Abstract
Aims
Selective serotonin re-uptake inhibitors (SSRIs), specifically citalopram and escitalopram, are thought to cause QTc prolongation, although studies have shown contradictory results. Nevertheless, a maximum citalopram dosage of 20 mg in high risk patients (e.g. >60 years of age) is recommended. We aimed to investigate the association between use of (individual) SSRIs and QTc in a population-based study in older adults.
Methods
This study, which was part of the prospective Rotterdam Study (period 1991–2012), included participants with up to five electrocardiograms (ECGs). We used linear mixed models to compare QTcF (QT corrected according to Fridericia) measured during use of individual SSRIs with QTcF measured during non-use of any antidepressant. For citalopram, analyses were additionally restricted to a maximum dosage of 20 mg in participants aged 60 years and older.
Results
We included 12 589 participants with a total of 26 620 ECGs of which 436 ECGs were made during SSRI use. The mean QTcF was similar during use of any drugs from the SSRI class and during non-use. After stratifying to individual SSRIs, ECGs recorded during use of citalopram had the longest QTc compared with ECGs recorded during non-use (+12.8 ms, 90% CI 7.5, 18.2). This result remained similar in the analysis comprising participants aged 60 years and older with a maximum prescribed daily dosage of 20 mg citalopram.
Conclusions
Although no SSRI class effect was observed, use of citalopram was associated with a longer QTcF, even after considering the recommended restrictions. Other SSRIs may not give a clinically relevant QTcF prolongation.
Keywords: electrocardiography, population surveillance, serotonin re-uptake inhibitors
What is Already Known about this Subject
Preliminary evidence suggests that multiple selective serotonin re-uptake inhibitors may prolong the QTc.
Daily dosage of citalopram has been restricted to 20 mg in patients aged 60 years and older.
What this Study Adds
We observed no SSRI class effect on QTcF prolongation.
Our data seem to confirm QTcF prolongation by citalopram. However, because of the low number of users, we cannot rule out that other individual SSRIs also have QTcF prolongation properties, although these are likely less clinically relevant.
In participants aged 60 years and older, an approximately 20 ms longer QTc was observed when using 20 mg or less daily of the drug citalopram.
Introduction
Sudden cardiac death accounts for approximately 50% of cardiovascular mortality and 20% of all deaths 1–3. Prolongation of the heart rate corrected QT interval (hereafter denoted as QTc) may induce ventricular arrhythmias, which is one of the causes of sudden cardiac death 4–6. Risk factors for QTc prolongation include female gender, old age, certain genetic variants (e.g. in the NOS1AP gene) and use of certain drugs 5,7–10.
One of the drug classes for which QTc prolonging properties are currently under debate, is selective serotonin re-uptake inhibitors (SSRIs). In vitro studies reported that a large number of the individual SSRIs have the potential to block the hERG channel 11–14, which could consequently increase the QTc. In line with this, many SSRIs are already listed as having QTc prolonging properties 15,16, which suggests that SSRIs as a class might increase QTc. However, in a large cross-sectional study, conducted in an electronic health care database, only citalopram and escitalopram were associated with a longer QTc 17. On the other hand, in a meta-analysis of randomized clinical trials on SSRI-induced QTc prolongation, the use of fluvoxamine and sertraline was also associated with QTc prolongation, although effect sizes were smaller than with citalopram and escitalopram 18. In 2011, the American Food and Drug Administration (FDA) announced that the daily dosage of citalopram should be restricted to a maximum of 40 mg daily in healthy adults and 20 mg maximum in high risk patients (e.g. >60 years of age) 19,20. However, the safety of use has not yet been determined within the limits of these restrictions.
Within the Rotterdam Study, the association between the use of different psychotropic drugs, including SSRIs, and QTc prolongation has been studied previously 21. However, no significantly longer QTc was observed in users of any of the individual SSRIs as well as SSRIs as a class, which was probably due to the low number of users. After this study was conducted, the Rotterdam Study cohort was further extended, more follow-up data became available 22 and SSRIs were increasingly dispensed 23. Furthermore, the FDA now recommends the use of QTc corrected with Fridericia (hereafter denoted as QTcF) instead of Bazett, because the latter overestimates the QTc in participants with higher heart rates 24–26. Therefore, we aimed to reinvestigate the association between SSRIs as a class and QTcF as well as the association between individual SSRIs and QTcF in this population-based cohort of middle-aged and older adults, and aimed to study QTcF prolongation within the limits of the FDA recommendations.
