Abstract
Aims
The aim of the present study was to conduct a meta-analysis of controlled trials assessing the impact of pharmaceutical care interventions (e.g. medication reviews) on medication underuse in older patients (≥65 years).
Methods
The databases MEDLINE and EMBASE were searched for controlled studies, and data on interventions, patient characteristics and exposure, and outcome assessment were extracted. Risk of bias was assessed using the Cochrane Collaboration’s ‘risk of bias’ table. Results from reported outcomes were synthesized in multivariate random effects meta-analysis, subgroup meta-analysis and meta-regression.
Results
From 954 identified articles, nine controlled studies, mainly comprising a medication review, were included (2542 patients). These interventions were associated with significant reductions in the mean number of omitted drugs per patient (estimate from six studies with 1469 patients: – 0.44; 95% confidence interval –0.61, –0.26) and the proportion of patients with ≥1 omitted drugs (odds ratio from eight studies with 1833 patients: 0.29; 95% confidence interval 0.13, 0.63). The only significant influential factor for improving success was the utilization of explicit screening instruments when conducting a medication review (P = 0.033).
Conclusion
Pharmaceutical care interventions, including medication reviews, can significantly reduce medication underuse in older people. The use of explicit screening instruments alone or in combination with implicit reasoning is strongly recommendable for clinical practice.
Keywords: elderly, inappropriate prescribing, medication reviews, medication underuse, meta-analysis, systematic literature review
What is Already Known about this Subject
Underuse of indicated medications is highly prevalent among older adults across all healthcare settings.
Associations with distinct patient characteristics such as age or drug numbers suggest that efficiency of pharmaceutical care interventions depend on those contextual factors.
Screening instruments allow for detection of underuse and can be implemented as part of pharmaceutical care interventions such as medication reviews.
What this Study Adds
Pharmaceutical care interventions significantly reduced medication underuse.
The usage of (explicit) screening instruments is significantly more effective than interventions without such instruments.
Introduction
Over the last few decades, the proportions of older adults have increased markedly in most populations 1,2. The increased life expectancy of older adults can be partly attributed to available pharmacological treatment options for acute and chronic diseases that help to alleviate ailments and prevent critical illness 3. However, potentially inappropriate prescribing (PIP) is common in older people, suggesting that this age group is especially susceptible to drug-related problems 4,5. Among the categories of PIP 4,6, medication underuse relates to potential prescribing omissions of drugs indicated for the treatment or prevention of a disease 7,8. In comparison with other forms of PIP (misuse, overuse), medication underuse is also frequent 9 but still poorly understood in its nature, and thus strategies to reduce it have not been established.
Interventions to reduce inappropriate prescribing are multifaceted 10,11 and may consist of medication reviews as part of a pharmaceutical care programme, educational programmes (e.g. prescriber education explaining pharmaceutical care) or clinical decision support systems. Medication reviews, defined as ‘the process where a health professional reviews the patient, the illnesses and the drug treatment’ 12, are most common for the identification of unmet therapeutic needs. Pharmaceutical care, including ‘the responsible provision of drug therapy’ 12 is ideally provided by a clinical pharmacist in the process of providing patient care in multidisciplinary teams. This requires elements from medication reconciliation, including the decisive steps of verification (i.e. collection of the patient’s medical and medication history) and clarification (i.e. determination of appropriateness) 13. Determination of appropriateness is generally facilitated by screening instruments based on either implicit (i.e. judgment-based) or explicit (i.e. criterion-based) criteria. Explicit screening instruments for assessing medication such as the Screening Tool to Alert Doctors to Right Treatments (START) 14,15 and Assessing Care of Vulnerable Elderly (ACOVE) 16–18 are commonly used, while implicit criteria such as the ‘Assessment of Underutilization’ (AOU) index are less often applied 18.
It is not clear what quantitative impact is attributable to interventions, or on what quantitative and qualitative population characteristics their effectiveness depends. Therefore, we aimed to quantify the effect of pharmaceutical care interventions on medication underuse and identify indicators of success, such as the application of explicit screening instruments in a multivariate random-effects meta-analysis. In addition, we explored the heterogeneity of factors that could potentially influence the outcome of pharmaceutical care interventions to reduce medication underuse by means of subgroup analyses and meta-regression.
Methods
Data sources and literature search strategy
In November 2014, we searched the database Medline (1966 onwards) for pharmaceutical care interventions targeting medication underuse, using terms that mapped to Medical Subject Headings in combination with keywords for nonmapping concepts (Supplementary Table 1). The search strategy was adapted to the database EMBASE, which was searched in December 2014. Additionally, we reviewed the bibliographies of articles retrieved from the database search.
