Abstract
Aim
Interleukin-6 (IL-6), a multifunctional cytokine, exists in several forms ranging from a low molecular weight (MW 20–30 kDa) non-complexed form to high MW (200–450 kDa), complexes. Accurate baseline IL-6 assessment is pivotal to understand clinical responses to IL-6-targeted treatments. Existing assays measure only the low MW, non-complexed IL-6 form. The present work aimed to develop a validated assay to measure accurately total IL-6 (complexed and non-complexed) in serum or plasma as matrix in a high throughput and easily standardized format for clinical testing.
Methods
Commercial capture and detection antibodies were screened against humanized IL-6 and evaluated in an enzyme-linked immunosorbent assay format. The best antibody combinations were screened to identify an antibody pair that gave minimum background and maximum recovery of IL-6 in the presence of 100% serum matrix. A plate-based total IL-6 assay was developed and transferred to the Meso Scale Discovery (MSD) platform for large scale clinical testing.
Results
The top-performing antibody pair from 36 capture and four detection candidates was validated on the MSD platform. The lower limit of quantification in human serum samples (n = 6) was 9.77 pg l–1, recovery ranged from 93.13–113.27%, the overall pooled coefficients of variation were 20.12% (inter-assay) and 8.67% (intra-assay). High MW forms of IL-6, in size fractionated serum samples from myelodysplastic syndrome and rheumatoid arthritis patients, were detected by the assay but not by a commercial kit.
Conclusion
This novel panoptic (sees all forms) IL-6 MSD assay that measures both high and low MW forms may have clinical utility.
Keywords: cancer, interleukin-6, panoptic assay, rheumatoid arthritis
What is Already Known about this Subject
IL-6 plays a key role in a wide range of normal and disease-related biological activities and is an attractive target for drug development
IL-6 exists in non-complexed and complexed forms with other proteins.
Current commercially available assays that do not detect all forms of IL-6 pose an impediment to understanding the full therapeutic effect of anti-IL-6 agents.
What this Study Adds
A validated panoptic IL-6 MSD assay was developed that measures low and high MW IL-6 in human serum samples.
The panoptic IL-6 MSD assay may provide insight into the role of IL-6 in normal and disease processes.
Introduction
Interleukin-6 (IL-6) is a pleiotropic cytokine that plays a key role in a wide range of diverse biological activities, such as immune regulation, haematopoiesis, oncogenesis, inflammatory processes, host immune defense mechanism, modulation of cellular growth, maintaining body temperature, stimulation of acute phase protein and maturation of neutrophils in the bone marrow 1,2. Elevated IL-6 production has been implicated in a large number of cancers, including multiple myeloma, endometrial cancer, lung cancer, renal cell carcinoma, cervical cancer, breast cancer, metastatic hormone-refractory prostate cancer, ovarian cancer and Castleman’s disease 3–8. IL-6 plays an important role in tumour behaviour, such as cell migration, invasion, growth of malignancies 9, proliferation, apoptosis 10, progression 11, angiogenesis and differentiation 12. IL-6 has also been implicated in autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus for its role in driving increased local and systemic inflammation 13. Given its role in these diseases, the reduction of supraphysiologic IL-6 concentrations represents an attractive target for drug development 14.
Multiple therapeutic approaches have been tried to reduce IL-6 activity, including targeting the IL-6 receptor (IL-6R), thereby blocking the binding site of IL-6. An alternative approach has been to target IL-6 directly and inhibit its action. IL-6 can bind to both membrane and soluble IL-6R and activate IL-6 signalling. Tocilizumab is an anti-human IL-6R monoclonal antibody that specifically binds to IL-6R and competitively inhibits IL-6 binding, thus inhibiting the biological activity of IL-6 15. Sirukumab (CNTO 136) is a human anti–IL-6 immunoglobulin G1 (IgG1) kappa antibody under development by Janssen Pharmaceuticals in collaboration with GlaxoSmithKline and is currently being evaluated in a phase III trial for the treatment of moderate to severe RA. Siltuximab (CNTO 328) is a humanized anti–IL-6 monoclonal antibody being developed by Janssen Pharmaceuticals for the treatment of multicentric Castleman’s disease and potentially smoldering multiple myeloma 8,16. Both antibodies in development bind to IL-6 and attenuate the downstream IL-6 signalling pathway.
Localized and systemic concentrations of IL-6 can vary depending on the stage of the disease. Elevated systemic IL-6 has been correlated with poor prognoses and shorter disease-free survival across a range of cancer types 17. With the awareness that there is a potential role for IL-6 as a prognostic marker and the development of new inhibitory therapies it is critical to measure accurately the circulating concentrations of IL-6.
Elevated IL-6 concentrations produced at the site of tissue damage can ultimately influence increases in systemic IL-6 burden that may drive acute phase response and other physiologic changes. The systemic role of IL-6 is heavily dependent on the transport of IL-6, which has been reported to exist in different forms or complexes. It has been shown that IL-6 exists not only as non-complexed monomers, dimers and trimers, but also in complex with various partner or chaperone proteins 18. Also, IL-6 is a 185-amino acid protein that is post-translationally modified by glycosylation and phosphorylation with five different molecular forms synthesized in the range of 21.5 to 28 kDa 19,20. Ndubuisi et al. 26 have shown that many enzyme-linked immunosorbent assays (ELISAs) adequately measure only the low molecular weight or non-complexed forms of IL-6 (25–30 kDa) and fail to recognize complexed IL-6 eluting in the range of 200 to 450 kDa. Using the standard, commercially available IL-6 assays (high sensitivity ELISA and Meso Scale Discovery [MSD]), complexed IL-6 is not quantified. Identification of specific combinations of antibodies that recognize complexed IL-6 is an unmet need and therefore, the primary goal of this study was to develop an assay to measure all forms of IL-6, which we termed the ‘panoptic IL-6 MSD assay’ or ‘one that sees all forms’.
To find the right combination of antibodies that could detect all forms of IL-6 or ‘total IL-6’, 36 commercially available antibodies were screened as capture antibodies in an ELISA plate-based format with the four best performing detection antibodies. Four of the best capture/detection antibody combinations were then screened to find a pair that gave minimum background and maximum recovery of IL-6 in the presence of 100% serum matrix. The top-performing antibody pair was transferred to the MSD platform to reduce serum volume requirements and increase the signal-to-noise ratio. In order to confirm that the panoptic IL-6 MSD assay recognized all forms of IL-6, serum samples were separated by size exclusion chromatography, and fractions were collected and assayed for IL-6 on the newly developed MSD assay. This panoptic IL-6 MSD assay may be useful to evaluate total IL-6 concentrations across normal and diseased indications for greater understanding of IL-6 functionality and response and/or resistance to anti–IL-6 therapeutics.