Methods
Research setting
The present study was embedded in the prospective Rotterdam Study, which aimed to investigate the occurrence of, and risk factors for different age-related diseases. The design and rationale of the Rotterdam Study has been described in more detail elsewhere 22,27. In short, the original cohort was initiated with a baseline visit between 1990 and 1993, and included inhabitants aged 55 years and older, who lived in the Ommoord district in Rotterdam, the Netherlands. In total, this cohort consisted of 7983 individuals (response rate 78%). From 2000 to 2001, participants who became 55 years of age or older, or moved into the district were asked to participate in an extension of the first cohort, which included 3011 individuals (response rate 67%). From 2006 until 2008 a second extension was initiated with a total of 3932 inhabitants from the same district, aged 45 years and older (response rate 65%). After baseline assessment, follow-up examinations were conducted every 4–5 years.
The Rotterdam Study was approved by the medical ethics committee according to the Wet Bevolkingsonderzoek ERGO (Population Study Act Rotterdam Study) executed by the Ministry of Health, Welfare and Sports of the Netherlands. All study participants provided written informed consent.
Study population
We included all participants from the Rotterdam Study with up to five electrocardiogram (ECG) measurements and for whom we had access to pharmacy dispensing records. ECGs that showed atrial fibrillation or a pacemaker rhythm were excluded from the analysis. ECG recordings during which participants were using other antidepressants than SSRIs were also excluded.
SSRI exposure assessment
Fully computerized pharmacy dispensing records were available from January 1 1991 onwards for more than 99% of the study population. Dispensing records included Anatomical Therapeutic Chemical (ATC) code, dispensing date, total amount of tablets/capsules per prescription, prescribed daily number of tablets/capsules and product name of the drug. Dispensing episodes were calculated by dividing the total number of filled tablets/capsules by the prescribed daily number. If the date of the ECG assessment fell within a dispensing episode of SSRI use (ATC code N06AB), the participant was considered a current SSRI user. The use of individual SSRIs was based on the full, seven digit, ATC code. The prescribed daily dosage was standardized by dividing the prescribed daily dosage by the defined daily dosage (PDD: DDD ratio) 22.
Electrocardiogram assessment
A standard 12-lead resting ECG was recorded with an ACTA Gnosis electrocardiograph (Esaote Biomedica, Florence, Italy) at a sampling frequency of 500 Hz and stored digitally. ECGs were processed by the Modular ECG Analysis System (MEANS) program, which has been described previously and validated and applied extensively 28–32. MEANS determines common onsets and offsets for all 12 leads together on one representative averaged beat, with the use of template matching techniques 30. The duration of the QT interval was measured from the start of the QRS complex to the end of the T wave, which is described in more detail elsewhere 33. The median RR and mean QT interval were computed after exclusion of beats that immediately preceded or followed premature ventricular complex 34.
Heart rate in beats min–1 was calculated by dividing 60 000 by the RR interval in milliseconds (ms). As QT and heart rate are highly correlated, QT intervals were corrected for heart rate (expressed as the RR interval) using the formula of Fridericia (QTcF: QT/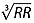 ) 35, which is advised by authorities 26,36.
) 35, which is advised by authorities 26,36.
Co-variables
We considered the following factors as potentially confounding factors at the date of ECG recording, in addition to age and gender: body mass index (BMI), use of drugs that affect the heart rate and/or QTc, diabetes mellitus, hypertension, myocardial infarction and heart failure. Weight and height were measured at the study centre. BMI was calculated as weight (in kg)/height2 (in m). Use of drugs associated with an altered heart rate was determined on the date of ECG examination and was based on the pharmacy dispensing records. The following drugs were considered heart rate altering: β-adrenoceptor blockers (ATC code C07), verapamil (ATC code C08DA01) and diltiazem (ATC code C08DB01). Definite QTc prolonging drugs are generally thought to increase the risk of torsade de pointes. This group of drugs comprises, among others, a number of antibiotics and antipsychotics 16. SSRIs on this list were not taken into account for this variable. Diabetes mellitus was defined as use of glucose-lowering drugs (ATC code A10), a non-fasting glucose concentration of more than 11.0 mmol l–1 or a fasting glucose concentration of more than 6.9 mmol l–1. Hypertension was defined as a systolic blood pressure of 140 mmHg or higher, a diastolic blood pressure of 90 mmHg or higher or use of blood pressure lowering medication for the indication hypertension 37. History of myocardial infarction was defined based on a combination of enzyme markers indicative of the presence of myocardial infarction, ECG measurements and symptoms. This information was retrieved from medical files 38. Heart failure was assessed based on typical signs and symptoms confirmed by objective cardiac dysfunction 38.
