Abstract
Aims
TNF-α inhibitors are considered relatively safe in pregnancy but experience is still limited. The aim of this study was to evaluate the risk of major birth defects, spontaneous abortion, preterm birth and reduced birth weight after first trimester exposure to TNF-α inhibitors.
Methods
Pregnancy outcomes of women on adalimumab, infliximab, etanercept, certolizumab pegol or golimumab were evaluated in a prospective observational cohort study and compared with outcomes of a non-exposed random sample. The samples were drawn from pregnancies identified by institutes collaborating in the European Network of Teratology Information Services.
Results
In total, 495 exposed and 1532 comparison pregnancies were contributed from nine countries. The risk of major birth defects was increased in the exposed (5.0%) compared with the non-exposed group (1.5%; adjusted odds ratio (ORadj) 2.2, 95% CI 1.0, 4.8). The risk of preterm birth was increased (17.6%; ORadj 1.69, 95% CI 1.1, 2.5), but not the risk of spontaneous abortion (16.2%; adjusted hazard ratio [HRadj] 1.06, 95% CI 0.7, 1.7). Birth weights adjusted for gestational age and sex were significantly lower in the exposed group compared to the non-exposed cohort (P = 0.02). As a diseased comparison group was not possible to ascertain, the influence of disease and treatment on birth weight and preterm birth could not be differentiated.
Conclusions
TNF-α inhibitors may carry a risk of adverse pregnancy outcome of moderate clinical relevance. Considering the impact of insufficiently controlled autoimmune disease on the mother and the unborn child, TNF-α inhibitors may nevertheless be a treatment option in women with severe disease refractory to established immunomodulatory drugs.
Keywords: TNF-α inhibitors, pregnancy outcome, birth defects, malformations, birth weight
What Is Already Known about this Subject
TNF-α inhibitors constitute an important treatment option of autoimmune disorders in women of childbearing age.
Large studies on TNF-α inhibitors during pregnancy are lacking.
Selected cases report on malformations in the offspring. However, overall, no increased risk of birth defects has been observed as yet.
What this Study Adds
Prenatal TNF-α inhibitor exposure for maternal chronic inflammatory conditions led to a) A moderately increased risk of birth defects without a distinct pattern of malformations, b) An increased risk of preterm birth and reduced birth weight, c) No increased risk of spontaneous abortion.
Introduction
Tumour necrosis factor alpha (TNF-α) inhibitors are approved for the treatment of moderate to severe forms of various chronic inflammatory conditions like inflammatory bowel disease (IBD) or rheumatoid arthritis (RA). The five approved TNF-α inhibitors, adalimumab (ADA), certolizumab pegol (CZP), etanercept (ETA), golimumab (GOL) and infliximab (IFX), are particularly indicated in cases of severe disease or following insufficient response to other disease modifying drugs (DMDs), partly in combination with methotrexate (MTX). Although they are regarded as relatively safe, recommendations remain inconclusive concerning the treatment of pregnant women or patients planning a pregnancy while under therapy 1. This represents a topic of urgent interest, given that women of childbearing age are one of the main groups affected by IBD 2. Thus, exposure during either planned or unintended pregnancy is considered a likely occurrence. Possible risks discussed after immunosuppressant therapy during pregnancy include teratogenicity, higher rates of spontaneous abortion, preterm birth, growth retardation and compromised fetal immunity 3,4.
Since all the approved TNF-α inhibitors are substances of high molecular weight, they are expected to require active transport to cross the human placenta. A similar process is known for IgG antibodies and is generally understood to only occur after the 20th week of gestation. Therefore, placental transfer during the embryonic period is not expected. Clinical experience is limited and varies widely between the five agents. In total, approximately 500 pregnancies exposed to TNF-α inhibitors have been published via registries, pharmacovigilance surveillance, case series 3,5–8, and cohort studies 9,10. Additionally a variety of case reports, abstracts and conference communications are available 1,11. While the main body of reports covers IFX, ADA, and ETA, there are only few cases with CZP and with GOL 12. Though malformations were described in a few pregnancies 5, these were heterogeneous and no distinct pattern was observed. Currently, there is no evidence of an increased malformation risk. This is in line with the preliminary results of the Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes (PIANO) study reporting 161 pregnancies exposed to TNF-α inhibitors 13.
A report on a child with VACTERL association (V: vertebral defects; A: anal atresia; C: cardiac anomalies; T: tracheal-oesophageal fistula; E: oesophageal atresia; R: radial and renal problems; L: limb anomalies) following exposure to ETA throughout pregnancy 14 led to considerable discussion 15. Interestingly, the authors were unable to ascertain additional children with VACTERL syndrome among 22 retrospectively recorded pregnancies with birth defects 7, though, in a somewhat unorthodox approach, they suggested isolated heart defects to be part of an incomplete VACTERL. An evaluation of the EUROCAT database accordingly did not confirm VACTERL to be associated with TNF-α inhibitor exposure 16.
A register evaluation including 71 prospectively recorded pregnancies on TNF-α inhibitors, among them 48 with ETA, nine with IFX and 14 with ADA, revealed a 27% miscarriage rate. Even after exclusion of pregnancies with MTX co-medication, the miscarriage rate remained high. However, the total number of evaluated pregnancies was small and the results have not been confirmed by other studies to date 8. Furthermore, TNF-α has been suggested to be an important factor in spontaneous abortion aetiology and data are available which have shown that ADA and ETA may prevent (recurrent) miscarriage 17,18.
After the 20th week of pregnancy there is an increasing placental transfer of monoclonal antibodies through an active process via the neonatal Fc receptor 19. This applies particularly to the full antibodies ADA and IFX 20,21. However, simultaneous measurements in the mother’s blood and the cord blood demonstrated that ETA, a TNF receptor-Fc fusion protein, also crosses the placenta 22. Placental transfer for CZP, a pegylated Fab fragment of a recombinant humanized anti-TNF-α monoclonal antibody, was also shown 20. The mechanism of transfer is not yet understood since it lacks an Fc portion which is essential for active transfer. There are no human reports on placental transfer for GOL as yet. However transfer can be assumed as it is a complete IgG1 antibody 23.
The aim of this collaborative prospective cohort study was to evaluate the risks of major birth defects and spontaneous abortion after first trimester exposure to TNF-α inhibitors. Secondary objectives were to evaluate the risks of preterm birth and reduced birth weight as well as the rate of electively terminated pregnancies. It was hypothesized that our study would add further evidence for the safety of TNF-α inhibitors during the first trimester of pregnancy.
Methods
The study was a prospective observational multicentre cohort study, i.e. neither the outcome of the pregnancy nor the results of prenatal diagnostic tests were known at the time of subject enrolment. The exposed and comparison cohort consisted of pregnancies enrolled at teratology information services (TIS) between 1998 and 2013. TIS offer risk assessment to health care professionals and pregnant women who spontaneously contact these services for consultation in pregnancy. The study was reported in accordance with the recommendations of the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement adapted to the needs of pregnancy outcome studies 24,25. Ethics approval was obtained from the ethics committee of the Charité Universitätsmedizin Berlin (no. EA4/013/13). The study was registered at the German Clinical Trials Register (no. DRKS00005036) and at ENCePP (http://www.encepp.eu/encepp/viewResource.htm?id=6138).