Methods
Ethics statement
In this study human plasma samples were purchased from a commercial vendor with no restrictions of use. All donors provided written informed consent prior to blood collection. The blood collection procedures and consent forms used by the vendor were approved by the Institutional Review Board of Schulman Associates, 4445 Lake Forest Drive, Suite 300, Cincinnati, Ohio 45242, USA.
Collection of serum samples
Commercially purchased normal human serum samples (Bioreclamation, Westbury, NY, USA) were used to develop and validate the various assays. Samples from patients with RA and multiple myeloma, breast, prostate, ovarian and pancreatic cancers were also purchased from Bioreclamation in order to establish the reference range for the panoptic IL-6 MSD assay. Blood samples were collected (usually in the morning) from the antecubital area of consenting healthy volunteers by licensed phlebotomists and allowed to clot in anticoagulant-free 450 to 500 ml dry collection bags (Terumo 1BB*D606A) at 4°C overnight. Sera were separated by centrifugation at 2800 g for 20 min at 5°C. The separated sera were transferred into transfer bags using a plasma extractor device to ensure no red blood cell contamination. The human sera were stored at 4°C for up to 1 week before being separated into aliquots in cryo tubes and frozen at −80°C until use.
ELISA for evaluation of capture antibodies
Thirty-six commercial antibodies were purchased from multiple vendors (Supplemental Table S1) and screened for their ability to bind IL-6 in human serum. In all experiments, recombinant human IL-6, generated in human cell lines and verified to contain multiple post-translational modifications, was utilized. In some experiments, recombinant IL-6 produced in Escherichia coli was evaluated as a comparison, since it lacked post-translational modifications. Using a standard plate-based ELISA format, antibodies were diluted in phosphate-buffered saline (PBS)/0.05% Tween-20 to 1 µg ml–1 and then plated on a 96-well microplate and incubated overnight at room temperature. The plates were washed seven times with PBS/0.05% Tween-20, and then 275 µl of 2% bovine serum albumin (BSA)/PBS was added to each well for blocking. Blocking reagent was incubated for 2.5 h at room temperature. Recombinant human IL-6 samples (Humanzyme, Chicago, IL, Cat# HZ-1019; GenWay Biotech Inc, San Diego, CA, USA, Cat# GWB-95DC02; Peprotech Sciences Inc, Toronto, ON, Canada, Cat# 200–06) were added to the plate in varying concentrations in diluent (PBS/0.05% Tween-20) and incubated for 2 h at room temperature. The plates were washed seven times with PBS/0.05% Tween-20 followed by addition of 1 µg ml–1 of detection antibody to each well and incubation for 1.5 h at room temperature, then again washed seven times with PBS/0.05% Tween-20 followed by addition of streptavidin-horseradish peroxidase (HRP) to each well and incubation for 30 min at room temperature. 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (100 µl) was then added to each well and the plates were read at 450 nm.
For both the standard plate-based ELISA and the commercial and panoptic IL-6 assays described next, the recovery of recombinant human IL-6 in spiked samples and the lower limit of quantification (LLOQ) in serum matrix were determined to evaluate potential capture-detection antibody pairs. To be defined as the LLOQ a standard had to be consistently linear, with a CV within 25%, and consistently produce acceptable spike recoveries between 75–125 % when spiked into the matrix.
Commercial IL-6 MSD assay
A commercially available assay for determining IL-6 concentrations in human serum was purchased from MSD (Rockville, MD, USA Cat# K151AKC). The assay was performed as per the manufacturer’s instructions and plates were read using an MSD Sector Imager 6000 instrument. The data were analyzed using SoftMax Pro 4.6 Enterprise Edition (Molecular Devices LLC, Sunnyvale, CA, USA).
Biotin conjugation of antibodies for MSD platform
The antibody labelling procedure utilized reagents that attach to primary amines on the antibody. The panoptic IL-6 MSD assay capture antibody was a mouse, anti-human IL-6 monoclonal antibody (Pierce Protein Biology Products, division of Thermo Fisher Scientific Inc [Thermo-Pierce], Rockford IL, USA Cat# M620 [clone 5IL6]) and was biotinylated in-house using the EZ-Link Sulfo-NHS-LC-Biotin reagent (Thermo-Pierce) at a 20: 1 molar excess. Excess biotin was quenched with NH4Cl and removed by centrifugal filtration. The panoptic IL-6 MSD assay detection antibody was a mouse, anti-human IL-6 monoclonal antibody (Invitrogen, division of Life Technologies Corporation [Invitrogen], Grand Island, NY, USA, a custom unlabelled version of clone 505E23C7) and was ruthenium labelled using SULFO-TAG™ NHS-Ester (MSD) at a 12: 1 molar excess. Free ruthenium was removed by centrifugal filtration. Labelled antibody concentrations were determined by bicinchoninic acid assay (Thermo-Pierce).
Panoptic IL-6 MSD assay
An assay for determining panoptic IL-6 concentrations in human serum was developed in-house using a sandwich ELISA setup on the MSD platform. A streptavidin-coated, high-bind MSD plate was blocked with assay buffer (1% BSA in PBS/0.05% Tween-20, 150 µl per well) for 30 min while on a shaking platform. The plate was washed three times with 400 µl of PBS/0.05% Tween-20 and 1 µg ml–1 of biotinylated Thermo-Pierce coating antibody was added to the plate (25 µl per well), which was then incubated for 30 min while shaking. A standard curve of recombinant human IL-6 (Humanzyme) with a range of 9.77 to 10000 pg ml–1 was created using assay buffer. The plate was washed as before and 25 µl of standard, control, and unknown samples were added in duplicates. The plate was incubated for 60 min while shaking and washed as before, 1 µg ml–1 of ruthenium-labeled Invitrogen antibody was added (25 µl per well) and the plate was incubated for 60 min while shaking. The plate was washed as before, 150 µl of 1X read buffer was added and the plate was read using an MSD Sector Imager 6000 instrument. The data were analyzed using SoftMax Pro 4.6 Enterprise Edition.
