMed] [Google Scholar]
- Abdulnour HA, Araya CE, Dharnidharka VR. Comparison of generic tacrolimus and Prograf drug levels in a pediatric kidney transplant program: brief communication. Pediatr Transplant. 2010;14:1007–11. doi: 10.1111/j.1399-3046.2010.01393.x. [DOI] [PubMed] [Google Scholar]
- Johnston A, Keown PA, Holt DW. Simple bioequivalence criteria: are they relevant to critical dose drugs? Experience gained from cyclosporine. Ther Drug Monit. 1997;19:375–81. doi: 10.1097/00007691-199708000-00002. [DOI] [PubMed] [Google Scholar]
- Johnston A. Equivalence and interchangeability of narrow therapeutic index drugs in organ transplantation. Eur J Hosp Pharm: Science and practice. 2013;20:302–07. doi: 10.1136/ejhpharm-2012-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo-Cabral CO, Garcia-Roca P, Reyes H, Lozada-Rojas L, Cruz-Antonio L, Medeiros M, Castaneda-Hernandez G. Limustin®, a non-innovator tacrolimus formulation, yields reduced drug exposure in pediatric renal transplant recipients. Pediatr Transplant. 2014;18:706–13. doi: 10.1111/petr.12335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


