Abstract
Aims
Cholangiocarcinoma (CCA) is the second most common primary liver cancer in the world. Due to the lack of effective treatments, the survival rate of CCA is low and it is usually considered difficult to diagnose early. To date, no effective strategies for the prevention of CCA have been developed. Statins are cholesterol-lowering agents which possess pleiotropic properties and the use of statins may reduce cancer risk. The aim of the study was to investigate the effect of statin use on the risk of CCA.
Methods
We used nationwide insurance data to perform a case–control study including 3174 CCA patients diagnosed in 2002–2011 and 3174 propensity score matched controls. Odds ratios (ORs) and 95% confidence intervals (CI) were calculated to assess the association between CCA risk and statin use by type of statin and dose.
Results
Patients with CCA were slightly younger than controls with mean ages of 67.4 (SD 12.3) and 68.5 (SD 13.2) years (P = 0.001), respectively, and had less users of statins (22.7 vs. 26.5%, P < 0.001). The overall adjusted OR of statin use associated CCA was 0.80 (95% CI 0.71, 0.90) and lowered for those with longer medications. The OR ranged from 0.65 to 0.77. Stronger dose–response association was seen when using lovastatin.
Conclusions
Statin use is associated with reduced risk of CCA and there is a dose–response relationship between the use of statins and risk of CCA.
Keywords: case–control study, cholangiocarcinoma, insurance data, propensity score matching, statins
What is Already Known about this Subject
No effective strategies for the prevention of cholangiocarcinoma (CCA) have been developed.
Statins are cholesterol-lowering agents which possess pleiotropic properties and the use of statins may reduce cancer risk.
What this Study Adds
Statin use is significantly associated with reduced risk of CCA (adjusted OR 0.80, 95% CI 0.71, 0.90).
The protective relationship between CCA and statins appears to havesome variation among individual statins.
Introduction
Cholangiocarcinoma (CCA) and hepatocellular carcinoma are the major primary liver cancers. CCA is composed of cells resembling those of the bile duct and is the second most common hepatic malignancy, accounting for about 3% of malignancies of the digestive system 1,2. CCA and hepatocellular carcinoma are morphologically, genomically and clinically highly heterogeneous with a dismal clinical outcome 3,4. CCA is considered difficult to diagnose early, due to the lack of effective treatments and the survival rate is low 1,5,6. The risk of CCA is increased with liver fluke infection, hepatitis B and C virus infection, cirrhosis, alcohol consumption and hepatolithiasis 2,7,8. At present, there are no definite strategies for CCA prevention, and epidemiological data indicate that chemoprevention provides no protective effect against the development of CCA 9,10.
Statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase 11. Traditionally, they are primarily used as cholesterol-lowering agents, but in recent years additional effects of statins have been revealed, including anti-inflammation, immunomodulation and anti-tumour effects 12–14. Statins have pleiotropic effects and may exert beneficial effects in the field of cancer chemoprevention 15–17. Statin use has been reported to have a promising anti-cancer effect with studies showing decreased risk of cancer in hepatocellular carcinoma 18,19, pancreatic cancer 20,21, oesophageal cancer 22, gastric cancer 23,24, colorectal cancer 25,26 and lung cancer 27,28. However, the putative anti-cancer effect of statins remains controversial 29,30. To date, the effect of statins on CCA has not been fully elucidated, and statins have not been considered or proved as chemoprevention agents in CCA 10.
Most patients with CCA are diagnosed and treated at an advanced stage, and often these patients are deemed poor candidates for curative surgery. Conventional chemotherapy and radiation therapy have not been shown to be effective in terms of long term survival, and endoscopic treatment with photodynamic therapy combined with stenting has been reported to be effective as a palliative but not curative treatment. There is an urgent need to develop novel chemopreventive and adjuvant strategies for CCA. Studies on statin use and risk of other cancer types, including hepatocellular carcinoma, indicate that the role of statins in chemoprevention for CCA warrants further investigation.