Size exclusion chromatography and fraction collection
Prior to testing on the MSD commercial IL-6 assay and the panoptic IL-6 MSD assay, human serum samples were separated by a size exclusion column and the fractions were concentrated by centrifugal filtration. Two hundred milliliters of human serum were thawed from −80°C for 10 min at room temperature. A 300 ml Superdex 200 (GE Healthcare, Waukesha, WI, USA Cat# 17-1043-01), packed into a 2.5 × 60 cm column (Bio-Rad, Hercules, CA, USA Cat# 737–2576), was assembled on a Bio-Rad BioLogic LP instrument. The column was pre-equilibrated with 1.5 column volumes of wash buffer, Tris HCl pH 7.5 and 150 m m NaCl. Human serum (200 ml) was applied directly on top of the resin and allowed to enter the column by gravity. An additional 3 ml of wash buffer was applied to the top of the resin bed and allowed to enter the column. A further 5 ml of wash buffer was then placed on top of the resin and a Bio-Rad column plunger was positioned on top. The Bio-Rad BioLogic instrument was programmed to apply a flow rate of 1.5 ml min–1 to the column. The first 100 ml was determined to be the void volume by analysis of the size exclusion profile of protein standards (Bio-Rad, Cat# 151–1901), and it was discarded. After the void volume, fractions of serum eluting from the column were collected every 3 min for a total of 60 fractions. Size exclusion samples were concentrated by centrifugal filtration (EMD Millipore Corporation, Billerica, MA, USA Cat# UFC801024) for 10 to 20 min at 3500 rev min–1 and 4°C, to less than 0.5 ml. The fractions were then stored at −20°C.
Human protein microarray analysis
In order to confirm the specificity of the antibody combination chosen for the panoptic IL-6 MSD assay, Invitrogen’s ProtoArrayTM Human Protein Microarray v5.0 containing 9000 human proteins spotted was utilized. Siltuximab (human-mouse chimera), Thermo-Pierce 5IL6 (mouse), GenWay 5E1 (mouse) and Epitomics EBI-R14-19 (rabbit) were profiled at two separate concentrations of 0.1 ng µl–1 and 1.0 ng µl–1. AlexaFluor®647 goat–anti-human IgG was used as the detection reagent for siltuximab, AlexaFluor®647 rabbit–anti-mouse IgG was used as the detection reagent for the Thermo-Pierce 5IL6 and GenWay 5E1 antibodies and AlexaFluor®647 goat-anti-rabbit IgG was used as the detection reagent for the Epitomics antibody.
Results
Identification of optimum antibodies for detecting total IL-6 in standard ELISA plate-based format
As shown in Supplement Table S1, 36 commercially available anti-IL-6 antibodies were investigated for their ability to bind recombinant human IL-6 (Humanzyme) in human serum as matrix (Figure1). Of the 36 anti-IL-6 antibodies screened, 11 antibodies were ruled out for assay development (four antibodies had no signal above background in any of the plates and seven antibodies had no to very low signals). Twenty-five antibodies produced signals above background and were chosen for additional testing (Figure2). Three of the 25 antibodies were either from ascites or were polyclonal and were ruled out for further assay development. The remaining antibody pairs were further tested for spike-recovery and LLOQ in serum matrix. In all cases, we used Humanzyme recombinant human IL-6 as a protein standard and a non-biotinylated version of Invitrogen anti-IL-6 antibody 505E23C7 as the detection antibody. Following individual evaluation, three anti–IL-6 detection antibodies were identified as potential candidates for further assay development, Thermo-Pierce 5IL6, Epitomics EBI-R14-19 and Ebiosciences 16YOR5/66. These top-performing antibodies were subsequently tested for their ability to immunoprecipitate all human IL-6 isoforms by two-dimensional gel (data not shown).
Figure 1.
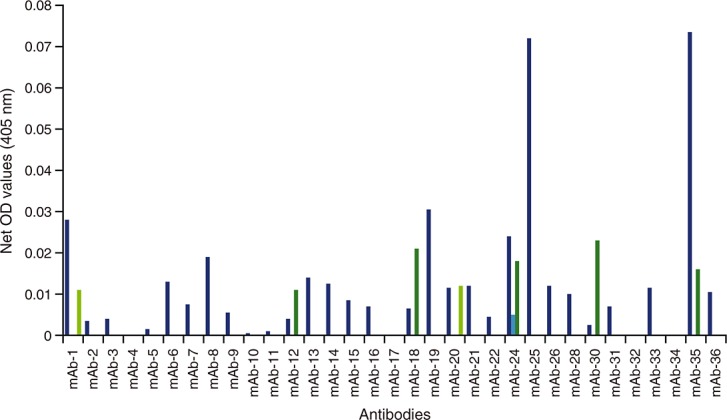
Capture antibodies screened for IL-6 capture in combination with four different detection antibodies. Each candidate antibody was used as a capture antibody to assess 125 pg ml–1 human recombinant IL-6. Detection antibodies from commercially available ELISA kits were used to determine which antibody pair would result in the best detection of IL-6 in human serum. The approach was repeated for human plasma samples, with similar results (data not shown). IL-6, interleukin 6; ELISA, enzyme-linked immunosorbent assay; OD, optical density; mAb, monoclonal antibody; det Ab, detection antibody;  Det Ab-1,
Det Ab-1,  Det Ab-2,
Det Ab-2,  Det Ab-3,
Det Ab-3,  Det Ab-4
Det Ab-4
Figure 2.