Statistical analyses
Study characteristics were determined at baseline, defined as the first eligible ECG recordings of the included participants.
We used linear mixed models to adjust for within-person correlations between repeated centre visits. We tested several covariance structures and selected the one with the lowest Akaike Information Criterion (AIC) 39.
To address the research question, we studied the effect of SSRIs on cross-sectional examinations of QTcF, QT (uncorrected for heart rate) and heart rate. These analyses assessed the difference of QTcF, QT and HR between the ECGs recorded during SSRI use and ECGs recorded during non-use. These analyses were done for SSRIs as a single exposure group as well as for individual SSRIs.
In a second analysis, we assessed whether the within-person change in QTcF between two consecutive ECG examination rounds was dependent on the use of (individual) SSRIs. The change in QTcF was calculated according to the following formula: QTcFvisit(t + 1) – QTcF(visit t). As participants could have had up to five ECG recordings included in the study, participants could have up to four ECG pairs. The effect of SSRI exposure on QTcF was studied at the second examination of an ECG pair and explored in two ways. First, use of (individual) SSRIs was considered irrespective of the participants’ exposure status during the first examination of an ECG pair. Second, use of (individual) SSRIs was considered when the first examination of an ECG pair was recorded during non-use of antidepressants. To interpret prolongation of QTcF attributed to the use of SSRIs, results were presented as β with respect to the ECGs pairs with non-use of antidepressants during the second visit (for both exposure definitions). These analyses were conducted for SSRIs as a single drug exposure group as well as for the individual SSRIs.
Our basic statistical model was adjusted for age and gender. In additional analyses, all potential covariables were included in the statistical model to study whether they confounded the association between SSRIs and QTcF.
We also conducted analyses in different subsamples. First, we restricted the analyses to ECGs of participants without cardiac conduction disorders (left or right bundle branch block, or second or third degree atrioventricular block) and/or left ventricular hypertrophy. Second, we restricted analyses to ECGs recorded in participants aged 60 years and older and with a maximum citalopram dose of 20 mg day–1. These restrictions are in line with the recommendations of the FDA 19,20.
All analyses were conducted using IBM SPSS Statistics (version 21.0, IBM Corp., Somers, NY, USA). The results were presented as the effect of (individual) SSRIs on ECG measures compared with ECGs or ECG pairs recorded during non-use of antidepressants with the 90% confidence interval (CI). No correction was made for multiple testing.
Results
Baseline characteristics
Table1 reports the general characteristics of the study population at baseline. In total, 12 589 participants were included with a total of 26 620 ECGs. At baseline, 57.9% was female and the mean age was 65.2 years (SD = 9.8). The mean heart rate was 70 beats min–1 and the mean QTcF was 421 ms.
Characteristics of the study population
| Total population n = 12 589 | |
|---|---|
| Number of ECGs | 26 620 |
| ECGs per participant, median (range) | 2 (1–5) |
| Females, n (%) | 7294 (57.9) |
| Age (years), mean (SD) | 65.2 (9.8) |
| Body mass index (kg m–2), mean (SD) | 26.9 (4.1) |
| Heart rate (beats min–1), mean (SD) | 70 (11) |
| QTc (ms), mean (SD) | 421 (22) |
| Diabetes mellitus, n (%) | 636 (5) |
| Hypertension, n (%) | 6645 (53) |
| Myocardial infarction, n (%) | 930 (7) |
| Heart failure, n (%) | 257 (2) |
| Right bundle branch block, n (%) | 314 (2.5) |
| Left bundle branch block, n (%) | 190 (1.5) |
| 2nd or 3rd degree atrioventricular block, n (%) | 12 (0.1) |
| Left ventricular hypertrophy, n (%) | 386 (3.1) |
| β-adrenoceptor blocking agents, n (%) | 1625 (12.9) |
| Verapamil | 80 (0.6) |
| Diltiazem | 156 (1.2) |
| Definite QTc-prolonging drugs, n (%)* | 261 (2) |
n, number of participants; ECG, electrocardiogram. Data presented at the first eligible ECG recording per participant.
selective serotonin re-uptake inhibitors were not taken into account in this variable.
Cross-sectional analyses
Of the tested covariance matrices, the ‘heterogeneous first order autoregressive covariance structure’ had the lowest AIC, which we therefore used for all analyses.
Table2 presents the differences in QTcF between ECGs recorded during SSRI use with ECGs recorded during non-use. Compared with ECGs recorded during non-use, ECGs recorded during SSRI use had a 2.9 ms longer QTcF (90% CI 1.3, 4.5) and a 6.2 ms longer uncorrected QT interval (90% CI 4.0, 8.4). Furthermore, heart rate was 1.7 beats min–1 slower (90% CI 0.9, 2.5) on ECGs recorded during use of SSRIs than on ECGs recorded during non-use of antidepressants.