The aim of the present study was to investigate whether individuals taking statins are at reduced risk of CCA using the National Health Insurance Research Database (NHIRD) of Taiwan. Comorbidities including diabetes, cirrhosis, hepatitis infection, inflammatory bowel disease, pancreatic disease and biliary disease were considered as covariates. The dose–response relationship with statins was evaluated in this study.
Methods
Data source
The National Health Insurance (NHI) programme of Taiwan is a compulsory health insurance programme, providing comprehensive medical coverage for all residents. Approximately 99% of 23.74 million people enrolled in the programme by 2009 31. The National Health Research Institutes (NHRI) has been in charge of managing NHIRD for claims data. Information on inpatient care, ambulatory care, dental care, prescription drugs and costs are available in the NHIRD. Data provided to researchers contained scrambled identification numbers associated with the relevant claims information, which includes the patient gender, date of birth, registry of medical services and medication prescriptions. Similar identification numbers were encrypted to all data files for linking data in accordance with privacy protocols. International Classification of Diseases-9-Clinical Modification (ICD-9-CM) codes were used to define diseases in the database. This study was approved by the Ethics Review Board of the China Medical University (CMU-REC-101-012).
Subject selection
Figure1 shows the procedure for selecting cases and controls, from two data sets of the NHIRD: 1) Cases were identified from the Registry of Catastrophic Illnesses Patient Database (RCIPD), containing health claims data for catastrophic illnesses from 1997 to 2011. Thirty categories of diseases requiring long term care were registered, including cancer. The insurance programme exempts beneficiaries from some obligations to reduce their financial burden. Cases comprised the CCA patients (ICD-9-CM codes 155.1 and 156.1) aged over 20 years newly diagnosed between 2002 and 2011. The CCA diagnosis date was defined as the index date. 2) Control subjects were identified from the Longitudinal Health Insurance Database 2000 (LHID 2000), a database containing the claims data from 1996 to 2011 for 1 million people randomly sampled from 2000 NHIRD enrolment records. The distribution of gender, age and health care costs of the LHID2000 was similar to that of all insured enrollees, as reported by the NHRI in Taiwan. Individuals with previous cancer (ICD-9-CM code 140-208) or incomplete information in both groups were excluded (Figure1). The CCA cases and controls were selected at a ratio of 1: 1 matching on propensity score and the diagnosis date. We used a logistic regression model to calculate the propensity score. The potential confounders to be considered were age, gender, medication with aspirin and metformin, Charlson comorbidity index score and comorbidities of diabetes (ICD-9-CM codes 250), cirrhosis (ICD-9-CM codes 571), chronic pancreatitis (ICD-9-CM codes 577.1), hepatitis B infection (ICD-9-CM codes V02.61, 070.20, 070.22, 070.30, 070.32), hepatitis C infection (ICD-9-CM codes V02.62, 070.41, 070.44, 070.51,070.54), gastric disease (ICD-9-CM codes 530-533), haemochromatosis (ICD-9-CM codes 275.0), inflammatory bowel disease (ICD-9-CM codes 556, 555), biliary tract disease (ICD-9-CM codes 751.69, 571.6, 574, 575.0-575.1, 121.0-121.1, 121.3), stroke (ICD-9-CM codes 430-438), CAD (ICD-9-CM codes 410-414), COPD (ICD-9-CM codes 491, 492, 496) and alcohol-related illness (ICD-9-CM codes 291, 303, 305, 571.0, 571.1, 571.2, 571.3, 790.3, A215, and V11.3).
Figure 1.