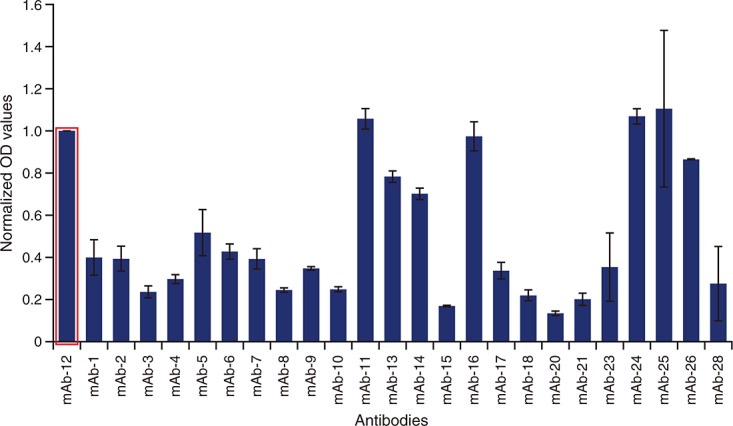
Comparison of capture antibodies in the second round of screening. Capture antibodies that passed the broad first round screening were further evaluated in standard ELISA format. Thermo-Pierce 5IL6 (mAb-12; circled) consistently emerged as a superior capture antibody and was included in all ELISAs to normalize ELISA plate runs. ELISA, enzyme-linked immunosorbent assay; OD, optical density; mAb, monoclonal antibody
Evaluation of optimal detection antibody
To identify the optimal anti-IL-6 detection antibody in detecting recombinant human IL-6 spiked into human serum, four anti-IL-6 detection antibodies from commercially available ELISA kits (R&D Systems, Thermo Pierce, PeproTech and Invitrogen) were compared in standard ELISA format using Thermo-Pierce anti-IL-6 antibody (5IL6) as a capture antibody (Figure3A). Invitrogen anti-IL-6 detection antibody proved superior in combination with Thermo-Pierce 5IL6. As the final antibody combinations were selected, three candidates (Thermo-Pierce 5IL6, Epitomics EBI-R14-19 and Ebiosciences 16YOR5/66) were evaluated in a standard ELISA format measuring recombinant human IL-6 in diluents. Thermo-Pierce 5IL6 demonstrated slightly higher sensitivity in the lower concentration range of recombinant human IL-6 (Figure3B).
Figure 3.
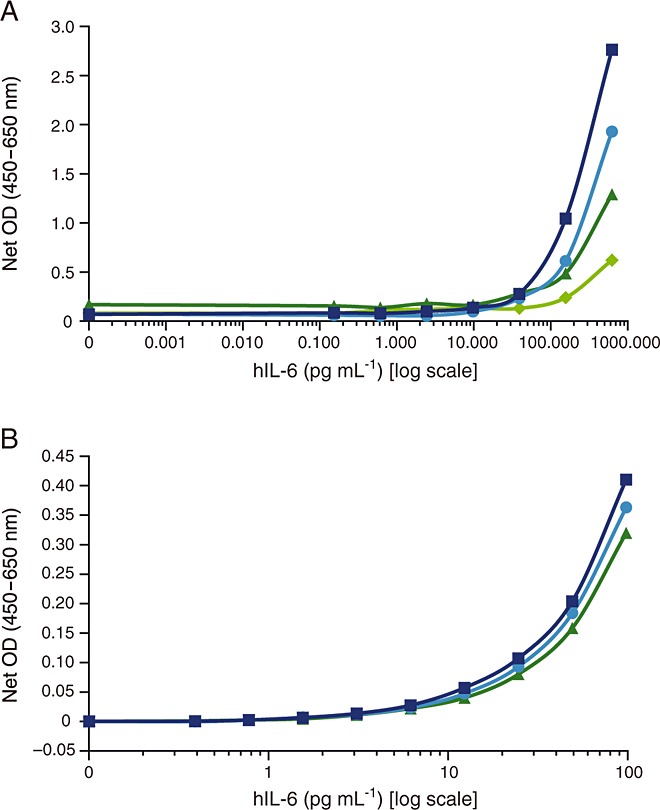
Comparison of top-performing detection and capture antibodies. (A) Four detection antibodies from commercial IL-6 kits were screened and compared for their ability to recognize human recombinant IL-6. Thermo-Pierce 5IL6 was used as a capture antibody. Invitrogen detection antibody (505E23C7) was selected as the detection antibody for future screenings. (B) The three top-performing capture monoclonal anti-IL-6 antibodies (Thermo-Pierce 5IL6, Ebiosciences 16YOR5/66 and Epitomics EBI-R14-19) were compared in a single analysis. The detection antibody was Invitrogen ELISA detection antibody 505E23C7. IL-6, interleukin 6; hIL6, human interleukin 6; ELISA, enzyme-linked immunosorbent assay; OD, optical density; det Ab, detection antibody,  R&D det mAb,
R&D det mAb,  Thermo-Pierce det mAb,
Thermo-Pierce det mAb,  PeproTech det mAb,
PeproTech det mAb,  Invitrogen det mAb,
Invitrogen det mAb,  Thermo-Pierce 5IL6,
Thermo-Pierce 5IL6,  Ebiosciences 16YOR5/66,
Ebiosciences 16YOR5/66,  Epitomics EBI-R14-19
Epitomics EBI-R14-19
Final selection of antibody pairs in panoptic assay development on MSD platform
Thermo-Pierce 5IL6 and Epitomics EBI-R14-19 were evaluated as capture antibodies with Invitrogen anti-IL-6 as the detection antibody on the MSD platform. These formats were compared against the MSD kit-based assays using the IL-6 standard provided in the kit as well as recombinant human IL-6 from Humanzyme. Serum samples from healthy human donors (n = 10), patients with cancer (n = 10) and patients with RA (n = 8) were evaluated using all five combinations of antibodies listed above. A majority of samples (four out of five) gave values that were below the LLOQ and only the combination of Thermo-Pierce 5IL6 (capture) with Invitrogen 505E23C7 (detection) demonstrated detection of a wide range of IL-6 values (Figure4). After additional analysis for standard spike-recovery in serum matrix and optimizing for minimizing background and LLOQ signal by standard laboratory methodology 21, the final antibody pair (Thermo-Pierce 5IL6 capture antibody and unbiotinylated Invitrogen 505E23C7 detection antibody) was then validated on an MSD platform.
Figure 4.
