In this series we draw attention to medicines that have entered the European market with an entirely new mechanism of action. Publication is not to be confused with endorsement of use in clinical practice. Copyright to the images belongs to Leiden University, but use of the images (also available at http://coo.lumc.nl/trc and in the app store) is free.
Introduction
Metastatic melanoma has very poor prognosis and until recently only limited therapeutic options were available. Mutations in the gene encoding BRAF kinase account for 40–60% of melanomas. The most common oncogenic mutation in BRAF kinase is V600E. The V600K mutation is much less common. Recently, three new compounds that target specific components in the RAS-RAF-MEK-ERK signal transduction pathway (the ERK pathway) have become available. These compounds are vemurafenib (Zelboraf®) 1,2, dabrafenib (Tafinlar®) 3 and trametinib (Mekinist®) 4.
Mechanism
The ERK pathway is an important regulator of several cellular processes, including proliferation, survival and differentiation. The ERK pathway plays an important role in the tumourigenesis of a variety of different cancers by causing dysregulation of the RAS-RAF-MEK-ERK signalling cascade. GTP-bound RAS activates the RAF proteins. BRAF, a member of the serine/threonine kinase family, activates the mitogen-activated protein/extracellular signal-regulated kinase (MEK), which in turn phosphorylates and activates ERK. Ultimately, this pathway activates transcription factors that drive cell proliferation, survival and differentiation (Figure1A).
Figure 1.
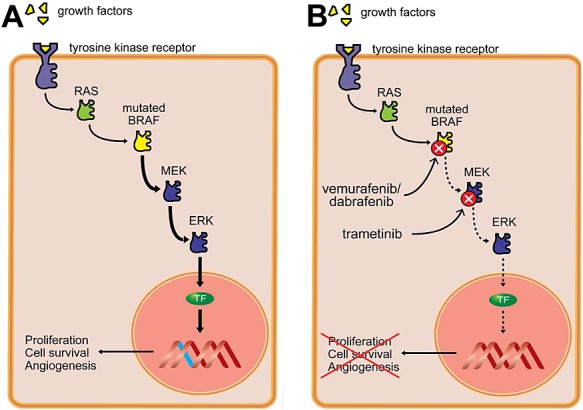
The RAS-RAF-MEK-ERK pathway and the mechanism of action of vemurafenib, dabrafenib and trametinib. Oncogenic mutations in the BRAF protein cause overactivation of this pathway, leading to increased cell proliferation, cell survival, and angiogenesis (A). Vemurafenib and dabrafenib selectively inhibit mutated BRAF proteins, whereas trametinib inhibits MEK and downstream ERK signalling. TF, transcription factor (B)
Vemurafenib and dabrafenib potently inhibit BRAF proteins containing the V600E or V600K mutation. Trametinib is an allosteric inhibitor of MEK (Figure1B).
Indication
Vemurafenib and dabrafenib are indicated for patients with non-resectable or metastatic melanoma associated with the BRAF V600E mutation. The MEK inhibitor trametinib is also indicated for melanoma associated with BRAF V600E or V600K mutations. Since 2014 trametinib has also been registered for use in combination with dabrafenib for the treatment of patients with BRAF(V600E) or BRAF(V600K) melanoma.
Clinical application
Vemurafenib and dabrafenib prolong both progression-free survival and overall survival of melanoma patients and are therefore promising options for patients with tumours that arise from a BRAF V600 mutation. In patients with metastatic melanoma, combined therapy with dabrafenib and trametinib increases progression-free survival compared with treatment with dabrafenib alone (median survival is 9.4 months vs. 5.8 months, respectively) 5. Compared with treatment with vemurafenib (which provides median progression-free survival of 7.3 months), combined therapy with dabrafenib and trametinib provides improved progression-free survival (median survival is 11.4 months) 6. However, response rates are highly variable and many patients develop resistance to ERK signalling inhibitors, possibly due to compensatory mechanisms such as EGFR overexpression or an adaptive transcriptional response 7. Other mechanisms that can confer resistance to treatment include increased activity of the PI3K/AKT/mTOR pathway and upregulation of the ERK signalling pathway 1,8.
Adverse effects
The most common adverse effects of vemurafenib are arthralgia, fatigue, rash, alopecia and photosensitivity. The most common adverse effects of dabrafenib include papilloma, headache, cough and nausea. Finally, the most common adverse effects of trametinib include rash, diarrhoea, fatigue, peripheral oedema and nausea. The prevalence of severe adverse events is similar between combination therapy and monotherapy 6.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- Flaherty KT, Yasothan U, Kirkpatrick P. Vemurafenib. Nat Rev Drug Discov. 2011;10:811–12. doi: 10.1038/nrd3579. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Zelboraf: EPAR – Product Information. March 19 2012. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002409/WC500124317.pdf (updated August 5 2014, last accessed 11 February 2015)
- European Medicines Agency. Tafinlar: EPAR – Product Information. September 18 2013. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002604/WC500149671.pdf (updated January 1 2015, last accessed 11 February 2015)
- European Medicines Agency. Mekinist: EPAR – Product Information. July 9 201. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002643/WC500169657.pdf (updated February 4 2015, last accessed 11 February 2015)
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA, III, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K, Weber J. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, Martin AM, Sun P, Crist W, Legos J, Rubin SD, Little SM, Schadendorf D. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- Sun C, Wang L, Huang S, Heynen GJ, Prahallad A, Robert C, Haanen J, Blank C, Wesseling J, Willems SM, Zecchin D, Hobor S, Bajpe PK, Lieftink C, Mateus C, Vagner S, Grernrum W, Hofland I, Schlicker A, Wessels LF, Beijersbergen RL, Bardelli A, Di NF, Eggermont AM, Bernards R. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118–22. doi: 10.1038/nature13121. [DOI] [PubMed] [Google Scholar]
- Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13:928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]