Patients
Data of exposed and comparison women were obtained from pregnancies identified by 11 institutions from nine countries collaborating within the European Network of Teratology Information Services (ENTIS): Australia (n = 24), Finland (n = 47), France (n = 580), Germany (n = 951), Italy (n = 91), The Netherlands (n = 196), Turkey (n = 12), Switzerland (n = 60) and the United Kingdom (n = 68).
The exposed group was defined as pregnant women who had been exposed to more than one dose of one of the five approved TNF-α inhibitors (ADA, CZP, ETA, GOL and IFX) at any time during the first 12 weeks after the last menstrual period (LMP). Exposure may have started earlier and can have continued longer. The comparison group consisted of non-exposed pregnant women identified through spontaneous TIS consultations for other conditions or exposures such as hair-dyeing, urinary tract infection, asthma or depression. Exclusion criteria for both groups were women with malignancies and exposure to known major teratogens or fetotoxicants, namely acitretin, isotretinoin, mycophenolate, thalidomide, valproic acid as well as angiotensin-II receptor blockers and ACE inhibitors, when used during the 2nd and/or 3rd trimester. Low dose MTX was not considered an exclusion criterion.
Each TIS identified pregnancies that met the study criteria. The target sample size to be reached was at least 200 exposed and 600 controls. From the eligible pool within each TIS, pregnancies for the non-exposed comparison group were randomly selected and frequency matched to the exposed cohort on year of ascertainment, aiming at an approximate 3:1 ratio per centre. The same method of ascertainment applied for each TIS. The data were then combined across centres.
Data were collected on exposure and outcome from structured telephone or face-to-face interviews and/or mailed questionnaires obtained from both the mother and/or her physician(s) after oral informed consent. Data were collected on demographics, maternal age, pregnancy history including previous number of children with birth defects, pre-pregnancy body mass index, medications including both prescription and over-the-counter, detailing specific dosages and dates of exposure and smoking and alcohol consumption. Details regarding the course and outcome of pregnancy were obtained 9 weeks after birth and focused on pregnancy complications and birth defects. In addition, details of delivery, pregnancy loss, and gestational age at pregnancy loss or at birth, sex, birth weight, length and head circumference were collected.
Outcomes
The primary endpoints were major birth defects and the risk of spontaneous abortion (SAB). Secondary endpoints included the rate of elective termination as well as an assessment of the reasons provided for terminating a pregnancy, and the risk of preterm birth as well as the infant’s birth weight. Fetal abnormalities in pregnancy losses including elective terminations of pregnancy (ETOPs) were considered in the calculation of birth defect rates.
Birth defects were classified as major or minor by two of the authors (CWS and CS) according to the EUROCAT classification system. The classifications were performed independently and blinded to exposure status. In case of disagreement between the two authors, consensus was achieved through discussion.
Concomitant use of MTX or other disease-modifying drugs (DMDs) was taken into account in all analyses.
Weeks of gestation were calculated by ultrasound during first trimester or, if not available, from the LMP. SAB was defined as spontaneous pregnancy loss of a fetus <500 g or if weight not known <23 completed weeks after LMP. Gestational age at delivery and birth weight were measured as continuous variables. Birth weight was adjusted for gestational age at birth and sex.
Statistical analysis
Logistic regression was used to evaluate the risk of major birth defects. Crude birth defect rates were calculated by dividing the number of infants and fetuses with birth defects by the number of all live-born infants plus the number of stillbirths/aborted fetuses with birth defects. Birth defects known to be of genetic aetiology were considered separately. The final analysis involved propensity score adjustment for bias reduction, classifying pregnant women into five strata defined by the quintiles of the propensity score 26. Propensity score estimation used boosted regression trees 27, including maternal age, alcohol consumption, smoking status, and numbers of previous pregnancies, miscarriages and previous infants with birth defects as covariates, and was repeated for each analysis. Maternal therapy with other DMDs was used as a covariate directly in the analysis, in addition to propensity score stratification, to account for effects of these concomitant medications. It was differentiated whether MTX was part of the DMDs therapy or not. Adjustment was used as described above in all multivariate analyses.
The cumulative incidences of spontaneous abortion and elective termination were assessed using event history analysis for cause-specific sub-distributions of competing risks while accounting for left truncation due to varying time of gestation at enrolment 28. Hazard ratios (HRs) were then estimated using Cox proportional hazards models.
The effect on the risk of preterm birth was assessed using logistic regression. For the comparison of birth weights between groups, live births from all centres were classified according to new-born birth weight percentile categories 29. A score was determined through standardization and included in a linear regression model as the dependent variable.
For all models that included covariates, missing values were addressed through multiple imputation using chained equations, assuming that the data were missing at random 30. Twenty imputed data sets were generated per outcome. The models of multiple imputations were based on the respective outcomes and the covariates used to estimate the propensity score. For each imputed data set, analyses were performed as described above. Results were then combined using Rubin’s rule 31.
Heterogeneity among contributing centres was tested using the Breslow-Day test of homogeneity for dichotomous outcomes and the analysis of variance (ANOVA) F test for continuous outcomes. Data from Finland, Australia and Turkey were excluded from heterogeneity analysis due to small numbers. All data analyses were performed at the Berlin Institute using R version 2.15.
Results
Cohort size, exposures and maternal characteristics
The study period comprised the period from 1998 until 2013. Follow-up of pregnancy outcome after maternal first trimester TNF-α inhibitor therapy meeting the study criteria was initiated in 629 cases and completed in 495 (79%). Causes for lost-to-follow-up were diverse such as moving house, changing doctors or simply lack of time or interest. In total, 172 ADA, 168 IFX, 140 ETA, 7 CZP, 3 GOL exposed plus five double exposed (three × ADA + ETA; two × ADA + IFX) pregnancies and 1532 comparison pregnancies were contributed from nine countries.
The most frequent treatment indications for a TNF-α inhibitor therapy were IBD (48.1%) and RA (26.9%) (Table1). Due to the approved treatment indications, the majority of women with ETA therapy were treated for RA (70%) followed by ankylosing spondylitis (18%) whereas IFX was mainly prescribed for IBD (86%). ADA was given for IBD in over half of the cases and in 47% for rheumatic disorders (RA, ankylosing spondylitis and psoriasis/psoriatic arthritis).
Table 1.