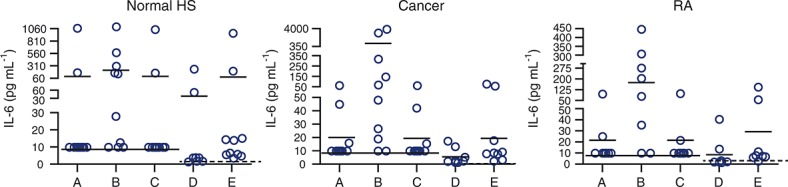
Final antibody pair selection for determining the best pair that gives the maximum spread of values. Capture/detection antibody pairs evaluated shown respectively: (A) CNTO328/Invitrogen 505E23C7, (B) Thermo-Pierce 5IL6/Invitrogen 505E23C7, (C) Epitomics EBI-R14-19/Invitrogen 505E23C7, (D) MSD IL-6 kit assay and (E) MSD IL-6 kit assay with the Humanzyme IL-6 standard. Solid line indicates LLOQ at 9.77 pg ml–1 and dotted line indicates LLOQ at 1.22 pg ml–1. IL-6, interleukin 6; HS, human serum; RA, rheumatoid arthritis; MSD, Meso Scale Discovery; LLOQ, lower limit of quantification
A further analysis demonstrated that siltuximab, Thermo-Pierce 5IL6, Epitomics and GenWay 5E1 all bound only IL-6 in the ProtoArray human protein array, with siltuximab having the highest signal, followed by Thermo-Pierce 5IL6, then GenWay 5E1 and finally Epitomics (data not shown). This also confirmed the choice of Thermo-Pierce 5IL6 for use in the panoptic IL-6 MSD assay.
Validation of panoptic IL-6 MSD assay
Validation parameters included assessment of standard curve, LLOQ, assay variability (inter-subject, inter-assay, intra-assay and inter-operator), spike-recovery, stability, assay ruggedness and dilutional linearity (Table1). Standard curves in the range of 9.77 to 10000 pg ml–1 and three controls (low, medium and high) were run on every MSD plate during the course of validation. The LLOQ for the assay was determined to be 9.77 pg ml–1, as assessed in normal human serum (n = 20). The higher values detected in some of the normal serum samples in the panoptic IL-6 MSD assay may have been due to undiagnosed conditions in these patients. The percent recovery ranged from 93.13% to 113.27%. The overall pooled inter-assay coefficient of variation was 20.12% and the overall pooled intra-assay coefficient of variation was 8.67%. The spike recovery at the low (150 pg ml–1) and high (2500 pg ml–1) concentrations of IL-6 demonstrated an average recovery of 85.04% in normal human serum and 100.32% in disease serum samples (multiple myeloma and breast cancer) using the method described by Chaturvedi et al. 21.
Table 1.
Validation parameters of panoptic IL-6 MSD assay and the commercial IL-6 MSD assay
| Validation parameters | Panoptic IL-6 MSD assay | Commercial IL-6 MSD assay |
|---|---|---|
| Linear standard curve | 9.77–10000.00 pg ml–1 | 1.22–2500.00 pg ml–1 |
| Lower limit of quantitation | 9.77 pg ml–1 | 1.22 pg ml–1 |
| Inter-patient variability | Normals (n = 20) <9.77–883.40 pg ml–1 | Normals (n = 34) <1.22–8.82 pg ml–1 |
| Inter-assay precision | 20.12% | 8.46% |
| Intra-assay precision | 8.67% | 5.07% |
| Inter-operator variability | 18.98% | 8.81% |
| Spike recovery (n = 6) | 85.04% | 89.89% |
| Freeze/thaw stability | Stable through five freeze/thaw cycles | Stable through three freeze/thaw cycles* |
| Short term antigen stability | Stable up to 6 days at 4°C | Stable up to 5 days at 4°C† |
| Assay ruggedness | Up to four plates can be run in sequence | Up to four plates can be run in sequence |
| Dilution linearity | Endogenous samples with concentrations >10000 pg ml–1 cannot be diluted linearly | Not performed |
IL-6, interleukin 6; MSD Meso Scale Discovery.
Freeze/thaw stability was not measured beyond three cycles.
Stability at 4°C was not measured beyond 5 days.
It was determined that serum samples can be exposed to five freeze/thaw cycles without significantly (described as 100 ± 25% recovery) affecting IL-6 concentration. IL-6 concentrations in serum ranged from <9.77 to 883.4 pg ml–1 in normal human serum (n = 20), 11.13 to 1901.96 pg ml–1 in breast cancer (n = 10), 40.75 to 473.39 pg ml–1 in RA (n = 10), <9.77 to 22.35 pg ml–1 in prostate cancer (n = 3), <9.77 to 1313.11 pg ml–1 in multiple myeloma (n = 3), <9.77 to 18.71 pg ml–1 in ovarian cancer (n = 2) and <9.77 pg ml–1 in pancreatic cancer (n = 1) samples (Figure5). Interestingly, the median values increased from 20.36 pg ml–1 in normal human serum to 48.50 pg ml–1 in breast cancer and 180.72 pg ml–1 in RA serum samples suggesting higher IL-6 concentrations in patients with disease Acceptable dilutional linearity (average recovery = 107.39%) was calculated up to and including a dilution of 1: 128 for pre-spiked samples. Samples with endogenous IL-6 concentrations that were higher than the upper range of the curve (i.e. 10 000 pg ml–1) could not be linearly diluted, and therefore, the concentrations for these samples should be reported as >10 000 pg ml–1 (upper limit of quantitation). There was acceptable variation (<25% coefficient of variation) between two different labelled lots of Thermo-Pierce biotin and detection antibody. Based on acceptable assay performance using a range of validation parameters (including low inter-assay and intra-assay variability, spike recovery and LLOQ) in human serum and plasma (data not shown) and the antigen stability for at least 6 days at 4°C and over multiple freeze/thaw cycles, this assay can be used for testing clinical study samples. The panoptic IL-6 MSD assay was also validated with human plasma samples and showed good concordance with human serum (data not shown).
Figure 5.
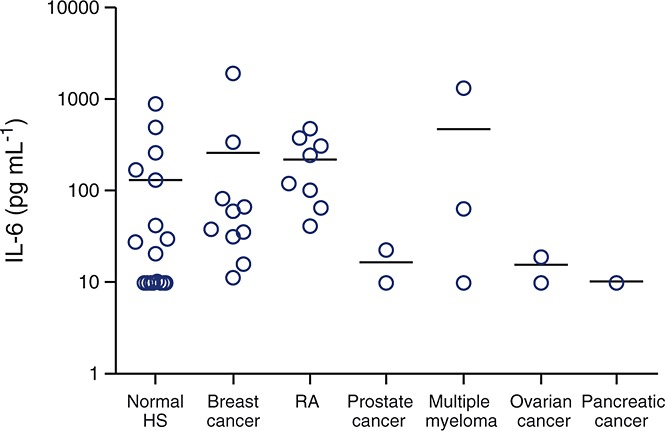
IL-6 concentrations in normal and disease sera samples as measured by panoptic IL-6, MSD assay; IL-6, interleukin 6; MSD, Meso Scale Discovery; HS, human serum; RA, rheumatoid arthritis
Comparison of IL-6 measurement between panoptic IL-6 MSD assay and commercial IL-6 MSD kit
The concentrations of IL-6 can be much higher than those detected by the commercially available assays compared with the panoptic IL-6 MSD assay. A rationale for this observation is that the Thermo-Pierce 5IL6 capture antibody is able to recognize a variety of IL-6 complexes of different molecular weights 22–24, whereas the commercially available MSD IL-6 kit assay only recognizes low molecular weight (i.e. uncomplexed) serum IL-6. This was further tested by separating serum IL-6 by size exclusion chromatography into fractions 18 and then measured using the panoptic and commercial IL-6 MSD assays to determine concentrations of IL-6 in each fraction.