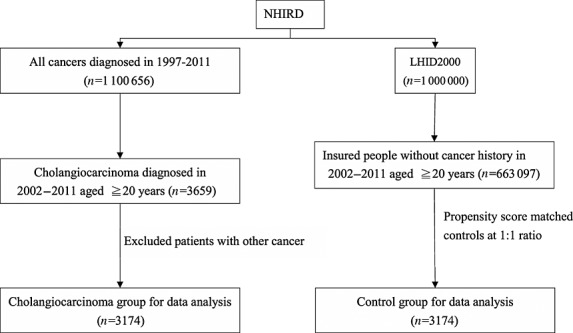
The flow chart for selecting cholangiocarcinoma cases and non-cholangiocarcinoma controls. NHRID: the National Health Insurance Research Database; LHID 2000: the Longitudinal Health Insurance Database 2000, a database containing the claims data from 1996 to 2011 for 1 million people randomly sampled from 2000 NHIRD enrollment records
Measurements of statins
The records of statin use were retrieved from ambulatory and inpatient claims data, including the cumulative defined daily dose (DDD) of each type of statin and day prescribed for cases and controls. Statins provided in the insurance programmes were simvastatin (ATC C10AA01), lovastatin (ATC C10AA02), pravastatin (ATC C10AA03), fluvastatin (ATC C10AA04), atorvastatin (ATC C10AA05) and rosuvastatin (ATC C10AA07). We calculated combined doses of all types of statin prescribed for the CCA case group and control group.
Statistical analysis
We compared proportionate distributions of gender, age (≤64 years, 65–74 years, ≥75 years), statin medication history and comorbidities between CCA cases and controls and examined the significance levels using a Chi-square test. The t-test was used to examine differences for continuous variables. The conditional logistic regression analysis was used to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs) for the association between CCA and statin uses. ORs associated with uses of all types of statin together and individual statins were analyzed. Dose–response relationships were also measured. The cumulative DDD for all statins were stratified into quartiles. For each type of statin, the cumulative DDD was partitioned into two levels by the median dose. All statistical analyses were performed using the SAS statistical package (version 9.2 for Windows; SAS Institute, Inc., Cary, NC, USA).
Results
Demographic data
We identified 3174 CCA cases diagnosed between 2002 and 2011 and 3174 controls of non-CCA patients also between 2002 and 2011. Of the 3174 CCA patients, 40.6% were younger than 64 years old and 50.9% of them were men (Table1). The mean ages of patients in the CCA and controls groups were 67.4 (±12.3) and 68.5 (± 13.2) years, respectively. Patients with CCA tended to have a lower prevalence of statin use than subjects in the control group (all P values <0.001).
Table 1.
Baseline characteristics compared between cholangiocarcinoma and control groups
| Control n = 3174 | CCA n = 3174 | ||||
|---|---|---|---|---|---|
| n | % | n | % | P value | |
| Age group (years) | 0.001 | ||||
| ≤64 | 1183 | 37.3 | 1289 | 40.6 | |
| 65–74 | 880 | 27.7 | 933 | 29.4 | |
| ≥75 | 1111 | 35.0 | 952 | 30.0 | |
| Mean (SD) (years)* | 68.5 | 13.2 | 67.4 | 12.3 | 0.001 |
| Gender | 0.78 | ||||
| Female | 1548 | 48.8 | 1559 | 49.1 | |
| Male | 1626 | 51.2 | 1615 | 50.9 | |
| Medications | |||||
| Statin | 840 | 26.5 | 720 | 22.7 | <0.001 |
| Aspirin | 1515 | 47.7 | 1534 | 48.3 | 0.63 |
| Metformin | 714 | 22.5 | 732 | 23.1 | 0.59 |
| CCI score† | 0.15 | ||||
| 0 | 1510 | 47.6 | 1596 | 50.3 | |
| 1 | 967 | 30.5 | 898 | 28.3 | |
| 2 | 350 | 11.0 | 350 | 11.0 | |
| 3 or more | 347 | 10.9 | 330 | 10.4 | |
| Baseline co-morbidities | |||||
| Diabetes | 767 | 24.2 | 772 | 24.3 | 0.88 |
| Cirrhosis | 1826 | 57.5 | 1818 | 57.3 | 0.84 |
| Chronic pancreatitis† | 35 | 1.10 | 34 | 1.07 | 0.90 |
| Hepatitis B infection | 569 | 17.9 | 537 | 16.9 | 0.29 |
| Hepatitis C infection | 320 | 10.1 | 323 | 10.2 | 0.90 |
| Gastric disease | 2152 | 67.8 | 2124 | 66.9 | 0.45 |
| Haemochromatosis† | 1 | 0.03 | 1 | 0.03 | 0.99 |
| Inflammatory bowel disease | 94 | 2.96 | 94 | 2.96 | 0.99 |
| Biliary tract disease | 1267 | 39.9 | 1303 | 41.1 | 0.36 |
| Stroke | 371 | 11.7 | 346 | 10.9 | 0.32 |
| CAD | 999 | 31.5 | 1021 | 32.2 | 0.55 |
| COPD | 686 | 21.6 | 713 | 22.5 | 0.41 |
| Alcohol-related illness | 263 | 8.29 | 271 | 8.54 | 0.72 |
Chi-square test,
t-test and
Fisher-exact test comparing subjects with and without CCA.
