Abstract
Aim
The aim of the study was to characterize the extent of indication bias resulting from the excessive use of NSAIDs on the days preceding a spontaneous abortion to relieve pain.
Methods
We used data from a retrospective cohort study assessing the risk for spontaneous abortions following exposure to NSAIDs. Three definitions of exposure for cases of spontaneous abortions were compared, from the first day of pregnancy until the day of spontaneous abortion and until 3 and 2 days before a spontaneous abortion. Statistical analysis was performed using multivariate time programmed Cox regression.
Results
A sharp increase was observed in the dispensation of indomethacin, diclofenac and naproxen, and a milder increase was found in the use of ibuprofen during the week before a spontaneous abortion. Non- selective COX inhibitors in general and specifically diclofenac and indomethacin were found to be associated with spontaneous abortions when the exposure period was defined until the day of spontaneous abortion (hazard ratio (HR) 1.15, 95% confidence interval (CI) 1.04, 1.28; HR 1.31, 95% CI 1.08, 1.59 and HR 3.33, 95% CI 2.09, 5.29, respectively). The effect disappears by excluding exposures occurring on the day before the spontaneous abortion for non-selective COX inhibitors and on the last week before the spontaneous abortion for indomethacin. In general, decreasing HRs were found with the exclusion of exposures occurring on the days immediately before the spontaneous abortion.
Conclusions
The increased use of NSAIDs during the last few days that preceded a spontaneous abortion to relieve pain associated with the miscarriage could bias studies assessing the association between exposure to NSAIDs and spontaneous abortions.
Keywords: ibuprofen, indication bias, miscarriage, NSAIDs, reverse causality bias, spontaneous abortions
What is Already Known about this Subject
Most studies examining the risk for adverse pregnancy outcomes following drug use are retrospective and therefore are prone to indication bias.
What this Study Adds
The use of NSAIDs during the week before a spontaneous abortion is sharply increased.
The increased use of NSAIDs during the last few days that preceded a spontaneous abortion to relieve pain associated with the miscarriage biases studies assessing the association between exposure to NSAIDs and spontaneous abortions.
Introduction
A frequent complication of pregnancy, spontaneous abortion, occurs in 15% of pregnancies 1. Its typical symptoms are abdominal and back pain, uterine cramping and vaginal bleeding. It is defined as a spontaneous termination of pregnancy prior to the 20th gestational week, but 80% of spontaneous abortions occur on the first 12 weeks of pregnancy, about half due to chromosomal anomalies 2–5. Subclinical spontaneous abortions are even more common, with an incidence of 22%–26% 3,4.The use of either prescription or over-the-counter non-steroidal anti-inflammatory drugs (NSAIDs) is increasing among women in the first trimester of pregnancy 6,7. This increased incidence of exposure to NSAIDs is likely due to the fact that ibuprofen and naproxen became available as over-the-counter drugs in the last 20 years and because half of pregnancies are unplanned 8, and therefore women do not realize they are pregnant at the time of exposure.
NSAIDs are indicated mainly for pain and fever 9, but indomethacin is also a tocolytic agent used to treat preterm labour 10 and the group of drugs termed ‘selective COX2 inhibitors’ (celecoxib, rofecoxib and etoricoxib) are mainly indicated for the treatment of inflammatory diseases and chronic musculoskeletal pain 11. Although evidence of fetal safety is inconclusive, some clinicians recommend their use during pregnancy 12. The results of previous studies that assessed the association between exposure to NSAIDs and spontaneous abortions were inconsistent 13–16. In those studies, a woman was defined as ‘exposed’ if a NSAID was dispensed from the first day of pregnancy until the day of spontaneous abortion.
This definition of exposure included dispensations that occurred during the last few days before the spontaneous abortion to treat abdominal cramps that resulted from the spontaneous abortion itself, or in an attempt to prevent an impending spontaneous abortion. In such cases, an ‘indication bias’ may occur, in which NSAIDs would be erroneously found to increase the risk for spontaneous abortions.
We recently published a study showing no association between exposure to NSAIDs during the first trimester of pregnancy and spontaneous abortions 17. Exposure in our study was defined as a dispensation of NSAIDs until 2 days before a spontaneous abortion.