Study selection
Medication underuse was defined as failure to prescribe or as absence of drugs that were indicated. Furthermore, medication underuse had to be clearly distinguished from other forms of PIP – i.e. overuse defined as prescribing or as the presence of more drugs than are clinically needed, and misuse defined as incorrect prescribing of needed drugs or the presence of potentially inappropriate medications (PIMs) 4. Based on this definition, we screened the titles and abstracts of publications and, where deemed appropriate, reviewed the full text to confirm that all of the following inclusion criteria were met: (1) investigation of patients aged 65 years or older; (2) quantification of medication underuse for several indications; (3) presentation of outcomes regarding medication underuse as the proportion of patients or care issues with ≥1 omitted drugs and/or the number of omitted drugs per patient after a pharmaceutical care intervention (e.g. medication review, drug utilization review); and (4) a controlled study design, in which a control group receives the usual care of the respective setting. Therefore, the control group either did not receive any medication review or was treated by staff who had not received study-related educational training. In these studies, the definition of an omitted drug can be based on clinical reasoning, guidelines, prescribing criteria or explicit screening lists. Studies were included if necessary information could be retrieved for all of these aspects. Studies were explicitly excluded if they did not distinguish between the different forms of inappropriate prescribing and therefore did not specifically address underuse. The search was limited to languages known by the authors – i.e. English, German, French and Spanish. Two investigators (AB and ADM) independently screened all identified titles and abstracts and full texts according to inclusion and exclusion criteria, which were predefined in the review protocol. Discrepancies were resolved by consulting a third reviewer (AL).
Data extraction and quality assessment
We extracted bibliographic details of the study and further information on study design, patient population, exposure assessment, type of intervention, reported outcomes, outcomes assessment, inclusion criteria and additional comments into a data extraction form. In cases of uncertainties or missing information, authors were contacted by e-mail and asked for this information. Finally, all included studies were assessed for risk of bias by two reviewers (ADM, AB) using the Cochrane Collaboration’s ‘risk of bias’ table 19. Accordingly, study results were assessed as being of low, unclear or high risk of bias. In cases of disagreement, a third reviewer (AL) was involved to reach consensus.
Data synthesis
Continuous measures and dichotomous outcomes were primarily reported unchanged for better interpretation. Thus, continuous measures were reported as means with their standard deviations, or changes from baseline in the case of baseline imbalances between intervention and control groups. Dichotomous outcomes were reported as odds ratios, or Mantel–Haenszel odds ratios in the case of baseline imbalances between intervention and control groups. Continuous and dichotomous measures alike were converted into standardized mean differences and Hedges’ g in order to obtain comparable effect sizes. Generally, point estimates and 95% confidence intervals were reported for all effect measures.
Meta-analysis relied on the random-effects model for combining studies to give pooled estimates of effect, with P < 0.05 (two-sided) considered statistically significant. Study heterogeneity was evaluated qualitatively by assessing differences in study populations, interventions, outcome measures and study design, whereas statistical heterogeneity was assessed using the Cochrane Q, τ2 and I2 statistics.
In univariate and multivariate approaches, restricted maximum likelihood was used as the variance estimator. Concerning multivariate analyses, the biserial correlation coefficient between continuous and dichotomous outcomes has been empirically determined as 0.66 in a cohort using START criteria 20. Based upon this estimate, within-study covariances were calculated according to Wei and Higgins 21, to be applied for multivariate random-effects meta-analysis 22,23. Sensitivity analyses included approaches assuming unknown within-study correlations using the method proposed by Riley and colleagues 24 and a Bayesian approach using non-informative priors based upon a uniform distribution for within-study covariances and a Wishart distribution for between-study covariances 25.
The identification of indicators of success of pharmaceutical care interventions was based on the outcomes of all studies. Upon conversion of odds ratios and mean number of omitted drugs, the common Hedges’ g adjusted standardized mean difference was applied using univariate random-effects models in order to allow for comparison of study effects in dependence of variables at the study level. Exploration of sources of heterogeneity included subgroup analyses for categorical factors indicating outpatient or inpatient outcome assessment, and use of explicit screening instruments as part of the intervention or not. Meta-regression was applied to investigate heterogeneity in terms of continuous factors (e.g. age, number of baseline omissions, follow-up time and number of drugs).
Results
Our broad search strategy (Supplementary Table 1) initially yielded 1108 records from the databases MEDLINE (Pubmed) and EMBASE (Figure1) and eight records through bibliographic search. After removing duplicates, 954 titles and abstracts were screened for indications of medication underuse. The remaining 39 records were examined for eligibility according to the predefined inclusion criteria. Thirty full-text records were excluded, mainly because of the lack of a control group or inadequately reported results (Figure1). Of the included nine studies 26–34, study characteristics were described (Supplementary Table 2) and assessed for their risk of bias (Supplementary Table 3). The most notable aspects contributing to risk of bias were unclear allocation concealment and absent blinding of personnel or outcome assessment, which can be partly attributed to the study design.