Data are presented as the number of subjects in each group, with percentages given in parentheses.
CCA cholangiocarcinoma
CCI score, Charlson comorbidity index score.
Risk of CCA and association with individual statins
Table2 shows the ORs of estimated CCA risk based on statin use. Use of a statin was associated with a significantly reduced risk of CCA (OR 0.80, 95% CI 0.71, 0.90). Results that measured for individual statins were all significant, ranging from 0.65 (95% CI 0.52, 0.82) for pravastatin and rosuvastatin to 0.77 (95% CI 0.61, 0.96) for fluvastatin.
Table 2.
Odds ratio and 95% confidence intervals of cholangiocarcinoma associated with statin uses
| Variable | Cases/Controls 3174/3174 | OR | (95% CI) |
|---|---|---|---|
| Medications | n1/n2 | ||
| All statins | 720/840 | 0.80 | (0.71, 0.90)*** |
| Individual statin | |||
| Simvastatin | 262/368 | 0.68 | (0.57, 0.80)*** |
| Lovastatin | 244/337 | 0.69 | (0.58, 0.83)*** |
| Pravastatin | 131/197 | 0.65 | (0.52, 0.82)*** |
| Fluvastatin | 143/183 | 0.77 | (0.61, 0.96)* |
| Atorvastatin | 357/489 | 0.69 | (0.60, 0.80)*** |
| Rosuvastatin | 141/210 | 0.65 | (0.52, 0.82)*** |
n, number of persons on the medicine.
P < 0.01,
P < 0.001
CCA risk by cumulative DDD dose of statin
Table3 shows the dose–response relationship between statin use and CCA risks, compared with non-users. Of all types of statin combined, the OR decreased from 0.86 (95% CI 0.70, 1.05) for those on < 50 cumulative DDD to 0.78 (95% CI 0.66, 0.9) for those on ≥ 150 cumulative DDD. For individual statins, CCA risk was the lowest in patients using ≧80 cumulative DDD of lovastatin (OR 0.55, 95% CI 0.41, 0.73), followed by ≧55 cumulative DDD of pravastatin (OR 0.64, 95% CI 0.44, 0.94), ≧80 cumulative DDD of simvastatin (OR 0.74, 95% CI 0.56, 0.98) and ≧65 cumulative DDD of atorvastatin (OR 0.78, 95% CI 0.62, 0.98). Compared with non-statin users, CCA risk was the lowest in patients using <300 cumulative DDD of fluvastatin (OR 0.68, 95% CI 0.47, 0.99). Fluvastatin was also associated with significant reduction in odds of CCA in <300 cumulative DDD (OR 0.68, 95% CI 0.47, 0.99).
Table 3.