Table 2.
Association between SSRIs and cross-sectional assessments of the heart rate corrected QT (QTcF) interval
| PDD: DDD* | DDD (mg)* | QTcF | QT | Heart rate | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | β (ms) | 90% CI | β (ms) | 90% CI | β (beats min–1) | 90% CI | |||
| Non-user | 26 184 | – | reference | – | reference | – | reference | ||
| Any SSRI | 436 | 2.9 | 1.3, 4.5 | 6.2 | 4.0, 8.4 | –1.7 | –2.5, –0.9 | ||
| Fluoxetine | 39 | 1.1 | 20 | 4.5 | –0.4, 9.3 | 9.4 | 2.7, 16.2 | –2.6 | –5.2, 0.0 |
| Citalopram | 35 | 1.1 | 20 | 12.8 | 7.3, 18.2 | 19.5 | 11.9, 27.1 | –3.7 | –6.5, –0.9 |
| Paroxetine | 263 | 1.0 | 20 | 1.7 | –0.4, 3.7 | 3.5 | 0.6, 6.4 | –0.9 | –1.9, 0.2 |
| Sertraline | 42 | 1.2 | 50 | 1.7 | –3.4, 6.9 | 9.2 | 2.0, 16.4 | –4.1 | –6.7, –1.5 |
| Fluvoxamine | 52 | 1.8 | 100 | 1.7 | –2.9, 6.3 | 5.7 | –0.7, 12.1 | –1.8 | –4.2, 0.5 |
| Escitalopram | 5 | 1.0 | 10 | 2.7 | –11.6, 16.9 | 2.5 | –17.4, 22.3 | –0.3 | –7.3, 6.7 |
β, mean difference in QTcF (corrected with Fridericia’s formula; in ms) QT (uncorrected, in ms) or heart rate (in beats min–1) between SSRI users and non-users (reference group); CI, confidence interval; DDD, defined daily dose; n, number of ECG measurements; PDD: DDD, ratio of the prescribed daily dosage and the defined daily dosage; SSRIs, selective serotonin re-uptake inhibitors.
The mean prescribed daily dosage with respect to the defined daily dosage. Analyses were adjusted for age and gender. Results presented as the difference with respect to non-users of antidepressants.
Data obtained from the World Health Organization 44.
Of the different studied individual SSRIs, ECGs recorded during citalopram use had a 12.8 ms longer QTcF (90% CI 7.3, 18.2) and a 19.5 ms longer uncorrected QT interval (90% CI 11.9, 27.1) than ECGs recorded during non-use of antidepressants. Heart rate was 3.7 beats min–1 lower on ECGs recorded during citalopram use than on ECGs recorded during non-use of antidepressants. Besides escitalopram, which was only prescribed during five ECGs, the upper limit of the 90% CI was not higher than 10 ms for the other studied SSRIs (Table2).
All results remained similar after exclusion of ECGs with cardiac pathologies and additional adjustment for co-variables (e.g. BMI and definite QTc-prolonging drugs). A total of 14 ECGs was recorded during use of citalopram with a maximum daily dosage of 20 mg, and in participants aged 60 years and older. In this subsample, QTcF was 15.5 ms longer (90% CI 6.8, 24.1) in citalopram users than in non-users of antidepressants.
Longitudinal analyses
Table3 shows the changes in QTcF and heart rate associated with different pairs of consecutive ECG recordings. In total, 13 225 pairs of ECGs were available for analyses with a median time interval between two consecutive visits of 4.3 years (interquartile range 2.4–5.0).
Table 3.