Drug treatment indication of TNF-α inhibitor exposed pregnancies (numbers)
| Drug treatment indication | Number (%) |
|---|---|
| Inflammatory bowel disease | 238 (48.1) |
| Ulcerative colitis | 36 (7.2) |
| Crohn‘s disease | 200 (40.4) |
| IBD (unclassified or unclassifiable) | 2 (0.4) |
| Rheumatoid arthritis | 133 (26.9) |
| Ankylosing spondylitis | 68 (13.7) |
| Psoriasis/psoriatic arthritis | 39 (7.9) |
| Miscellaneous | 13 (2.6) |
| Unknown | 4 (0.8) |
| Total | 495 |
The overall median treatment duration during pregnancy was 6.9 weeks (IQR 4.0–25.0). The median gestational week at time of last drug administration was week 7.4 (IQR 4.0–24.0) for ADA, week 5.0 (IQR 4.0–7.4) for ETA and week 22.6 (IQR 5.0–32.0) for IFX.
For maternal characteristics see Table2. Low dose MTX exposure occurred in 7.5% of the exposed pregnancies and in 0.1% of the comparison cohort. In total, almost half of the patients in the TNF-α inhibitor group were concomitantly treated with other DMDs, such as MTX (n = 37), mesalazine/sulfasalazine (n = 49), azathioprine/mercaptopurine (n = 55), leflunomide (n = 5), hydroxychloroquine (n = 8), ciclosporin (n = 4) and systemic glucocorticoids (n = 167). Therapy with DMDs took place in 5.6% of the patients in the non-exposed comparison cohort.
Table 2.
Maternal characteristics by cohorts
| TNF-α inhibitor (n = 495) | Comparison (n = 1532) | |
|---|---|---|
| Maternal age, n | 481 | 1431 |
| Age (years) median (IQR) | 30 (27–34) | 31 (28–35) |
| min–max (years) | 16–42 | 14–46 |
| BMI (pre-pregnancy), n | 291 | 883 |
| BMI (kg m–2) median (IQR) | 22 (20.3–24.9) | 22.6 (20.6–25.6) |
| Smoking, n | 384 | 1202 |
| No n (%) | 311 (81) | 1040 (86.5) |
| <= 5 cigarettes/day n (%) | 21 (5.5) | 59 (4.9) |
| >5 cigarettes/day n (%) | 52 (13.5) | 103 (8.6) |
| Alcohol, n | 366 | 1147 |
| No n (%) | 342 (93.4) | 1075 (93.7) |
| <= 1 drink/day n (%) | 14 (3.8) | 55 (4.8) |
| >1 drink/day n (%) | 10 (2.7) | 17 (1.5) |
| Mother’s years of schooling, n | 177 | 576 |
| <= 9 years n (%) | 14 (7.9) | 39 (6.8) |
| >9 and < = 13.5 years n (%) | 104 (58.8) | 291 (50.5) |
| Academic degree n (%) | 59 (33.3) | 246 (42.7) |
| Other DMDs, n | 495 | 1532 |
| No n (%) | 250 (50.5) | 1447 (94.5) |
| Other than MTX n (%) | 208 (42.0) | 84 (5.5) |
| Including MTX n (%) | 37 (7.5) | 1 (0.1) |
| Previous pregnancies, n | 451 | 1336 |
| 0 n (%) | 217 (48.1) | 559 (41.8) |
| 1 n (%) | 133 (29.5) | 421 (31.5) |
| 2 n (%) | 53 (11.8) | 204 (15.3) |
| 3 or more n (%) | 48 (10.6) | 152 (11.4) |
| Previous deliveries, n | 449 | 1327 |
| 0 n (%) | 272 (60.6) | 684 (51.5) |
| 1 n (%) | 120 (26.7) | 438 (33.0) |
| 2 n (%) | 40 (8.9) | 139 (10.5) |
| 3 or more n (%) | 17 (3.8) | 66 (5.0) |
| Previous miscarriages, n | 434 | 1295 |
| 0 n (%) | 357 (82.3) | 1066 (82.3) |
| 1 n (%) | 60 (13.8) | 164 (12.7) |
| 2 or more n (%) | 17 (3.9) | 65 (5.0) |
| Previous children with birth defect, n | 414 | 1240 |
| 0 n (%) | 405 (97.8) | 1218 (98.2) |
| 1 n (%) | 8 (1.9) | 20 (1.6) |
| 2 or more n (%) | 1 (0.2) | 2 (0.2) |
| Gestational week at first contact, n | 495 | 1532 |
| Median gestational week (IQR) | 8.1 (6–12.9) | 9 (6.3–14.3) |
BMI, body mass index; DMDs disease-modifying drugs; IQR, interquartile range.
Birth defects
The proportion of infants with birth defects is shown in Table3. There was a significantly increased risk of major birth defects (21 of 421 [5 %]) in the TNF-α inhibitor group compared with the non-exposed comparison cohort (21 of 1,385 [1.5%]) (adjusted odds ratio [OR adj] 2.20 [95% CI 1.01, 4.8]). Concomitant maternal therapy with other DMDs including therapy with MTX and/or systemic glucocorticoids did not explain the increased risk of birth defects after TNF-α inhibitor exposure. The number of major birth defects by substance was nine of 150 (6.0%) after intrauterine ADA exposure, seven of 156 (4.5%) after IFX and six of 111 (5.4%) after ETA exposure.
Table 3.
Rate of birth defects by cohort
| TNF-α inh. (n = 419) | Comparison (n = 1383) | ORadj (95% CI) | |
|---|---|---|---|
| All birth defects (%) | 52/423 (12.3) | 87/1393 (6.2) | 1.64 (1.1, 2.6) |
| Major birth defects (%) | 21/421 (5.0) | 21/1385 (1.5) | 2.20 (1.0, 4.8) |
| Minor birth defects (%) | 27/419 (6.4) | 55/1383 (4.0) | 1.27 (0.7, 2.3) |
| Genetic anomalies (%) | 4/421 (0.9) | 11/1391 (0.8) | 1.80 (0.5, 6.9) |
Varying denominators are due to twin pregnancies and to varying numbers of stillbirths/abortions with malformations in the numerator and denominator.
A detailed description of major birth defects of the TNF-α inhibitor exposed infants/fetuses is shown in Table4. Of note, there was no distinct pattern of birth defects among the TNF-α inhibitor exposed infants. However, there were more cardiac defects than expected, but in most cases they were associated with other birth defects.
Table 4.