Odds ratio and 95% confidence intervals of cholangiocarcinoma associated with cumulative DDD dose of statin uses
| Case numbers/control numbers | Odds ratio | (95% CI) | |
|---|---|---|---|
| Non-use of statins | 2454/2334 | 1.00 | (reference) |
| All statins* | |||
| <50 DDD | 210/227 | 0.86 | (0.70, 1.05) |
| 50 − 149 DDD | 157/187 | 0.79 | (0.64, 0.99)* |
| ≥ 150 DDD | 353/426 | 0.78 | (0.66, 0.91)** |
| P for trend | <0.001 | ||
| Simvastatin† | |||
| <80 DDD | 131/163 | 0.76 | (0.58, 0.99)* |
| ≥80 DDD | 118/148 | 0.74 | (0.56, 0.98)* |
| Lovastatin† | |||
| <80 DDD | 136/160 | 0.74 | (0.57, 0.96)* |
| ≥80 DDD | 107/162 | 0.55 | (0.41, 0.73)*** |
| Pravastatin† | |||
| <55 DDD | 62/77 | 0.72 | (0.48, 1.08) |
| ≥55 DDD | 64/90 | 0.64 | (0.44, 0.94)* |
| Fluvastatin† | |||
| <300 DDD | 64/89 | 0.68 | (0.47, 0.99)* |
| ≥300 DDD | 73/71 | 1.22 | (0.81, 1.84) |
| Atorvastatin† | |||
| <65 DDD | 165/196 | 0.84 | (0.66, 1.08) |
| ≥65 DDD | 164/199 | 0.78 | (0.62, 0.98)* |
| Rosuvastatin† | |||
| <35 DDD | 68/63 | 1.01 | (0.66, 1.56) |
| ≥35 DDD | 57/74 | 0.75 | (0.50, 1.12) |
The cumulative DDD dose is partitioned into three segments for the first quartile, second quartile and third and fourth quartiles combined.
The cumulative DDD dose is partitioned into two segments by median.
P < 0.05,
P < 0.01
Discussion
This population-based nationwide study demonstrated a dose–response protective relationship between the use of statins and odds of CCA. Overall, the CCA risk reduced by 20% based on measuring all statins combined. ORs measured by the cumulative DDD of individual statins also showed lowered risk of CCA for most subgroups of individual statins. The stronger dose related reduction in CCA risk is more likely for those who have taken lovastatin and pravastatin. The beneficial association was also seen for those on simvastatin, lovastatin and fluvastatin. Our results demonstrated an important role of statin use associated with the reduced risk of CCA.
To the best of our knowledge, this is the first study demonstrating a protective relationship of statin use on CCA in a dose-dependent manner. The data were obtained from a large computerized database so the studied population was sufficient in size and highly representative. CCA patients and controls were selected by matching propensity score and diagnosis date to reduce bias. As the data on statin use were collected from a computerized database containing all available prescription information, the potential for bias with regard to medication was minimized. The statin prescriptions were required to be in accordance with national health insurance regulations with a definite diagnosis of increased serum lipid profiles.
The use of cholesterol-lowering agents has increased as part of the effort to improve nutrition, lifestyle habits and health care. Prolonged control of LDL cholesterol with statin treatment may be warranted in all patients at high risk of any type of major vascular event 32. Besides their cholesterol-lowering effect, statins also exhibit pleiotropic properties including anti-inflammation, immunomodulation and anti-tumour effects 12,13,17. In addition, statin use could reduce the risk of cancer 14,17, and this effect has been demonstated in multiple organ systems, including the digestive system 18,23,25,26,33, respiratory system 27,28, haematological system 34 and genitourinary system 35, as well as in breast 36 and skin tissues.
Whether statins reduce the risk of various cancers is controversial 29,30,37,38 particularly with regard to the duration of statin use. Short term use of statins may not reduce cancer risk 16. Our study did show a non-significant protection relationship on CCA for those on all statins of < 50 cumulative DDD. However, studies on cholesterol-reducing strategies suggest that the long term use of statins is recommended to protect against vascular events 32. Thus, it appears that long term statin use may not be a cause for concern. However, a definitive baseline duration of statin use for cancer prevention or anti-cancer effect has not been established. In addition to the use of statins in cancer prevention, the combined treatment of statins and chemotherapy agents could be a promising new strategy for the treatment of certain tumour types 16.
The mechanism of statin use for reducing cancer risk is not well understood. Interestingly, the risk of cancer may be related to increased serum lipid concentrations or the pleiotropic properties of statins or both 37. The mechanism by which lower cholesterol confers health benefits may involve its effects on membrane lipid organization possibly resulting in various molecular effects that might be effective for preventing and treating the progression of malignant tumours 39. Most of the molecular mechanisms reported in the literature involve the effect of statins on inhibition of cell proliferation and stimulation of apoptosis, and these results have also been demonstrated in cholangiocytes 40,41.