The objective of the current study was to characterize and quantify the extent of indication bias by comparing three different definitions of exposure periods to NSAIDs.
Methods
A detailed description of the study databases and definitions of the variables has been published previously 17. In brief, we conducted a population-based retrospective cohort study that included all pregnancies to women 15–45 years of age registered with Clalit Health Services maintenance organization (Southern district of Israel) who conceived between January 2004 and December 2009 and ended with birth or a spontaneous abortion at the Soroka Medical Center (SMC, Beer-Sheva, Israel).
The cohort was created by combining three computerized databases, in which data were entered according to each patient’s unique Israeli internal ministry ID number. The Obstetrics and Gynecology Deliveries database contains information regarding all births that occurred at SMC including gestational age at the time of delivery, medical diagnoses during and before pregnancy (ICD9 coded), delivery results and demographic information. The hospitalization database at SMC contains information regarding all spontaneous abortions that were diagnosed at SMC, including letters of admission and discharge, operating room reports, gestational age at the time of diagnosis, medical diagnoses before and during pregnancy and demographic information. The Clalit Health Services drug dispensation database contains electronically recorded drug dispensations at community pharmacies during and before pregnancy, including the date of dispensation, name of the drug, the specific Anatomical Therapeutic Chemical (ATC) code and the defined daily dose dispensed.
All medical diagnoses were made by a specialist gynaecologist, who had full access to the patient’s community medical record including imaging and laboratory test results, and reviewed by a medical record secretary.
The diagnosis of spontaneous abortion was based on ICD9 codes 634 and 632. The entire Israeli population is medically insured and according to the Israeli guidelines of preventive medicine, a free ultrasound imaging and determination of gestational age is indicated for all pregnant woman at the beginning of pregnancy. As mentioned, the patient’s community electronic medical record was available at the time of admission such that the diagnosis of spontaneous abortion could be made with certainty.
Other medical diagnoses were based on the following ICD-9 codes: hypercoagulation diseases (286.4, 289.81, 286.53), obesity (278,649.1), diabetes mellitus (250, 357.2), hypothyroidism (244), recurrent spontaneous abortions (646.33, 629.81), tobacco use (305.1, 649), inflammatory diseases (710, 714, 720) and presence of an intrauterine contraceptive device (V4551, Z975).
Exposure was defined according to documented dispensations of NSAIDs in the comminuty drug dispensation database for all pregnancies in the cohort. A pregnancy that resulted in birth was defined as ‘exposed’ if a NSAID was dispensed from the first day of the last menstrual period until the 20th gestational week. For pregnancies that resulted in a spontaneous abortion, exposures were defined in three different ways:
Dispensation of a NSAID from the first day of the last menstrual period to the end of the 20th week, or to the day of admission for spontaneous abortion.
Dispensation of a NSAID until 1 day before admission for spontaneous abortion.
Dispensation of a NSAID until 2 days before admission for spontaneous abortion.
Both univariate and multivariate statistical analyses were performed using time programmed Cox regression models. All multivariate models were adjusted for diagnosis of diabetes mellitus, hypothyroidism, hypercoagulable and inflammatory disorders and recurrent miscarriages, self-reported tobacco use during pregnancy, the presence of an intrauterine contraceptive device, maternal age, ethnic group (i.e. Jewish vs. Bedouin Muslim) and the year of the pregnancy. Hazard ratios (HR) with 95% confidence intervals (CI) were calculated.
Results
There were 66 547 pregnancies during the study period. Of these, 6508 (9.9%) ended in spontaneous abortion. For 1090 (14.3%) cases of spontaneous abortions, data regarding gestational age at the time of abortion were missing and, therefore, those cases were excluded from the analysis.
The median gestational age at the diagnosis of spontaneous abortion was 64 days (9 completed gestational weeks) and 80% of spontaneous abortions occurred before the 12th gestational week.