Changes in heart rate corrected QT (QTcF) interval stratified by exposure changes between two consecutive electrocardiogram (ECG) measurements
| Exposure definition 1 | Exposure definition 2 | |||||
|---|---|---|---|---|---|---|
| n | change (ms) | 90% CI | n | change (ms) | 90% CI | |
| Non-user | 13 036 | – | reference | 12 881 | – | reference |
| Any SSRI | 189 | 1.6 | –0.3, 3.5 | 114 | 1.1 | –1.6, 3.8 |
| Fluoxetine | 14 | –5.9 | –12.9, 1.2 | 11 | –3.9 | –12.6, 4.9 |
| Citalopram | 10 | 21.5 | 12.1, 30.8 | 5 | 28.9 | 15.3, 42.5 |
| Paroxetine | 120 | 2.2 | –0.1, 4.6 | 69 | 1.9 | –1.7, 5.6 |
| Sertraline | 19 | –4.4 | –10.4, 1.7 | 12 | –5.8 | –14.5, 2.9 |
| Fluvoxamine | 24 | 0.1 | –5.2, 5.4 | 12 | –10.8 | –19.3, –2.3 |
β, mean difference in QTcF (corrected with Fridericia’s formula; in ms) and heart rate (in beats min–1) between SSRI users and non-users (reference group); CI, confidence interval; n, number of ECG pairs; SSRIs, selective serotonin re-uptake inhibitors. Escitalopram could not be studied because of a too low number of ECGs for participants who were using it. Non-users at both ECG assessments were used as the reference. All analyses were adjusted for age and gender. Results are presented as the difference in change with respect to paired ECGs of non-use. Exposure definition 1 refers to the use of (individual) SSRIs at the subsequent visit irrespective of use at the previous visit. Exposure definition 2 refers to the use of (individual) SSRIs at the subsequent visit when no antidepressants were used at the previous visit.
ECG pairs with SSRI use at the second recording had no longer QTcF when compared with ECG pairs with non-use during the second recording. The results were similar for both definitions of exposure (β estimates are 1.6 and 1.1 ms, respectively, for the sample irrespective of previous exposure and for the sample with non-use during the previous visit).
Of the individual SSRIs, citalopram use at the second recording of an ECG pair was associated with a 21.5 ms (90% CI 12.1, 30.8) longer QTcF than ECG pairs during non-use at the second recording irrespective of antidepressant use during the first round of an ECG pair. The difference in QTcF was somewhat more explicit when no antidepressant use was allowed during the first visit of an ECG pair (28.9 ms, 90% CI 15.3, 42.5). No prolonged QTcF was observed for the other SSRIs. For these SSRIs, the upper limit of the 90% CIs was not above 6 ms for both definitions of exposure during the first visit of an ECG pair.
Results remained similar after additional adjustment for the covariables (e.g. BMI and definite QTc-prolonging drugs) and exclusion of ECGs with cardiac pathologies. In participants aged 60 years and older, use of citalopram during the second ECG recording with a maximum daily dosage of 20 mg was associated with a 22.2 ms longer QTcF (90% CI 11.4, 33.0). Due to a limited number of users, this analysis was not conducted when no antidepressant use was allowed during the first visit of an ECG pair.
Discussion
In our study population of middle-aged and elderly participants, QTcF during use of SSRIs as a class was not different from the QTcF during non-use of antidepressants. In the analyses where individual SSRIs were studied separately, only use of citalopram was consistently associated with a longer QTcF in the cross-sectional and longitudinal analysis. This result was similarly observed in a subsample of participants aged 60 years and older who were prescribed citalopram with a maximum daily dose of 20 mg.
The association between use of SSRIs as one exposure class and QTc has been studied in our study population before, but with a smaller sample size 21. Nevertheless, the observed difference between ECGs recorded during use of SSRIs and during non-use of antidepressants in the present study population was considered too small to be of any clinical importance by regulatory authorities 40. Furthermore, the upper limits of the confidence intervals were not above 5 ms. Therefore, our study adds to the evidence that SSRIs as a class, including drugs that share a similar pharmacological target, do not or at best minimally, prolong the QTcF.
For the individual SSRIs, this study is in line with a large cross-sectional study that used electronic health care records 17, but is at variance with other, often smaller, studies 18,41. Except for fluvoxamine, all other SSRIs have been listed to have QTc-prolonging properties 15,16, and have been observed to have the potential to block the HERG channel 11–14. This discrepancy might be explained by variation in patient populations or by a too low number of users of specific SSRIs in some of these studies, including ours. However, except for escitalopram which was only prescribed during five ECG recordings, all other SSRIs had an upper limit of the confidence interval below 10 ms. For paroxetine, the SSRI with the largest number of users, the upper limit of the confidence interval was below 5 ms, which means that there is a 90% probablility that paroxetine has no QTc-prolonging properties 40. For the other SSRIs, this means that there is, at most, moderate QTcF prolongation, which is considered as minimally clinically relevant 40. Thus, despite the fact that most SSRIs have been observed to block the hERG channel 11–14, in our naturalistic setting, no QTcF prolongation was observed for most SSRIs that was of clinical importance. Nevertheless, we cannot rule out that SSRIs like fluoxetine and sertraline have some QTcF increasing effects since the number of users was limited and the upper limit of the 90% CI in the cross-sectional analysis was above 5 ms.