To investigate the distinction between the two assays, RA (n = 7) and myelodysplastic syndrome (n = 6) samples were run on Superdex 200 columns as described above and collected into 60 fractions. The immunologic property of IL-6 was tested in each of the gel filtration eluate fractions on both the MSD and panoptic IL-6 MSD assay. Complete analysis for each fraction is shown in Figure6. Figure6A shows IL-6 quantification in each fraction measured using the commercial IL-6 MSD assay, confirming earlier observations that many commercial IL-6 assays effectively quantify the low molecular weight, non-complexed forms of IL-6 (i.e. from 10–50 kDa) and less effective at quantifying the high molecular weight, complexed forms of IL-6. In contrast to the commercial MSD assay, the panoptic IL-6 MSD assay quantified both high molecular weight, complexed IL-6 in the range of 1650 to 500 kDa and low molecular weight, non-complexed IL-6 (Figure6B). Both assays detected a high molecular weight form of IL-6 in one sample (MDS 3) although at lower levels in the commercial IL-6 assay. The reasons for detection of higher molecular weight IL-6 by the commercial assay in this sample were not apparent. It may have been due to unknown biological features specific to this patient or that the commercial assay showed partial inconsistent efficacy in some situations.
Figure 6.
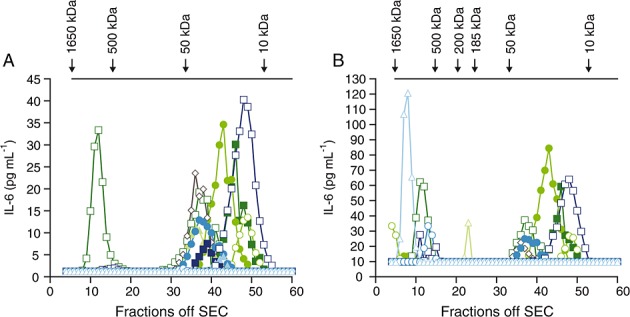
Superdex 200 gel filtration and immunologic characterization of IL-6 in serum from patients with RA (n = 7) and patients with MDS (n = 6). (A) IL-6 concentrations of each fraction assayed using commercial MSD assay and (B) IL-6 concentrations of each fraction assayed using the panoptic IL-6 assay. IL-6, interleukin 6; MDS, myelodysplastic syndrome; MSD, Meso Scale Discovery; SEC, size exclusion column,  RA 1,
RA 1,  RA 2,
RA 2,  RA 3,
RA 3,  RA 4,
RA 4,  RA 5,
RA 5,  RA 6,
RA 6,  RA 7,
RA 7,  MDS 1,
MDS 1,  MDS 2,
MDS 2,  MDS 3,
MDS 3,  MDS 4,
MDS 4,  MDS 5,
MDS 5,  MDS 6
MDS 6
Thirteen human serum samples from donors with RA (n = 7) or myelodysplastic syndrome (MDS, n = 6) were purchased from a commercial vendor and tested for serum IL-6 concentrations using both the panoptic IL-6 MSD assay and the MSD IL-6 kit assay (Table2). Seven of the 13 samples (53.84%, # 1, 2, 3, 4, 5, 8 and 9) demonstrated high IL-6 concentrations by the panoptic MSD assay compared with the commercial MSD assay and five of the 13 samples (38.46%, # 6, 7, 11, 12 and 13) had almost similar quantifiable IL-6 measurements using both the assays. However, there was only one sample (7.69%, sample #10) that demonstrated higher IL-6 values by the commercial MSD assay compared with the panoptic IL-6 MSD assay.
Table 2.
IL-6 measurement using the panoptic MSD assay and the commercial MSD assay prior to gel filtration column
| Disease | Sample number | Panoptic IL-6 MSD assay (pg ml–1) | Commercial IL-6 MSD assay (pg ml–1) |
|---|---|---|---|
| RA 1 | Sample #01 | 1866.25 | 2.9 |
| RA 2 | Sample #02 | 381.42 | 5.73 |
| RA 3 | Sample #03 | 446.72 | 46.89 |
| RA 4 | Sample #04 | 76.32 | 10.19 |
| RA 5 | Sample #05 | 54.95 | 40.63 |
| RA 6 | Sample #06 | 21.77 | 21.44 |
| RA 7 | Sample #07 | 73.32 | 84.09 |
| MDS 1 | Sample #08 | 764.2 | 4.26 |
| MDS 2 | Sample #09 | 193.15 | 24.9 |
| MDS 3 | Sample #10 | 43.84 | 104.76 |
| MDS 4 | Sample #11 | 35.19 | 48.92 |
| MDS 5 | Sample #12 | 64.84 | 77.3 |
| MDS 6 | Sample #13 | 35.33 | 39.43 |
IL-6, interleukin 6; MSD, Meso Scale Discovery; RA, rheumatoid arthritis; MDS, myelodysplastic syndrome
Discussion
IL-6 is a pleiotropic cytokine linked to multiple biological pathways and pathologies, and because of its central role in inflammation, IL-6 and IL-6R have become attractive targets for drug development 25. Tocilizumab is a monoclonal antibody that targets IL-6R and has been approved for use in Japan, Europe and the United States for treatment of RA 26. It has also received approval for the treatment of Castleman’s disease in Japan 27. Sirukumab is a monoclonal antibody that targets IL-6 and is currently in phase III clinical development for adults with moderate to severe RA. Siltuximab, an anti-IL-6 antibody, is currently being evaluated in high risk, smoldering multiple myeloma and has been approved for the treatment of multicentric Castleman’s disease in the European Union and United States.