Figure1 presents the amount NSAID dispensation in the weeks preceding the diagnosis of spontaneous abortion. A sharp increase was observed in the dispensation of indomethacin, diclofenac and naproxen during the week before a spontaneous abortion. Among these, 66% of the dispensations of indomethacin, 12.6% of the dispensations of diclofenac and 5.6% of the dispensations of naproxen were during the week before the miscarriage to women who were diagnosed with spontaneous abortions. A milder increase was observed in the dispensations of ibuprofen where 7.7% of the dispensations occurred in the last week before the spontaneous abortion. No increase was observed in the use of etodolac or COX2 selective inhibitors during the week before the spontaneous abortion.
Figure 1.
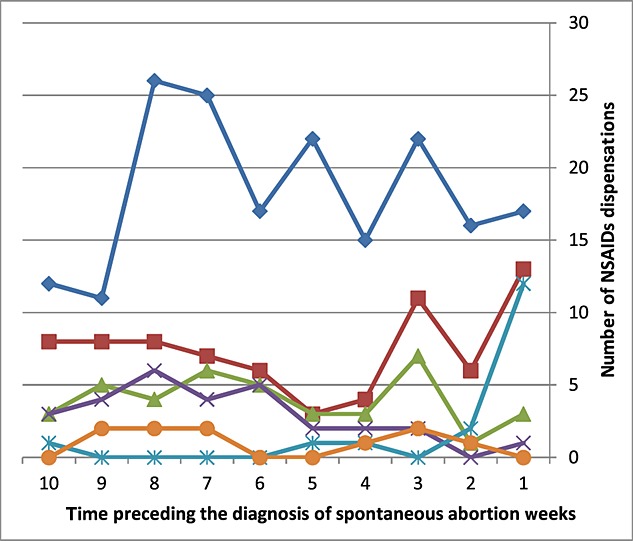
Number of NSAID dispensations in the weeks preceding the diagnosis of spontaneous abortion.  ibuprofen
ibuprofen  diclofenaac
diclofenaac  naproxen
naproxen  etodolac
etodolac  indomethacin
indomethacin  COX2 selective inhibitors
COX2 selective inhibitors
Table1 presents the hazard ratios for spontaneous abortions following exposure to NSAIDs for the three different exposure definitions (until the day of spontaneous abortion and until 3 and 2 days before the spontaneous abortion).
Table 1.
Adjusted risk (hazard ratios and 95% CI) for spontaneous abortions following exposure to NSAIDs for three different exposure definitions
| Exposure to | Number of exposed*, median gestational age at exposure | Mean defined daily dose (DDD) dispensed | Number of events (spontaneous abortions) | Until the day of spontaneous abortion | Until 2 days before spontaneous abortion | Until 3 days before spontaneous abortion |
|---|---|---|---|---|---|---|
| Non-selective COX inhibitors | 4424 | 5.1 | 373 | 1.15 (1.04, 1.28) | 1.10 (0.99, 1.22) | 1.08 (0.97, 1.20) |
| 31 | ||||||
| Ibuprofen | 2,732 | 1.6 | 215 | 1.09 (0.95, 1.24) | 1.06 (0.93, 1.22) | 1.06 (0.92, 1.22) |
| 40 | ||||||
| Diclofenac | 919 | 7.3 | 93 | 1.31 (1.08, 1.59) | 1.19 (0.97, 1.46) | 1.16 (0.94, 1.43) |
| 23 | ||||||
| Naproxen | 671 | 8.6 | 53 | 1.01 (0.77, 1.33) | 0.97 (0.74, 1.28) | 0.95 (0.72, 1.26) |
| 27 | ||||||
| Etodolac | 272 | 19.38 | 4 | 1.32 (0.94, 1.84) | 1.28 (0.91, 1.79) | 1.28 (0.91, 1.79) |
| 20.5 | ||||||
| Indomethacin | 132 | 7.2 | 15 | 3.33 (2.09, 5.29) | 2.82 (1.70, 4.69) | 2.64 (1.56, 4.47) |
| 89 | ||||||
| COX2 selective inhibitors | 71 | 14.4 | 11 | 1.43 (0.79, 2.59) | 1.43 (0.79, 2.59) | 1.43 (0.79, 2.60) |
| 25 | ||||||
| Unexposed | 60 962 | 6132 |
259 pregnancies were exposed to two medications, 26 were exposed to three medications and two were exposed to four medications during the exposure period
Significantly decreasing HRs were found with the exclusion of exposures that occurred on the days immediately before the spontaneous abortion for non-selective COX inhibitors and specifically for diclofenac and indomethacin. Decreasing HRs were also found for ibuprofen, naproxen and etodolac. For indomethacin, the effect disappeared only when excluding exposures that occurred during the week before the spontaneous abortion (HR 1.72, 95% CI 0.89, 3.31).