There are several potential limitations to the present study. First, because this study was a retrospective cohort study based on computerized data, most of patients were presumed to comply with their prescribed medications. However, the true compliance rates among patients taking statins could not be evaluated. Second, unmeasured confounding factors, such as body mass index, smoking, alcohol intake, drug history and parasite infection, which may be associated with the effects of drugs and CCA, were not included in our database. Third, as this was a retrospective study, a definite diagnosis of CCA was not possible for all patients. However, experts must review the certificate for CCA with the aid of imaging or pathological results. Fourth, there was likely a range of variance in the registration of diagnosis of CCA as well as co-morbid diseases, but the effect of variance would be limited.
In conclusion, statin use may associated with reduced risk of CCA by 20% in this population-based nationwide study. The dose-dependent relationship between the use of statins and risk of CCA demonstrates a benefit of long term usage. However, the role of statin use for reducing cancer risk in chemoprevention, and its synergic effect with chemotherapy, as well as the baseline recommended duration of statin use require further study. The long term effect of statins on the incidence of CCA and other cancers also warrants further research.
Competing Interests
All authors state that they have no conflicts of interest.
Contributors
Conception and design: Yen-Chun Peng, Cheng-Li Lin, Chia-Hung Kao; Administrative support: Chia-Hung Kao; Collection and assembly of data: Yen-Chun Peng, Cheng-Li Lin, Chia-Hung Kao; Data analysis and interpretation: Yen-Chun Peng, Cheng-Li Lin, Chia-Hung Kao; Manuscript writing: All authors; Final approval of manuscript: All authors.
Financial support
This study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. No additional external funding was received for this study.
References
- Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–14. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–85. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–5. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucman-Rossi J, Nault JC, Zender L. Primary liver carcinomas can originate from different cell types: a new level of complexity in hepatocarcinogenesis. Gastroenterology. 2013;145:53–5. doi: 10.1053/j.gastro.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–22. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33–42. doi: 10.1038/ncpgasthep0389. [DOI] [PubMed] [Google Scholar]
- Chaiteerakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, Kim WR, Gores GJ, Roberts RO, Olson JE, Therneau TM, Roberts LR. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology. 2013;57:648–55. doi: 10.1002/hep.26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TH, Yuan RH, Chen YL, Yang WC, Hsu HC, Jeng YM. Viral hepatitis is associated with intrahepatic cholangiocarcinoma with cholangiolar differentiation and N-cadherin expression. Mod Pathol. 2011;24:810–9. doi: 10.1038/modpathol.2011.41. [DOI] [PubMed] [Google Scholar]
- Burr NE, Talboys RJ, Savva S, Clark A, Phillips M, Metcalfe M, Dennison A, Robinson R, Lewis MP, Rhodes M, Rushbrook S, Hart AR. Aspirin may prevent cholangiocarcinoma: a case-control study from the United Kingdom. Dig Dis Sci. 2014;59:1567–72. doi: 10.1007/s10620-014-3056-z. [DOI] [PubMed] [Google Scholar]
- Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–87. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 2005;46:1855–62. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, Dalton SO, Sørensen HT, Olsen JH. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643–7. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: A systematic review and meta-analysis. Eur J Cancer. 2008;44:2122–32. doi: 10.1016/j.ejca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Osmak M. Statins and cancer: current and future prospects. Cancer Lett. 2012;324:1–12. doi: 10.1016/j.canlet.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–94. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–32. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623–30. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- Carey FJ, Little MW, Pugh TF, Ndokera R, Ing H, Clark A, Dennison A, Metcalfe MS, Robinson RJ, Hart AR. The differential effects of statins on the risk of developing pancreatic cancer: a case-control study in two centres in the United Kingdom. Dig Dis Sci. 2013;58:3308–12. doi: 10.1007/s10620-013-2778-7. [DOI] [PubMed] [Google Scholar]