In August 2011, the FDA issued a warning concerning citalopram. The FDA recommended a maximum citalopram dosage of 40 mg day–1 in normal patients and 20 mg in high risk patients (e.g. >60 years of age) 19,20. In the present study, we additionally restricted analyses to ECGs recorded in participants aged 60 years and older to assess the association between a citalopram-dosing regimen of maximum 20 mg day–1 and QTcF. In the cross-sectional analysis, we observed a 15.5 ms longer QTcF on ECGs during citalopram use than on ECGs during no use of antidepressants. In the longitudinal analysis an approximately 20 ms longer QTcF was observed in participants aged 60 years and older with a citalopram dosage equal or less than 20 mg day–1. As also the lower limit of the confidence interval was above 10 ms, this result may suggest that there is a considerably increased risk of arrhythmias 40. However, in contrast to what our data suggest, the clinical importance of the restrictions for citalopram are under debate, as patients treated with a daily dose of citalopram of at least 40 mg have not been associated with an increased risk of sudden cardiac death or cardiac mortality 42,43.
Our study has a number of strengths. First, we were able to study the within-person change in QTcF dependent on the use of individual SSRIs because for most participants, more than one ECG was available. Although the longitudinal analysis is still observational, it can provide additional arguments in favour of a causal relationship. As similar results were observed in the cross-sectional and longitudinal analysis with respect to the effect of citalopram use on QTcF measures, the observed effects seem to be caused by the drug instead of by other factors. Second, pharmacy dispensing data were registered prospectively and irrespective of disease status, which minimizes the risk of information and/or recall bias. And finally, all ECGs were processed using the MEANS program. MEANS calculates the QT interval and heart rate automatically, which reduces intra- and inter-observer variability in assessment of the QTcF 29–31. This study also has some limitations. First, some of the individual SSRI drugs were dispensed in low numbers. Therefore, it was not possible to conduct dose–response analyses. Nevertheless, numbers were sufficient to observe that citalopram gave a clinically relevant prolongation in QTcF, and that paroxetine is most likely not associated with any clinically relevant prolongation in QTcF. Second, the median time period between two consecutive ECG recordings was 4.3 years, which is long for the calculation of the change in QTcF interval. Therefore, a possible acute effect of (individual) SSRIs on QTcF cannot be ruled out. Third, due to the non-randomized nature of the data, the analyses are subject to confounding factors. We examined several possible confounders and adjusted or restricted our analyses to eliminate potential confounding as much as possible, and this did not influence the results.
In conclusion, this study showed that, while no SSRI class effect on QTcF was observed, the use of citalopram was associated with a longer QTcF while paroxetine was not associated with a longer QTcF. Even with the recommended dosage restriction in participants aged 60 years and older, still a considerably longer QTcF interval was observed in citalopram users.
Author contribution
Data analysis: NMM, RN, NA. Interpretation of the data: NMM, RN, NA, MNN, MEvdB, BHS, LEV. Data acquisition: AH, JAK, BHS. Study design: AH, BHS. Drafting the manuscript: MNN, RN, NA, MNN, MEvdB, LEV. Commenting of initial versions of the manuscript and approval of the final version: NMM, RN, NA, MNN, MEvdB, AH, JAK, BHS, LEV.
Competing Interests
There are no competing interests to declare.
The Rotterdam Study is supported by the Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research (NWO), the Netherlands Organization for Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Netherlands Genomics Initiative (NGI), the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission (DG XII) and the Municipality of Rotterdam. This work was supported by grants from ZonMw (Priority Medicine Elderly grants 113101002 to L.E.V).