Description of major birth defects in the TNF-α inhibitor cohort (n = 21)
| Substance and exposure time (after LMP) | Treatment indication | Co-medication (trimester or exact gestational period) | Gestational age at birth/elective termination (in weeks after LMP) and birth weight, sex | Birth defects |
|---|---|---|---|---|
| ADA from before pregnancy until week 5 + 6 days | Psoriatic arthritis | None | Week 38 + 5 days, 2770 g, female | Hexadactyly both feet, atrial septal defect |
| ADA from before pregnancy until week 38 + 5 days | Crohn’s disease | Prednisolone (1–3), ASS low-dose (1–3), omeprazole (1–3), lansoprazole (1), enoxaparin (1–3) | Week 38 + 5 days, 2540 g, male | Oesophageal atresia type IIIb (Vogt) with tracheo-oesophageal fistula; ventricular septal defect, syndactyly D2/3 both feet, peripheral pulmonary stenosis, PFO |
| ADA from before pregnancy until week 2 + 1 day | Ankylosing spondylitis | Amoxicillin (3), betamethasone (lung maturation, 3) | Week 32 + 3 days, 1840 g, female | Atrial septal defect with left-right shunt and aneurysm, cavum septum pellucidum on both sides, haemangioma at left flank |
| ADA only once in week 3 | Rheumatoid arthritis | Prednisone (1–3), levothyroxine (1–3) | Week 39 + 4 days, 3330 g, female | Ventricular septal defect, hip dysplasia type 2c (Graf) |
| ADA only once in week 1 + 5 days | Psoriasis | Ciclosporin (1), insulin (3) | Week 39 + 3 days, 3490 g, male | Hexadactyly |
| ADA from before pregnancy until week 7 | Ankylosing spondylitis | Ethinylestradiol/chlormadinone (1) | Week 34 + 3 days, 1990 g, male | Haemangioma right temple, (umbilical hernia) |
| ADA started during week 12 until week 14 (two injections) | Crohn’s disease | Prednisolone (1) | Spontaneous abortion in week 15 | Imperforate anus |
| ADA from before pregnancy until week 40 + 5 days | Ankylosing spondylitis | Sulfasalazine (1), fluoxetine(1), alprazolam (1) | Week 40, 3750 g, male | Amniotic band sequence: talipes and amputation of four fingers of the right hand |
| ADA and ETA from before pregnancy until week 2 + 2 days | Rheumatoid arthritis | Low dose methotrexate (from before pregnancy until week 2 + 2 days), prednisolone (1–3), omeprazole (1–3), ibuprofen (1–2). | Week 40 + 2 days, 2520 g, male | Cystic adenomatoid malformation of the right lung dorsobasal (multiple cysts of different sizes, totally 2.3 cm x 4.2 cm, surgery done), incomplete right bundle branch block, pericardial effusion, PFO and PDA, slight persistent pulmonary hypertension |
| ETA from before pregnancy until week 8 | Rheumatoid arthritis | Prednisone (1–3), fluconazole (1) | Week 35 + 3 days, 2200 g, male | Hypoplastic left heart, hypospadias glandis |
| ETA from before pregnancy until week 3 + 5 days | Rheumatoid arthritis | Prednisolone (1–3), thyroxine, ibuprofen (as needed) | Week 37 + 3 days, 2860 g, male | Agenesis of left kidney |
| ETA from before pregnancy until week 4 | Ankylosing spondylitis, Crohn’s disease | Prednisolone (1–3) | Week 36, 2920 g, female | WPW-syndrome with heart failure |
| ETA from before pregnancy until week 5 | Rheumatoid arthritis | None | Week 36 + 4 days, 3200 g, male | Bilateral hydronephrosis |
| ETA from before pregnancy until week 32 + 6 days | Behcet’s disease | Dalteparin (1–3) | Week 32 + 6 days, 2260 g, female | Atrial septal defect, (inguinal hernia) |
| IFX from before pregnancy until week 36 + 4 days | Crohn’s disease | None | Week 38; 2450 g, female | Aneurysmatic subaortic ventricular septal defect |
| IFX from before pregnancy until week 27 + 5 days | Crohn’s disease | Prednisolone (1–3), methylprednisolone (2–3), azathioprine (2–3), corticoid for lung maturation (2–3), antibiotics (2–3) | Week 27 + 5 days, 860 g, female | Two rapidly growing facial haemangiomata (propranolol therapy), (left inguinal hernia, PFO spontaneously closed) |
| IFX from before pregnancy until week 34 + 4 days | Crohn’s disease | Azathioprine (1–3), acyclovir (1), anti-D-immunoglobulins, human insulin (2–3), cefuroxime | Week 40 + 4 days, 3950 g, male | Hydronephrosis and obstructive megaureter right at birth. (Later silent kidney right and hypertrophy of left kidney) |
| IFX only once in week 3 + 6 days | Crohn’s disease | Low dose methotrexate (from before pregnancy until week 4 + 4 days) | Week 38 + 6 days, 2590 g, male | Pelviureteric junction obstruction (surgery required) |
| IFX from before pregnancy until week 25 (weeks 1, 9, 17, 25) | Crohn’s disease | Hydrocortisone rect. (1–2), azathioprine (1–3), mesalazine (1–3), pantoprazole (1–2), isoniazid and pyridoxine (1–2), betamethasone (lung maturation; 3), ibuprofen (1), citalopram (1) | Week 33 + 4 days, 2400 g, male | Hypospadias glandis, hepatic cyst; small aortic-pulmonary collateral |
| IFX from before pregnancy until week 5 + 2 days | Crohn’s disease | Contraceptive pill (until week 5 + 3 days) | Spontaneous abortion week 13 + 5 days (pericentric inversion of chromosome 9). | Megacystis, bilateral talipes |
| IFX from before pregnancy until week 23 + 5 days | Ulcerative colitis | None | Week 33 + 1 day, 1985 g, male (twin pregnancy, other twin healthy) | Facial haemangioma (cryo-therapy) |
LMP last menstrual period; PFO persistent foramen ovale; PDA persistent ductus arteriosus; WPW-syndrome:Wolf Parkinson White syndrome
Pregnancy and neonatal outcome
Table5 gives a summary of pregnancy outcomes of the exposed and non-exposed cohort. The crude rate of live births was slightly lower in the study cohort compared with the comparison group due to more frequent SABs and ETOPs. Cumulative incidences of live birth, SABs, ETOPs and stillbirths are illustrated in Figure1. The risk of SAB was not increased in the TNF-α inhibitor cohort (adjusted hazard ratio [HRadj] 1.06, 95% CI 0.7, 1.7) whereas ETOPs occurred more frequently (HRadj 1.69, 95% CI 1.0, 2.9). Concomitant low dose MTX therapy increased the chance of having an elective termination (HRadj 2.15, 95% CI 1.2, 3.9) as well as the risk of spontaneous abortion (HRadj 1.60, 95% CI 1.0, 2.6).
Table 5.
Pregnancy outcomes by cohort
| TNF-inhibitor | Comparison | |
|---|---|---|
| Pregnancies n | 495 | 1532 |
| SAB n (% after excl. of ETOPs) | 43 (9.3) | 116 * (7.9) |
| Stillbirth n | 5 | 7 * |
| ETOP n (%) | 34 (6.9) | 57 (3.7) |
| voluntary | 28 | 44 |
| maternal disease | 4 | 3 |
| fetal reasons | 1 | 9 |
| unknown reason | 1 | 1 |
| Live birth n (%) | 413 (83.4) | 1355 (88.4) |
| Live-born children n | 419 † | 1383† |
SAB, spontaneous abortion; ETOP, elective termination of pregnancy
including twin pregnancies with one live born infant and one fetal loss/stillbirth
including live born infants from twin pregnancies
Figure 1.