Given the future of IL-6 therapies, it is important to measure accurately total IL-6 burden (i.e. both non-complexed and complexed to other proteins). On examining the literature, there are large discrepancies in trying to define the accurate reference range for healthy donors and patients with disease. There are multiple methods in use for IL-6 measurement, including bioassays, radioimmunoassay or standard ELISA 28–30. Other factors adding to the discrepancies could include the time of blood draw 30, the presence of IL-6 binding proteins in serum 18 and post-translational modifications of IL-6 29, which might affect the ability of the capture antibody to recognize all forms of IL-6. In addition, antibodies used in these assays recognize different epitopes of IL-6 and may contribute to variability in reported reference ranges. Another important point is that besides human IL-6, viral IL-6 may play a role in the pathogenesis of other diseases (e.g. HIV or HHV-8 related Castleman’s disease), which may confound the accurate measurement of IL-6 burden. Additional work needs to be performed in order to understand if this assay differentiates between the two types of IL-6.
During the course of development of the panoptic IL-6 MSD assay, we realized that many commercial assays use the standard E. coli-derived recombinant IL-6 that does not contain post-translational modifications similar to those found in humans. It remains unclear what significance or biological implications these modifications have, but they certainly play a significant role in the ability of antibodies to recognize human IL-6 in serum 18. Endogenous circulating IL-6 contains multiple isoforms, such as phosphorylated, O-glycosylated and N-glycosylated, to name a few 31,32. At least 12 different isoforms of IL-6 that have post-translational modifications, such as glycosylation and phosphorylation from human monocytes and fibroblasts, have been seen 33. Recent work from our laboratory has shown that IL-6 from healthy or diseased samples may contain more than 20 different isoforms with multiple post-translational modifications as identified on two dimensional gels (data not shown). Given the enormous evidence for several isoforms of IL-6, it was important to use humanized IL-6 instead of E. coli-derived recombinant IL-6 based on its similar migration pattern on 2-D gels in order to obtain optimum results for the assay.
Although C-reactive protein is a sensitive surrogate biomarker for IL-6 activity 34, the association between serum IL-6 and C-reactive protein can differ depending on the study and test employed. Thus, there is a need for a more accurate way to quantify IL-6 burden in healthy donors vs. diseased patients (e.g. those with cancer, RA or cardiovascular disease). For this reason, we sought to develop the panoptic IL-6 MSD assay, which can measure all forms of IL-6, complexed and non-complexed. It is unclear how IL-6 post-translational modifications (i.e. independently of chaperone/complex proteins) affect IL-6 biological activity, but it is conceivable that post-translational modifications can directly impact IL-6 bioactivity and the extent of formation of secondary complexes with other proteins. There are significant efforts toward developing drugs that interfere with either IL-6 or IL-6R across multiple disease states. Therefore, it will be crucial to develop an assay that can detect all forms of IL-6. While the non-complexed forms of IL-6 may be most relevant in some situations, both complexed and non-complexed IL-6 may be critical in others. Therefore, we developed and validated a novel assay that enables evaluation of IL-6 in both complex and non-complex forms. The designation ‘panoptic assay’ differentiates it from other tests that claim to detect all forms of IL-6. However, we do not make the claim that the panoptic assay is the definitive solution to this complicated issue. More data need to be generated using all IL-6 assays in order to draw any meaningful, statistically significant conclusions.
Obtaining these data will be facilitated by the successful transfer of the panoptic IL-6 MSD assay to a central laboratory where it is currently being used to measure total systemic IL-6 in ongoing clinical studies. The potential clinical utility of the assay has begun to be explored beyond its initial restricted application in drug registration trials. A recent study conducted in a clinical centre that treats multicentric Castleman’s disease patients used the assay to investigate the effect of IL-6R polymorphisms in normal and affected individuals 35. Reliable quantification of total systemic IL-6 was an essential prerequisite in order to understand associations between IL-6R polymorphisms and the disease state. Examples of these types of studies and future evaluations may provide more insight into the role of various forms of IL-6 in clinical settings.
In conclusion, IL-6 is a pleiotropic molecule with biological significance in numerous malignancies and immunologic diseases. There are currently multiple drugs in development or approved for clinical use that target IL-6, IL-6R or closely related pathways. Utilization of these drugs requires careful monitoring of IL-6 concentrations. We have developed a novel panoptic IL-6 MSD assay that measures both high and low molecular weight IL-6. The biological significance of these multiple forms of IL-6 is not yet understood, but tools such as the described panoptic IL-6 MSD assay will hopefully provide insight into the role of IL-6 in normal and disease processes.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare support from Janssen Pharmaceutical Companies of Johnson and Johnson for the submitted work. All authors were employees of Janssen Pharmaceutical Companies of Johnson and Johnson for the previous 3 years and had no other relationships or activities that could have influenced this work. Brett Hall is now an employee of MedImmune, LLC.
Editorial assistance was provided by Nancy Bella, PharmD and Christopher J. Jones, PhD, of MedErgy. The study was funded by the Janssen Pharmaceutical Companies of Johnson and Johnson.