References
- Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–97. doi: 10.7326/0003-4819-119-12-199312150-00006. [DOI] [PubMed] [Google Scholar]
- Myerburg RJ, Interian A, Jr, Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997;80:10F–19F. doi: 10.1016/s0002-9149(97)00477-3. [DOI] [PubMed] [Google Scholar]
- Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest - the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–9. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–5. [PubMed] [Google Scholar]
- Straus SM, Sturkenboom MC, Bleumink GS, Dieleman JP, van der Lei J, de Graeff PA, Kingma JH, Stricker BH. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26:2007–12. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- van Noord C, Rodenburg EM, Stricker BH. Invited commentary: sex-steroid hormones and QT-interval duration. Am J Epidemiol. 2011;174:412–5. doi: 10.1093/aje/kwr170. [DOI] [PubMed] [Google Scholar]
- Newton-Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, Bis JC, Marciante K, Rivadeneira F, Noseworthy PA, Sotoodehnia N, Smith NL, Rotter JI, Kors JA, Witteman JC, Hofman A, Heckbert SR, O’Donnell CJ, Uitterlinden AG, Psaty BM, Lumley T, Larson MG, Stricker BH. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet. 2009;41:399–406. doi: 10.1038/ng.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Gut B, Wendt-Nordahl G, Kiehn J. The antidepressant drug fluoxetine is an inhibitor of human ether-a-go-go-related gene (HERG) potassium channels. J Pharmacol Exp Ther. 2002;300:543–8. doi: 10.1124/jpet.300.2.543. [DOI] [PubMed] [Google Scholar]
- Lee SH, Sung MJ, Lee HM, Chu D, Hahn SJ, Jo SH, Choe H, Choi BH. Blockade of HERG human K+ channels by the antidepressant drug paroxetine. Biol Pharm Bull. 2014;37:1495–504. doi: 10.1248/bpb.b14-00244. [DOI] [PubMed] [Google Scholar]
- Lee HA, Kim KS, Hyun SA, Park SG, Kim SJ. Wide spectrum of inhibitory effects of sertraline on cardiac ion channels. The Korean J Physiol Pharmacol: Official Journal of The Korean Physiol Soc, Korean Soc Pharmacol. 2012;16:327–32. doi: 10.4196/kjpp.2012.16.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchel HJ, Pabbathi VK, Hofmann G, Paul AA, Hancox JC. Inhibitory actions of the selective serotonin re-uptake inhibitor citalopram on HERG and ventricular L-type calcium currents. FEBS Lett. 2002;512:59–66. doi: 10.1016/s0014-5793(01)03320-8. [DOI] [PubMed] [Google Scholar]
- De Ponti F, Poluzzi E, Montanaro N. Organising evidence on QT prolongation and occurrence of Torsades de Pointes with non-antiarrhythmic drugs: a call for consensus. Eur J Clin Pharmacol. 2001;57:185–209. doi: 10.1007/s002280100290. [DOI] [PubMed] [Google Scholar]
- Woosley RL. 2014. Drugs that prolong the QTc interval and/or induce torsade de pointes. Available at http://www.crediblemeds.org/everyone/composite-list-all-qtdrugs/ (last accessed 22 August )
- Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, Erb JL, Churchill SE, Kohane IS, Iosifescu DV, Smoller JW, Perlis RH. QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ. 2013;346:f288. doi: 10.1136/bmj.f288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SR, Kostis WJ, Celano CM, Januzzi JL, Ruskin JN, Noseworthy PA, Huffman JC. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J Clin Psychiatry. 2014;75:e441–9. doi: 10.4088/JCP.13r08672. [DOI] [PubMed] [Google Scholar]
- American Food and Drug Administration. 2014. FDA drug safety communication: abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). Available at http://www.fda.gov/drugs/drugsafety/ucm269086.htm (last accessed 9 September )
- Food American, Administration Drug. 2014. FDA drug safety communication: abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). Available at: http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm (last accessed 9 September )
- van Noord C, Straus SM, Sturkenboom MC, Hofman A, Aarnoudse AJ, Bagnardi V, Kors JA, Newton-Cheh C, Witteman JC, Stricker BH. Psychotropic drugs associated with corrected QT interval prolongation. J Clin Psychopharmacol. 2009;29:9–15. doi: 10.1097/JCP.0b013e318191c6a8. [DOI] [PubMed] [Google Scholar]
- Hofman A, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, Klaver CC, Nijsten TE, Peeters RP, Stricker BH, Tiemeier HW, Uitterlinden AG, Vernooij MW. The Rotterdam Study: 2014 objectives and design update. Eur J Epidemiol. 2013;28:889–926. doi: 10.1007/s10654-013-9866-z. [DOI] [PubMed] [Google Scholar]
- Aarts N, Noordam R, Hofman A, Tiemeier H, Stricker BH, Visser LE. Utilization patterns of antidepressants between 1991 and 2011 in a population-based cohort of middle-aged and elderly. Eur Psychiatry. 2014;29:365–70. doi: 10.1016/j.eurpsy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study) Am J Cardiol. 1992;70:797–801. doi: 10.1016/0002-9149(92)90562-d. [DOI] [PubMed] [Google Scholar]