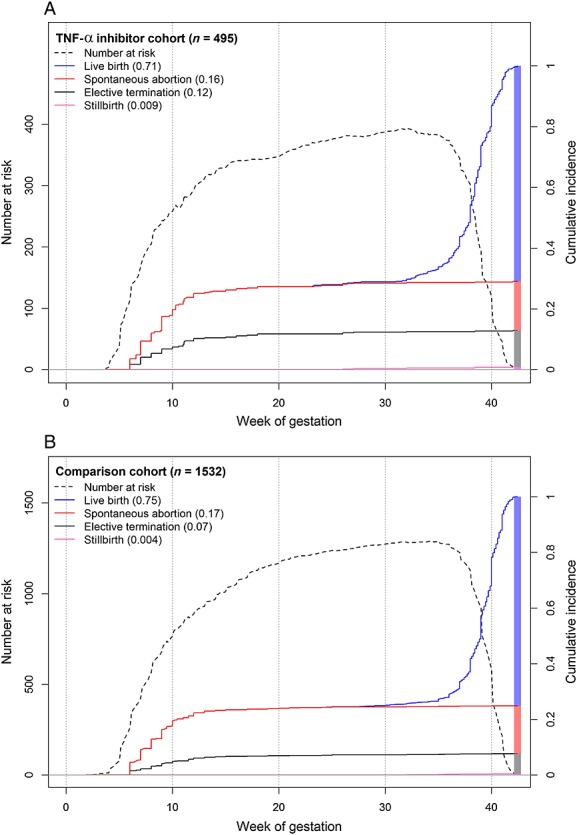
Cumulative incidence rates of fetal loss and live births by cohort. Cumulative incidences of pregnancy outcomes of the TNF-α inhibitor cohort (A) and of the comparison cohort (B) are plotted one above the other. Cumulative incidences for live births are drawn in blue, for spontaneous abortions in red, for elective terminations in black and for stillbirth in pink (y-axis labelling on the right). Of note, the final cumulative incidences add up to 1 covering all possible outcomes. The dotted line represents the number at risk over time (y-axis labelling on the left)
There were significantly more preterm births in the TNF-α inhibitor cohort than in the comparison cohort (ORadj 1.69, 95% CI 1.1, 2.5) and also more infants with low birth weight (Table6). Although the median birth weight of both cohorts was in the normal range, it was lower in the study group (Table6). After adjustment for sex and gestational age at birth, the difference between the cohorts remained significant (P = 0.02). Infants with birth weights ≤ 50th percentile were overrepresented in the exposed cohort (Figure2). The differences in infants’ median birth weight were marginal between ADA (3080 g), ETA (3110 g) and IFX (3200 g). As the median birth weight is influenced by gestational age and by infant’s sex, we compared adjusted weight scores between the three compounds. ETA exposed infants had a score of only –0.24 (IQR –1.0, 0.2), IFX of –0.30 (IQR –0.9, 0.6) and ADA of –0.43 (IQR –0.9, 0.3). Excluding twins from the analyses did not result in notably different findings. There were no significant differences between the TIS with regard to any of the study endpoints.
Table 6.
Child characteristics by cohort
| TNF-α inhibitor (n = 419/407)* | Comparison (n = 1383/1324) | |
|---|---|---|
| Gestational week (GW) at birth, n | 403/391 | 1373/1314 |
| GW, including twins median (IQR) | 38.71 (37.4–40) | 39.43 (38.3–40.3) |
| GW, excluding twins median (IQR) | 38.86 (37.6–40) | 39.57 (38.4–40.4) |
| Preterm birth (<37 weeks), n | 403/391 | 1373/1314 |
| Preterm, including twins n (%) | 71 (17.6) | 123 (9.0) |
| Preterm, excluding twins n (%) | 63 (16.1) | 93 (7.1) |
| Infant’s weight, n | 409/399 | 1357/1299 |
| Weight in g, including twins median (IQR) | 3125 (2745–3450) | 3350 (3020–3660) |
| Weight in g, excluding twins median (IQR) | 3130 (2797.5–3460) | 3374 (3080–3680) |
| Infants with LBW (excluding twins) n (%) | 51 (12.8) | 14 (1.1) |
LBW low birth weight (<2500 g) according to the WHO definition (http://www.who.int/maternal_child_adolescent/documents/9789241548366.pdf). IQR, interquartile range.
numbers with and without twins
Figure 2.
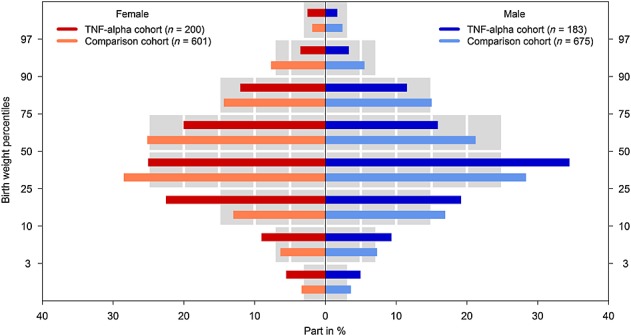
Birth weights according to centile categories and sex by cohort. Coloured bars give the proportions of singletons of both study cohorts according to centile categories. Grey bars represent the proportion of new-borns from the German perinatal survey 29. (The differences in numbers of infants compared with Table4 are due to missing values in the gestational week at delivery, sex of the infant or birth weight)
Discussion
This study evaluated a cohort of 495 prospectively ascertained pregnancies exposed to TNF-α inhibitors during at least the first trimester. Based on animal experiments 32 and human data published to date, this study was expected to further confirm the safety of this group of biologic agents. However, our data revealed an increased rate of birth defects, a significantly lower birth weight and a higher rate of preterm births in the TNF-α inhibitor exposed cohort.
Birth defects
The significant increase in the number of major birth defects is the most striking result of our study. However, the lower limit of the confidence interval was 1.01. The co-medication does not explain this finding. Although low dose MTX was shown to increase the risk of birth defects 33, the therapy with low dose MTX or other immunomodulatory drugs in this study could not account for the higher number of birth defects in exposed pregnancies. Furthermore, among the 21 infants with major birth defects, only two were prenatally exposed to low dose MTX. In both cases these exposures occurred in very early pregnancy and before the vulnerable time window. Pregnancies exposed to other established teratogens were excluded from both cohorts.
We did not observe a distinct pattern of malformations which would be expected for typical teratogens. Likewise, the previously reported suspicion that TNF-α inhibitors may cause a VACTERL association 7,14 could not be confirmed by our study. Indeed, in one infant exposed to ADA throughout pregnancy a VACTERL association could be debated (#2, Table6). The child had oesophageal atresia with tracheo-oesophageal fistula, a ventricular septal defect and syndactyly of both second and third toes. The only core feature of a VACTERL association is the tracheo-oesophageal fistula 34. The muscular ventricular septal defect was mild and might have closed later. 2/3 syndactyly of the toes occurs frequently and is most often inherited 35. Since the presence of at least three typical malformations is required to make the diagnosis 34, we do not consider this to be a true case of VACTERL association.