Contributors
The study was conceived and designed by Kate Sasser, Brett Hall and Shalini Chaturvedi and performed by Shalini Chaturvedi, Derick Siegel and Jaehong Park. Data were analyzed by all the authors. Reagents, materials and analysis tools were provided by Janssen Research and Development. The manuscript was written by Kate Sasser, Brett Hall and Shalini Chaturvedi with editorial support from Nancy Bella and Christopher Jones.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web site:
Supporting Information
References
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992;62:S60–5. doi: 10.1016/0090-1229(92)90042-m. [DOI] [PubMed] [Google Scholar]
- Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: the role of interleukin 6 (IL-6) BJU Int. 2014;113:986–92. doi: 10.1111/bju.12452. [DOI] [PubMed] [Google Scholar]
- Salgado R, Junius S, Benoy I, Van DP, Vermeulen P, Van ME, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–6. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, Shen Z, Patel N, Smith ES, Wang W, Prabhala R, Tai YT, Tassone P, Anderson KC, Munshi NC. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–52. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauta VM. Interleukin-6 and the network of several cytokines in multiple myeloma: an overview of clinical and experimental data. Cytokine. 2001;16:79–86. doi: 10.1006/cyto.2001.0982. [DOI] [PubMed] [Google Scholar]
- Maccio A, Madeddu C. The role of interleukin-6 in the evolution of ovarian cancer: clinical and prognostic implications--a review. J Mol Med (Berl ) 2013;91:1355–68. doi: 10.1007/s00109-013-1080-7. [DOI] [PubMed] [Google Scholar]
- Van Rhee F, Wong RS, Munshi N, Rossi JF, Ke XY, Fossa A, Simpson D, Capra M, Liu T, Hsieh RK, Goh YT, Zhu J, Cho SG, Ren H, Cavet J, Bandekar R, Rothman M, Puchalski TA, Reddy M, van de Velde H, Vermeulen J, Casper C. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15:966–74. doi: 10.1016/S1470-2045(14)70319-5. [DOI] [PubMed] [Google Scholar]
- Santer FR, Malinowska K, Culig Z, Cavarretta IT. Interleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cells. Endocr Relat Cancer. 2010;17:241–53. doi: 10.1677/ERC-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchi K, Fujiwara H, Okamura S, Okamura H, Umehara S, Todo M, Furutani A, Yoneda M, Shiozaki A, Kubota T, Ichikawa D, Okamoto K, Otsuji E. Overexpression of interleukin-6 suppresses cisplatin-induced cytotoxicity in esophageal squamous cell carcinoma cells. Anticancer Res. 2011;31:67–75. [PubMed] [Google Scholar]
- Keller ET, Wanagat J, Ershler WB. Molecular and cellular biology of interleukin-6 and its receptor. Front Biosci. 1996;1:d340–57. doi: 10.2741/a136. [DOI] [PubMed] [Google Scholar]
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- Ripley BJ, Goncalves B, Isenberg DA, Latchman DS, Rahman A. Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann Rheum Dis. 2005;64:849–53. doi: 10.1136/ard.2004.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–26. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- Paul-Pletzer K. Tocilizumab: blockade of interleukin-6 signaling pathway as a therapeutic strategy for inflammatory disorders. Drugs Today (Barc) 2006;42:559–76. doi: 10.1358/dot.2006.42.9.1025692. [DOI] [PubMed] [Google Scholar]
- Zaki MH, Nemeth JA, Trikha M. CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer. 2004;111:592–5. doi: 10.1002/ijc.20270. [DOI] [PubMed] [Google Scholar]
- Visconti L, Nelissen K, Deckx L, van Den AM, Adriaensen W, Daniels L, Mathei C, Linsen L, Hellings N, Stinissen P, Buntinx F. Prognostic value of circulating cytokines on overall survival and disease-free survival in cancer patients. Biomark Med. 2014;8:297–306. doi: 10.2217/bmm.13.122. [DOI] [PubMed] [Google Scholar]
- Ndubuisi MI, Patel K, Rayanade RJ, Mittelman A, May LT, Sehgal PB. Distinct classes of chaperoned IL-6 in human blood: differential immunological and biological availability. J Immunol. 1998;160:494–501. [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihl MP, Heinimann K, Rudiger JJ, Eickelberg O, Perruchoud AP, Tamm M, Roth M. Identification of a novel IL-6 isoform binding to the endogenous IL-6 receptor. Am J Respir Cell Mol Biol. 2002;27:48–56. doi: 10.1165/ajrcmb.27.1.4637. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5:128–34. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–32. [PubMed] [Google Scholar]
- May LT, Viguet H, Kenney JS, Ida N, Allison AC, Sehgal PB. High levels of ‘complexed’ interleukin-6 in human blood. J Biol Chem. 1992;267:19698–704. [PubMed] [Google Scholar]
- May LT, Patel K, Garcia D, Ndubuisi MI, Ferrone S, Mittelman A, Mackiewicz A, Sehgal PB. Sustained high levels of circulating chaperoned interleukin-6 after active specific cancer immunotherapy. Blood. 1994;84:1887–95. [PubMed] [Google Scholar]
- Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21:1248–57. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- Venkiteshwaran A. Tocilizumab. MAbs. 2009;1:432–8. doi: 10.4161/mabs.1.5.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M, Ogata A, Kishimoto T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol. 2014;26:88–96. doi: 10.1016/j.smim.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti CS, Donoval B, Doncel GF, Donaghay M, Grivel JC, Guzman E, Hayes M, Herold B, Hillier S, Lackman-Smith C, Landay A, Margolis L, Mayer KH, Pasicznyk JM, Pallansch-Cokonis M, Poli G, Reichelderfer P, Roberts P, Rodriguez I, Saidi H, Sassi RR, Shattock R, Cummins JE., Jr Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem. 2008;80:4741–51. doi: 10.1021/ac702628q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledur A, Fitting C, David B, Hamberger C, Cavaillon JM. Variable estimates of cytokine levels produced by commercial ELISA kits: results using international cytokine standards. J Immunol Methods. 1995;186:171–9. doi: 10.1016/0022-1759(95)00184-c. [DOI] [PubMed] [Google Scholar]
- Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63:879–84. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May LT, Santhanam U, Sehgal PB. On the multimeric nature of natural human interleukin-6. J Biol Chem. 1991;266:9950–5. [PubMed] [Google Scholar]
- Gross V, Andus T, Castell J, Vom BD, Heinrich PC, Gerok W. O- and N-glycosylation lead to different molecular mass forms of human monocyte interleukin-6. FEBS Lett. 1989;247:323–6. doi: 10.1016/0014-5793(89)81361-4. [DOI] [PubMed] [Google Scholar]
- Tanner JE, Goldman ND, Tosato G. Biochemical and biological analysis of human interleukin 6 expressed in rodent and primate cells. Cytokine. 1990;2:363–74. doi: 10.1016/1043-4666(90)90067-4. [DOI] [PubMed] [Google Scholar]
- Van Rhee F, Fayad L, Voorhees P, Furman R, Lonial S, Borghaei H, Sokol L, Crawford J, Cornfeld M, Qi M, Qin X, Herring J, Casper C, Kurzrock R. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010;28:3701–8. doi: 10.1200/JCO.2009.27.2377. [DOI] [PubMed] [Google Scholar]
- Stone K, Woods E, Szmania SM, Stephens OW, Garg TK, Barlogie B, Shaughnessy JD, Jr, Hall B, Reddy M, Hoering A, Hansen E, Van RF. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054610. . Interleukin-6 receptor polymorphism is prevalent in HIV-negative Castleman’s disease and is associated with increased soluble interleukin-6 receptor levels.: e54610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