- Chiu HF, Chang CC, Ho SC, Wu TN, Yang CY. Statin use and the risk of pancreatic cancer: a population-based case-control study. Pancreas. 2011;40:669–72. doi: 10.1097/MPA.0b013e31821fd5cd. [DOI] [PubMed] [Google Scholar]
- Alexandre L, Clark AB, Bhutta HY, Holt S, Lewis MP, Hart AR. Statin use is associated with reduced risk of histologic subtypes of esophageal cancer: a nested case-control analysis. Gastroenterology. 2014;146:661–8. doi: 10.1053/j.gastro.2013.11.046. [DOI] [PubMed] [Google Scholar]
- Singh PP, Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann Oncol. 2013;24:1721–30. doi: 10.1093/annonc/mdt150. [DOI] [PubMed] [Google Scholar]
- Chiu HF, Ho SC, Chang CC, Wu TN, Yang CY. Statins are associated with a reduced risk of gastric cancer: a population-based case-control study. Am J Gastroenterol. 2011;106:2098–103. doi: 10.1038/ajg.2011.277. [DOI] [PubMed] [Google Scholar]
- Jacobs EJ, Rodriguez C, Brady KA, Connell CJ, Thun MJ, Calle EE. Cholesterol-lowering drugs and colorectal cancer incidence in a large United States cohort. J Natl Cancer Inst. 2006;98:69–72. doi: 10.1093/jnci/djj006. [DOI] [PubMed] [Google Scholar]
- Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–92. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- Cheng MH, Chiu HF, Ho SC, Yang CY. Statin use and the risk of female lung cancer: a population-based case-control study. Lung Cancer. 2012;75:275–9. doi: 10.1016/j.lungcan.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Khurana V, Bejjanki HR, Caldito G, Owens MW. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest. 2007;131:1282–8. doi: 10.1378/chest.06-0931. [DOI] [PubMed] [Google Scholar]
- Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol. 2006;24:4808–17. doi: 10.1200/JCO.2006.06.3560. [DOI] [PubMed] [Google Scholar]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029849. : e29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TM. Taiwan’s National Health Insurance system: high value for the dollar. In: Okma KGH, Crivelli L, editors. Six Countries, Six Reform Models: The Health Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. New Jersey: World Scientific; 2009. pp. 71–204. [Google Scholar]
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514–21. doi: 10.1200/JCO.2012.44.6831. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Holford TR, Leaderer B, Zahm SH, Boyle P, Morton LM, Zhang B, Zou K, Flynn S, Tallini G, Owens PH, Zheng T. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15:419–28. doi: 10.1023/B:CACO.0000027506.55846.5d. [DOI] [PubMed] [Google Scholar]
- Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch Intern Med. 2005;165:2264–71. doi: 10.1001/archinte.165.19.2264. [DOI] [PubMed] [Google Scholar]
- Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635–7. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi S, Glynn RJ, Avorn J, Mogun H, Schneeweiss S. Statins and the risk of lung, breast, and colorectal cancer in the elderly. Circulation. 2007;115:27–33. doi: 10.1161/CIRCULATIONAHA.106.650176. [DOI] [PubMed] [Google Scholar]
- Murai T, Maruyama Y, Mio K, Nishiyama H, Suga M, Sato C. Low cholesterol triggers membrane microdomain-dependent CD44 shedding and suppresses tumor cell migration. J Biol Chem. 2011;286:1999–2007. doi: 10.1074/jbc.M110.184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T, Yang F, Wise CE, Meng F, Priester S, Munshi MK, Guerrier M, Dostal DE, Glaser SS. Simvastatin stimulates apoptosis in cholangiocarcinoma by inhibition of Rac1 activity. Dig Liver Dis. 2011;43:395–403. doi: 10.1016/j.dld.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamigaki M, Sasaki T, Serikawa M, Inoue M, Kobayashi K, Itsuki H, Minami T, Yukutake M, Okazaki A, Ishigaki T, Ishii Y, Kosaka K, Chayama K. Statins induce apoptosis and inhibit proliferation in cholangiocarcinoma cells. Int J Oncol. 2011;39:561–8. doi: 10.3892/ijo.2011.1087. [DOI] [PubMed] [Google Scholar]