Due to the lack of active placental IgG transport mechanisms during early pregnancy it is assumed that embryonic exposure to TNF-α inhibitors is minimal during the first trimester, making direct teratogenic effects unlikely. However, an indirect effect on the normal embryonic development is debatable. Animal experiments suggest that TNF-α plays a dual role in embryogenesis in activating some defence mechanism on the one hand and inducing embryonic death in cases of developmental damage on the other hand. All this could contribute to the prevention of birth defects in live-births 36. TNF-α inhibitors may disturb these processes to a certain extent.
One could speculate that a detection bias contributed to the higher rate of birth defects in the study group, a phenomenon discussed also in relation to other drugs suspected to be teratogens 37. More careful prenatal and postnatal screenings result in higher detection rates of fetal birth defects. However, the malformations reported in our study are heterogeneous and not specifically dependent on extended (ultrasound) diagnostics.
The rate of 1.5% major birth defects in the comparison cohort is lower than the prevalence of all non-chromosomal anomalies of 2.2% recorded by EUROCAT for the years 2008-2012 38. Therefore, it could be discussed whether a selection bias has contributed to the increased malformation risk. Taking into account differences in maternal characteristics between cohorts, adjustment reduced the crude OR of 3.5 to 2.2 (95% CI 1.0, 4.8), which at least in part removed a potential selection bias.
Not all women received TNF-α inhibitors during the entire period of organogenesis. It is, therefore, debatable whether the exposed cohort is informative for all organ specific vulnerable windows within the first trimester. To answer this question, treatment duration and half-lives of individual TNF-α inhibitors need to be taken into account. The latter refers to the fact that halting of TNF-α inhibitor therapy does not coincide with the end of fetal exposure. Of the IFX exposed women 66.5% received their last infusion after gestational week 10, whereas 26.5% stopped therapy before week 6 (Figure3). Considering that IFX has a half-life of 8 to 12.3 days and is detectable in maternal blood for up to 8 to 12 weeks after the last dosage, we can be confident that the vast majority of women had detectable IFX concentrations during all sensitive organ-specific periods. ETA in contrast, has a far shorter half-life of 70 h and is usually administered twice weekly. The median week of the last ETA injection was week 5. Therefore it is assumed that only a minority of women had detectable ETA concentrations during the entire first trimester (Figure3). Considering ADA’s half-life of 14 days in women receiving injections every other week, it seems likely that 50% of the exposed women had significant ADA serum concentrations up to week 11 and 42% up to week 13 (Figure3).
Figure 3.
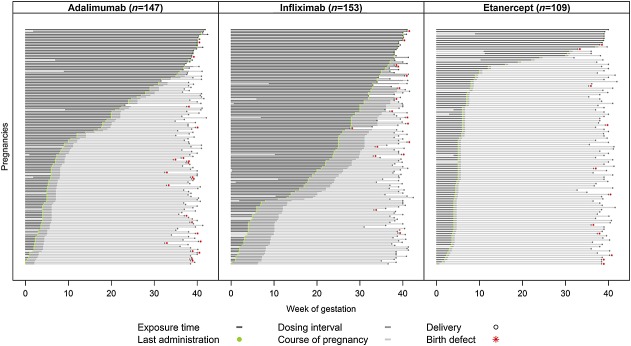
Duration of prenatal ADA, IFX or ETA exposure in live-born infants. Each pregnancy is represented by a single line, showing assumed fetal exposure times, i.e. treatment duration plus 1 dosing interval. The differences in exposure times among the three TNF-α-inhibitors are obvious
Birth weight and preterm birth
The lower birth weights of exposed infants could either be due to drug toxicity during pregnancy or to the underlying disease and its activity. The tendency towards lower birth weight was most prominent in ADA-exposed infants (SDS -0.43) and least in ETA-exposed infants (SDS -0.24).
Studies on pregnancy outcome in women with IBD have revealed an increased risk of low birth weight, small-for-gestational age and preterm birth, particularly when there were disease flares during pregnancy 39. Maternal RA seems to have a less pronounced influence on an infant’s birth weight 40 which is consistent with the results of our ETA subgroup, since the majority of women with ETA therapy were treated for RA. However, an association between higher disease activity of maternal RA during pregnancy and lower birth weight could also be shown 41. Following this, cessation of maternal TNF-α inhibitor therapy might have led to an increased number of flares, to higher disease activity with increased inflammatory processes and, in consequence, to compromised fetal growth.
The higher rate of preterm births may be due to toxicity of the compounds or inadequate disease control. IFX has the lowest rate with 16.0% (24/150), followed by ADA with 17.4% (25/144) and ETA with 17.9% (19/106). A higher rate of preterm birth among pregnant women with IBD 39 and with RA 40 has been discussed in relation to higher disease activity 39,41 and discontinuation of medication 42.
Strengths and limitations
Strengths of the TIS studies include the prospective approach, the possibility to evaluate a range of pregnancy outcomes and to control for a variety of potential confounders. Drug exposure is documented in real time, and the lost-to-follow-up rate is relatively small. Exposed and unexposed women are ascertained with similar procedures over the same time period. Thus, comparison is made between the average TIS population with and without exposure to the studied TNF-α inhibitors 43.
One limitation is that exposed pregnancies were only compared with a general comparison cohort. Usually, TNF-α inhibitors are used only in cases of severe disease and after failure of classical DMDs. TIS data on disease activity are incomplete due to the focus on drug toxicity. Therefore, an appropriate diseased comparison group could not be provided. This limitation is unlikely to confound the evaluation of teratogenicity because neither Crohn’s disease 44,45 nor rheumatoid arthritis are suspected to increase the risk of birth defects 40. However, high disease activity and flares during pregnancy might have an influence on pregnancy outcome in terms of preterm birth and other complications 39,41,46. The design of this study does not allow differentiating the influence of the underlying disease and treatment on birth weight and preterm birth. It is likely that disease activity, the mother’s medication as well as the interaction of these factors influence the outcome of pregnancy.
Strengths and limitations of prospective observational pregnancy outcome studies have been discussed in detail in other papers 25. Using pregnancies from the respective TIS population as controls has the advantage of similar procedures of ascertainment across cohorts and contributing centres. Women assigned to the control cohort have contacted the TIS because of fear of embryotoxicity of agents that proved to be of no risk during consultation. Therefore, the control group does not necessarily represent the general pregnant population but rather a subset of particularly concerned and/or health-oriented patients with non-teratogenic drug exposure. A recent publication demonstrated that this approach does not substantially bias the results of pregnancy outcome studies 47.