Discussion
Our study shows increased use mostly of indomethacin, but also of diclofenac, naproxen and ibuprofen, during the week before a spontaneous abortion. Moreover, a decreasing association was found between exposure to those drugs and spontaneous abortions after the exclusion from the study of exposures that occurred on the days immediately before the abortion. This analysis confirms the existence of indication bias.
One of the major symptoms preceding a spontaneous abortion is lower abdominal or back pain, symptoms which could have been treated with over-the-counter NSAIDs, without consulting a medical authority. The prescription of these drugs during that period, therefore, creates an ‘indication bias’, and a spurious association is found between NSAID dispensation and spontaneous abortions.
Indomethacin, indicated not only for pain but also to postpone preterm labour, was found to be more strongly associated with spontaneous abortions than other NSAIDs and in addition the association disappears only when excluding exposure that occurred during the last 4 days before the abortion.
To strengthen the evidence that the association that was found between exposure to NSAIDs and spontaneous abortions arises as a result of an indication bias, we also examined the association between COX2 selective inhibitors, which are usually prescribed for inflammatory diseases and not indicated to treat acute pain or used as tocolytic agents, and spontaneous abortions. No increase was observed in the use of COX2 selective inhibitors in the week prior to the abortion, and no change in the association between COX2 selective inhibitors and spontaneous abortions was observed by omitting recent exposures.
Nakhai-Pour et al. 16 found an increased risk for spontaneous abortions among women who were prescribed with non-aspirin NSAIDs (OR 2.43, 95% CI 2.12, 2.79). In this study, exposure was defined as a prescription of NSAIDs from the first gestational day until the day of spontaneous abortion and, as such, it could be biased by indication. Moreover, although the association was significant for all specific NSAIDs, the odds ratios were relatively higher for non-selective NSAIDs, which are indicated mainly for pain and fever, compared with COX2 selective inhibitors, which are usually indicated for inflammatory diseases 11.
Nielsen et al. published the results of a case control study assessing the association between NSAIDs prescription and spontaneous abortions 18. Exposure was defined as a prescription of NSAIDs during the last 12 weeks prior to discharge from the hospital for cases of spontaneous abortions, or during the first trimester for births. Their results showed an increasing risk for spontaneous abortions the shorter the period between the prescription of NSAIDs and discharge from the hospital (from OR 1.26 for prescriptions issued 10 weeks before discharge to OR 6.99 for prescriptions issued during the week prior to the abortion). These definitions of exposure and results provide strong support for an indication bias.
A second analysis of the data was performed by these authors by re-defining exposure after including gestational age data 14. Although found not significant, the risk for spontaneous abortions was substantially higher among exposures that occurred during the last week before the spontaneous abortion, a finding that could have been the result of indication bias.
One limitation of our study is the lack of information regarding the indication for dispensation of NSAIDs, mostly due to the fact that ibuprofen is marketed as an over the counter drug without the need for prescription. As in other studies, this limitation can lead to indication bias, as presented by the current study.
Exposure was defined as the dispensation of NSAIDs from the first day of pregnancy until the days before the diagnosis of spontaneous abortion. While half of the spontaneous abortions that occur prior to the 12th gestational week are a result of chromosomal abnormalities, genetic assessment was lacking regarding cases of spontaneous abortions in our study. Nonetheless, a secondary analysis was performed after defining exposure only from the 12th gestational week onwards and yielded similar results.
Although the results prove the associations between exposure to NSAIDs and spontaneous abortions are a result of an indication bias, a minor possibility still exists that this association is genuine.