- Sarma JS, Sarma RJ, Bilitch M, Katz D, Song SL. An exponential formula for heart rate dependence of QT interval during exercise and cardiac pacing in humans: reevaluation of Bazett’s formula. Am J Cardiol. 1984;54:103–8. doi: 10.1016/0002-9149(84)90312-6. [DOI] [PubMed] [Google Scholar]
- Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:982–91. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7:403–22. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- Darpo B, Agin M, Kazierad DJ, Layton G, Muirhead G, Gray P, Jorkasky DK. Man versus machine: is there an optimal method for QT measurements in thorough QT studies? J Clin Pharmacol. 2006;46:598–612. doi: 10.1177/0091270006286900. [DOI] [PubMed] [Google Scholar]
- de Bruyne MC, Kors JA, Hoes AW, Kruijssen DA, Deckers JW, Grosfeld M, van Herpen G, Grobbee DE, van Bemmel JH. Diagnostic interpretation of electrocardiograms in population-based research: computer program research physicians, or cardiologists? J Clin Epidemiol. 1997;50:947–52. doi: 10.1016/s0895-4356(97)00100-5. [DOI] [PubMed] [Google Scholar]
- van Bemmel JH, Kors JA, van Herpen G. Methodology of the modular ECG analysis system MEANS. Methods Inf Med. 1990;29:346–53. [PubMed] [Google Scholar]
- Willems JL, Abreu-Lima C, Arnaud P, van Bemmel JH, Brohet C, Degani R, Denis B, Gehring J, Graham I, van Herpen G. The diagnostic performance of computer programs for the interpretation of electrocardiograms. N Engl J Med. 1991;325:1767–73. doi: 10.1056/NEJM199112193252503. , et al. [DOI] [PubMed] [Google Scholar]
- Willems JL, Arnaud P, van Bemmel JH, Bourdillon PJ, Degani R, Denis B, Graham I, Harms FM, Macfarlane PW, Mazzocca G, Meyer J, Zywiets C. A reference data base for multilead electrocardiographic computer measurement programs. J Am Coll Cardiol. 1987;10:1313–21. doi: 10.1016/s0735-1097(87)80136-5. , for the Common Standard for Qunatitative Electrocardiography (CSE) Working Party. [DOI] [PubMed] [Google Scholar]
- Kors JA, van Herpen G. Methodology of QT-interval measurement in the modular ECG analysis system (MEANS) Ann Noninvasive Electrocardiol. 2009;14(Suppl 1):S48–53. doi: 10.1111/j.1542-474X.2008.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanchen D, Leening MJ, Locatelli I, Cornuz J, Kors JA, Heeringa J, Deckers JW, Hofman A, Franco OH, Stricker BH, Witteman JC, Dehghan A. Resting heart rate and the risk of heart failure in healthy adults: the Rotterdam Study. Circ Heart Fail. 2013;6:403–10. doi: 10.1161/CIRCHEARTFAILURE.112.000171. [DOI] [PubMed] [Google Scholar]
- Fridericia L. Die Systolendauer im Elektrokardiogramm bei normalen Menschen und bei Herzkranken. Acta Med Scand. 1920;53:469–86. [Google Scholar]
- American Food and Drug Administration. 2015. Guidance for Industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073153.pdf (last accessed 27 June )
- World Health Organization. International society of hypertension guidelines for the management of hypertension. Guidelines subcommittee. J Hypertens. 1999;17:151–83. [PubMed] [Google Scholar]
- Leening MJ, Kavousi M, Heeringa J, van Rooij FJ, Verkroost-van Heemst J, Deckers JW, Mattace-Raso FU, Ziere G, Hofman A, Stricker BH, Witteman JC. Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol. 2012;27:173–85. doi: 10.1007/s10654-012-9668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfinger R. Covariance structure selection in general mixed models. Commun Stat-Simul C. 1993;22:1079–106. [Google Scholar]
- American Food and Drug Administration. 2005. Guidance for Industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073153.pdf (last accessed 27 June )
- van Haelst IM, van Klei WA, Doodeman HJ, Warnier MJ, De Bruin ML, Kalkman CJ, Egberts TC. QT interval prolongation in users of selective serotonin reuptake inhibitors in an elderly surgical population: a cross-sectional study. J Clin Psychiatry. 2014;75:15–21. doi: 10.4088/JCP.13m08397. [DOI] [PubMed] [Google Scholar]
- Vieweg WV, Hasnain M, Howland RH, Hettema JM, Kogut C, Wood MA, Pandurangi AK. Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am J Med. 2012;125:859–68. doi: 10.1016/j.amjmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Zivin K, Pfeiffer PN, Bohnert AS, Ganoczy D, Blow FC, Nallamothu BK, Kales HC. Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40 mg. Am J Psychiatr. 2013;170:642–50. doi: 10.1176/appi.ajp.2013.12030408. [DOI] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology. 2015. Guidelines for ATC classification and DDD assignment. Available at http://www.whocc.no/atcddd/ (last accessed 27 June )