Although this is the largest study published to date on pregnancy outcomes following administration of TNF-α inhibitors during pregnancy, the sample size is still limited in power particularly with regard to individual agents. However, the prospective approach and similar procedures of ascertainment across cohorts makes substantial bias in the assessment of exposure and the outcome data unlikely.
In conclusion TNF-α inhibitors may carry a risk of adverse pregnancy outcome of moderate clinical relevance. Given the results of our study and elsewhere published data on first trimester exposure, IFX is the TNF-α inhibitor with the largest evidence for safety in pregnancy. In contrast, GOL and CZP should only be used with special consideration of the still limited experience. Considering the impact of insufficiently controlled autoimmune disease on the mother and the unborn child, TNF-α inhibitors may nevertheless be a treatment option in women with severe disease refractory to established immunomodulatory drugs. Above all effective disease control resulting in low disease activity is an important prerequisite for a favourable pregnancy outcome.
Competing Interests
This work was supported by the German Ministry of Health (BMG, grant number IIA5-2512FSB053) and the German Federal Institute for Drugs and Medical Devices (BfArM, grant number V-6308/68605/2011-2014). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare (aside from the aforementioned) no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Contributors
Conception and design of the study: C.W-S., M.O., E.W., R.M., and C.S.; collection of data: C.W-S, M.O., N.B., D.B., B.C-M., J.L.R., L.E.R., A.P., H.M., G.E., D.K., M.KD.; analysis and interpretation of data: C.W-S., M.O., E.W., R.M., and C.S.; drafting of the manuscript: C.W-S; M.O.; reviewing and approving the submitted version of the manuscript: C.W-S., M.O., E.W. N.B., D.B., B.C-M., J.L.R., L.E.R., A.P., H.M., G.E., D.K., M.KD, R.M., and C.S.
We would like to thank the network of French pharmacovigilance centres (Amiens, Angers, Caen, Clermont-Ferrand, Dijon, Grenoble, Lille, Limoges, Lyon, Marseille, Montpellier, Paris Fernand-Widal, Poitiers, Rouen, Saint-Étienne, Strasbourg, Toulouse and Tours) for contributing cases. Furthermore, we would like to thank colleagues from the Berlin Institute for counselling patients and their physicians and thoroughly documenting cases and controlling data quality of the study population. The latter especially relates to Mary Panse. We would like to thank Dr Maria Hoeltzenbein from Berlin, Germany, Professor Maurizio Clementi from Padua, Italy and Dr Herbert Juch from Graz, Austria, for their expert opinion concerning VACTERL association. We also would like to acknowledge the activity of PD Dr Teich from Leipzig, Germany, motivating his patients to participate in this study. Many thanks also to Dr Vial, Lyon, France for his valuable comments to the manuscript.
References
- Nielsen OH, Loftus EV, Jr, Jess T. Safety of TNF-α inhibitors during IBD pregnancy: a systematic review. BMC Med. 2013;11:174. doi: 10.1186/1741-7015-11-174. . doi: 10.1186/1741-7015-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39:690–7. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula N, Al-Dabbagh R, Dhillon A, Sands BE, Marshall JK. Anti-TNFalpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20:1862–9. doi: 10.1097/MIB.0000000000000092. [DOI] [PubMed] [Google Scholar]
- Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case Report: Fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603–5. doi: 10.1016/j.crohns.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Johnson DL. Emerging data on the use of anti-tumor necrosis factor-alpha medications in pregnancy. Birth Defects Res A Clin Mol Teratol. 2012;94:607–11. doi: 10.1002/bdra.23033. [DOI] [PubMed] [Google Scholar]
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426–38. doi: 10.1038/ajg.2013.171. [DOI] [PubMed] [Google Scholar]
- Carter JD, Ladhani A, Ricca LR, Valeriano J, Vasey FB. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635–41. doi: 10.3899/jrheum.080545. [DOI] [PubMed] [Google Scholar]
- Verstappen SM, King Y, Watson KD, Symmons DP, Hyrich KL. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823–6. doi: 10.1136/ard.2010.140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MJ, Chaparro M, Domenech E, Barreiro-de AM, Bermejo F, Iglesias E, Gomollon F, Rodrigo L, Calvet X, Esteve M, Garcia-Planella E, Garcia-Lopez S, Taxonera C, Calvo M, Lopez M, Ginard D, Gomez-Garcia M, Garrido E, Perez-Calle JL, Beltran B, Piqueras M, Saro C, Botella B, Duenas C, Ponferrada A, Manosa M, Garcia-Sanchez V, Mate J, Gisbert JP. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:433–40. doi: 10.1038/ajg.2012.430. [DOI] [PubMed] [Google Scholar]
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, Ornoy A. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: A prospective, comparative, observational study. Reprod Toxicol. 2014;43:78–84. doi: 10.1016/j.reprotox.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Marchioni RM, Lichtenstein GR. Tumor necrosis factor-alpha inhibitor therapy and fetal risk: a systematic literature review. World J Gastroenterol. 2013;19:2591–602. doi: 10.3748/wjg.v19.i17.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A, Clark M, Harrison DD, Geldhof A, Nissinen R, Sanders M. Pregnancy outcomes in women exposed to the tumor necrosis factor inhibitor, Golimumab. Ann Rheum Dis. 2014;73:232. [Abstract]. [Google Scholar]
- Mahadevan U, Martin CF, Sandler RS, Kane SV, Dubinsky M, Lewis JD, Sandborn WJ, Sands BE. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immuno-modulators and biologic therapy. Gastroenterology. 2012;142:S149. [Abstract]. [Google Scholar]
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014–7. [PubMed] [Google Scholar]
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465–6. doi: 10.3899/jrheum.081083. [DOI] [PubMed] [Google Scholar]
- Crijns HJ, Jentink J, Garne E, Gispen-de Wied CC, Straus SM. de Jong-van den Berg LT. The distribution of congenital anomalies within the VACTERL association among tumor necrosis factor antagonist-exposed pregnancies is similar to the general population. J Rheumatol. 2011;38:1871–4. doi: 10.3899/jrheum.101316. [DOI] [PubMed] [Google Scholar]