In conclusion, the increased use of NSAIDs during the last days before a spontaneous abortion to relieve pain could bias studies assessing the association between exposure to NSAIDs and spontaneous abortions. This indication bias could be avoided by excluding exposures that occurred on the last few days prior to the event.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf )available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- Prernoll M, Benson R. Early pregnancy risks. In: Alan HD, Lauren N, editors. Current Diagnosis & Treatment, Obstetrics & Gynecology. 10th ed. New York: McGraw-Hill; 2007. p. 259. [Google Scholar]
- Harlap S, Shiono PH. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet. 1980;2:173–76. doi: 10.1016/s0140-6736(80)90061-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577–84. doi: 10.1016/s0015-0282(02)04694-0. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Hoffman BL, Schorge JO, Halvorson LM, Bradhaw KD, Cunningham FG. First- Trimester Abortion. In: Hoffman BL, Schorge JO, Halvorson LM, Bradhaw KD, Cunningham FG, editors. Williams Gynecology. 2nd ed. New York: McGraw Hill; 2011. p. 170. . In:, eds. [Google Scholar]
- Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193:771–77. doi: 10.1016/j.ajog.2005.02.100. [DOI] [PubMed] [Google Scholar]
- Daniel S, Matok I, Gorodischer R, Koren G, Uziel E, Wiznitzer A, Levy A. Major malformations following exposure to nonsteroidal antiinflammatory drugs during the first trimester of pregnancy. J Rheumatol. 2012;39:2163–9. doi: 10.3899/jrheum.120453. [DOI] [PubMed] [Google Scholar]
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478–85. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser T, Smyth E, FitzGerald G. Anti-inflammatory, antipyretic,and analgesic agents; pharmacotherapy of gout. In: Brunton L, Chabner B, Knollmann B, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011. pp. 959–1004. [Google Scholar]
- Roman APM. Late Pregnancy Complications. In: Alan HD, Lauren N, editors. Current Diagnosis & Treatment, Obstetrics & Gynecology. 10th ed. New York: McGraw-Hill; 2007. p. 273. [Google Scholar]
- Pongparadee C, Penserga E, Lee DJ, Chen SL, Gill RS, Hamid A, Kumthornthip W, Liu Y, Meliala L, Misbach HJ, Tan KH, Yeap SS, Yeo SN, Lin HY. Current considerations for the management of musculoskeletal pain in Asian countries: a special focus on cyclooxygenase-2 inhibitors and non-steroid anti-inflammation drugs. Int J Rheum Dis. 2012;15:341–47. doi: 10.1111/j.1756-185X.2012.01769.x. [DOI] [PubMed] [Google Scholar]
- Guillermo R, Munther AK. 2013. Pregnancy and Rheumatic Disease; Available at: https://www.rheumatology.org/Practice/Clinical/Patients/Diseases_And_Conditions/Pregnancy_and_Rheumatic_Disease/. Accessed 02/25, 2015.
- Keim SA, Klebanoff MA. Aspirin use and miscarriage risk. Epidemiology. 2006;17:435–39. doi: 10.1097/01.ede.0000221693.72971.b3. [DOI] [PubMed] [Google Scholar]
- Nielsen GL, Skriver MV, Pedersen L, Sorensen HT. Danish group reanalyses miscarriage in NSAID users. BMJ. 2004;328:109. doi: 10.1136/bmj.328.7431.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DK, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ. 2003;327:68. doi: 10.1136/bmj.327.7411.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhai-Pour HR, Broy P, Sheehy O, Berard A. Use of nonaspirin nonsteroidal anti-inflammatory drugs during pregnancy and the risk of spontaneous abortion. CMAJ. 2011;183:1713–720. doi: 10.1503/cmaj.110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel S, Koren G, Lunenfeld E, Bilenko N, Ratzon R, Levy A. Fetal exposure to nonsteroidal anti-inflammatory drugs and spontaneous abortions. CMAJ. 2014 doi: 10.1503/cmaj.130605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen GL, Sorensen HT, Larsen H, Pedersen L. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case–control study. BMJ. 2001;322:266–70. doi: 10.1136/bmj.322.7281.266. [DOI] [PMC free article] [PubMed] [Google Scholar]