- Winger EE, Reed JL, Ashoush S, Ahuja S, El-Toukhy T, Taranissi M. Treatment with adalimumab (Humira) and intravenous immunoglobulin improves pregnancy rates in women undergoing IVF. Am J Reprod Immunol. 2009;61:113–20. doi: 10.1111/j.1600-0897.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- Winger EE, Reed JL. Treatment with tumor necrosis factor inhibitors and intravenous immunoglobulin improves live birth rates in women with recurrent spontaneous abortion. Am J Reprod Immunol. 2008;60:8–16. doi: 10.1111/j.1600-0897.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol. 2009;104:228–33. doi: 10.1038/ajg.2008.71. [DOI] [PubMed] [Google Scholar]
- Mahadevan U, Wolf DC, Dubinsky M, Cortot A, Lee SD, Siegel CA, Ullman T, Glover S, Valentine JF, Rubin DT, Miller J, Abreu MT. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286–92. doi: 10.1016/j.cgh.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinkova Z, de Haar C, de Ridder L, Pierik MJ, Kuipers EJ, Peppelenbosch MP, van der Woude CJ. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther. 2011;33:1053–8. doi: 10.1111/j.1365-2036.2011.04617.x. [DOI] [PubMed] [Google Scholar]
- Berthelsen BG, Fjeldsoe-Nielsen H, Nielsen CT, Hellmuth E. Etanercept concentrations in maternal serum, umbilical cord serum, breast milk and child serum during breastfeeding. Rheumatology (Oxford) 2010;49:2225–7. doi: 10.1093/rheumatology/keq185. [DOI] [PubMed] [Google Scholar]
- Martin PL, Oneda S, Treacy G. Effects of an anti-TNF-alpha monoclonal antibody, administered throughout pregnancy and lactation, on the development of the macaque immune system. Am J Reprod Immunol. 2007;58:138–49. doi: 10.1111/j.1600-0897.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Schaefer C, Ornoy A, Clementi M, Meister R, Weber-Schoendorfer C. Using observational cohort data for studying drug effects on pregnancy outcome - methodological considerations. Reprod Toxicol. 2008;26:36–41. doi: 10.1016/j.reprotox.2008.05.064. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9:403–25. doi: 10.1037/1082-989X.9.4.403. [DOI] [PubMed] [Google Scholar]
- Meister R, Schaefer C. Statistical methods for estimating the probability of spontaneous abortion in observational studies--analyzing pregnancies exposed to coumarin derivatives. Reprod Toxicol. 2008;26:31–5. doi: 10.1016/j.reprotox.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Voigt M, Rochow N, Hesse V, Olbertz D, Schneider KT, Jorch G. Short communication about percentile values of body measures of newborn babies. Z Geburtshilfe Neonatol. 2010;214:24–9. doi: 10.1055/s-0029-1241833. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- Rubin DB. Multiple imputation for nonresponsive in surveys. New York: Wiley; 1987. [Google Scholar]
- Arsenescu R, Arsenescu V, de Villiers WJ. TNF-alpha and the development of the neonatal immune system: implications for inhibitor use in pregnancy. Am J Gastroenterol. 2011;106:559–62. doi: 10.1038/ajg.2011.5. [DOI] [PubMed] [Google Scholar]
- Weber-Schoendorfer C, Chambers C, Wacker E, Beghin D, Bernard N, Shechtman S, Johnson D, Cuppers-Maarschalkerweerd B, Pistelli A, Clementi M, Winterfeld U, Eleftheriou G, Pupco A, Kao K, Malm H, Elefant E, Koren G, Vial T, Ornoy A, Meister R, Schaefer C. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: a prospective multicenter cohort study. Arthritis Rheumatol. 2014;66:1101–10. doi: 10.1002/art.38368. [DOI] [PubMed] [Google Scholar]
- Solomon BD. VACTERL/VATER Association. Orphanet J Rare Dis. 2011;6:56. doi: 10.1186/1750-1172-6-56. . doi: 10.1186/1750-1172-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S. Syndactyly: phenotypes, genetics and current classification. Eur J Hum Genet. 2012;20:817–24. doi: 10.1038/ejhg.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toder V, Fein A, Carp H, Torchinsky A. TNF-alpha in pregnancy loss and embryo maldevelopment: a mediator of detrimental stimuli or a protector of the fetoplacental unit? J Assist Reprod Genet. 2003;20:73–81. doi: 10.1023/A:1021740108284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Oz B, Einarson T, Einarson A, Boskovic R, O’Brien L, Malm H, Berard A, Koren G. Paroxetine and congenital malformations: meta-Analysis and consideration of potential confounding factors. Clin Ther. 2007;29:918–26. doi: 10.1016/j.clinthera.2007.05.003. [DOI] [PubMed] [Google Scholar]
- EUROCAT. Prevalence Tables. Available at http://www.eurocat-network.eu/accessprevalencedata/prevalencetables (last accessed 14-8-2014)
- Broms G, Granath F, Linder M, Stephansson O, Elmberg M, Kieler H. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis. 2014;20:1091–8. doi: 10.1097/MIB.0000000000000060. [DOI] [PubMed] [Google Scholar]
- Reed SD, Vollan TA, Svec MA. Pregnancy outcomes in women with rheumatoid arthritis in Washington State. Matern Child Health J. 2006;10:361–6. doi: 10.1007/s10995-006-0073-3. [DOI] [PubMed] [Google Scholar]
- de Man YA, Hazes JM, van der Heide H, Willemsen SP, de Groot CJ, Steegers EA, Dolhain RJ. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 2009;60:3196–206. doi: 10.1002/art.24914. [DOI] [PubMed] [Google Scholar]
- Langen ES, Chakravarty EF, Liaquat M, El-Sayed YY, Druzin ML. High rate of preterm birth in pregnancies complicated by rheumatoid arthritis. Am J Perinatol. 2014;31:9–14. doi: 10.1055/s-0033-1333666. [DOI] [PubMed] [Google Scholar]
- Chambers C. The role of teratology information services in screening for teratogenic exposures: challenges and opportunities. Am J Med Genet C Semin Med Genet. 2011;157C:195–200. doi: 10.1002/ajmg.c.30303. [DOI] [PubMed] [Google Scholar]
- Ban L, Tata LJ, Fiaschi L, Card T. Limited risks of major congenital anomalies in children of mothers with IBD and effects of medications. Gastroenterology. 2014;146:76–84. doi: 10.1053/j.gastro.2013.09.061. [DOI] [PubMed] [Google Scholar]
- van der Woude CJ, Kolacek S, Dotan I, Oresland T, Vermeire S, Munkholm P, Mahadevan U, Mackillop L, Dignass A. European evidenced-based consensus on reproduction in inflammatory bowel disease. J Crohns Colitis. 2010;4:493–510. doi: 10.1016/j.crohns.2010.07.004. [DOI] [PubMed] [Google Scholar]
- de Man YA, Bakker-Jonges LE, Goorbergh CM, Tillemans SP, Hooijkaas H, Hazes JM, Dolhain RJ. Women with rheumatoid arthritis negative for anti-cyclic citrullinated peptide and rheumatoid factor are more likely to improve during pregnancy, whereas in autoantibody-positive women autoantibody levels are not influenced by pregnancy. Ann Rheum Dis. 2010;69:420–3. doi: 10.1136/ard.2008.104331. [DOI] [PubMed] [Google Scholar]
- Wacker E, Navarro A, Meister R, Padberg S, Weber-Schoendorfer C, Schaefer C. Does the average drug exposure in pregnant women affect pregnancy outcome? Vol. 24. Pharmacoepidemiol Drug Saf: A comparison of two approaches to estimate the baseline risks of adverse pregnancy outcome; 2015. pp. 353–60. [DOI] [PubMed] [Google Scholar]