Figure 1.
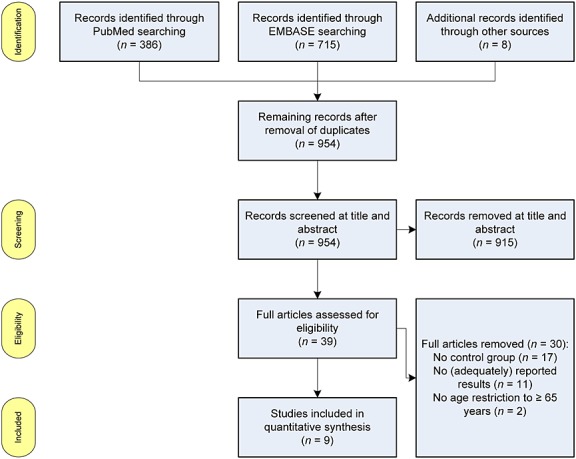
PRISMA flow chart
After extraction of the reported results, six studies 28–30,32–34, two of which were based on the same data source 29,30, reported the outcome as a mean number of omitted drugs, while seven studies 26–28,30,31,33,34 presented the outcome as a proportion of patients or pharmaceutical care issues with ≥1 omission of indicated drugs. Given the fact that studies were heterogeneous regarding the setting and statistics, pooled estimates from a random-effects meta-analysis are provided in Figure2. Because four studies 28,30,33,34 reported both outcomes (i.e. mean number of omitted drugs and the proportion of patients or pharmaceutical care issues with ≥1 omission of indicated drugs), we analysed the effects in a multivariate meta-analysis. The multivariate pooled estimate of the mean number of omitted drugs revealed a significant reduction of 0.44 omitted drugs per patient (95% confidence interval 0.61, 0.20) (Figure2A). The pooled estimate of odds ratios calculated from proportions was significant in the univariate and the multivariate approach (Figure2B). In sensitivity analyses, multivariate point estimates calculated by the alternative Riley model 24 and the Bayesian approach with unknown within-study correlations revealed an odds ratio of 0.32 (95% confidence interval 0.14, 0.73; Bayesian 95% credible interval 0.12, 0.71). A mean reduction in omitted drugs per patient by –0.36 (95% confidence interval–0.62, –0.11) was found in the Riley model and 0.44 in the Bayesian approach (Bayesian 95% credible interval –0.93, 0.04). Of note, the multivariate estimate might be less precise due to the uncertainty imposed by studies not reporting both outcomes.
Figure 2.
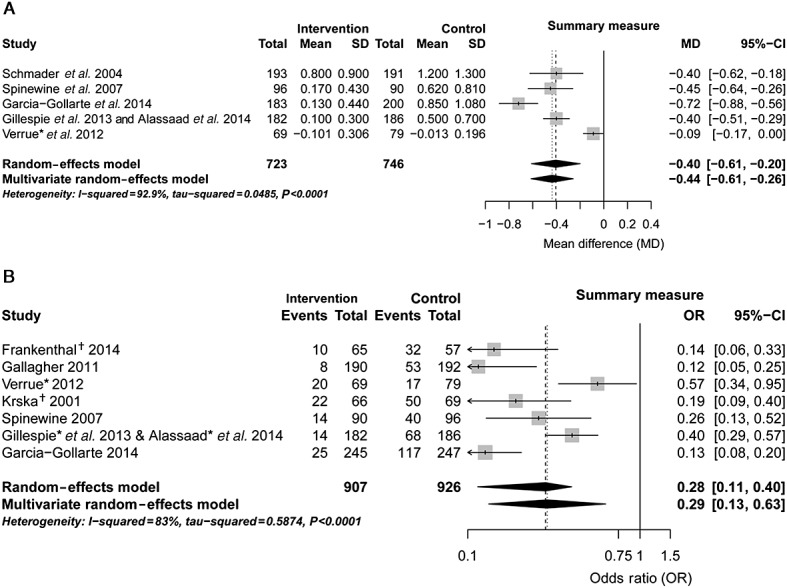
Forest plots of intervention effects on the continuous mean of omitted drugs per patient (A) and the proportion of patients with ≥1 omission (B). (* mean changes from baseline or Mantel–Haenszel odds ratio were calculated, accounting for baseline imbalances; † number of potential prescribing omissions (PPOs) was used instead of number of patients unavailable). CI, confidence interval
The subgroup analysis revealed no significant difference between the inpatient (including long-term care) and outpatient setting (P = 0.877) (Figure3A). However, grouping the studies according to the application of explicit screening instruments as part of the intervention yielded significantly different pooled subgroup estimates (P = 0.033) (Figure3B). Thus, the application of explicit screening instruments (i.e. START, ACOVE) is more effective than an unstructured intervention without a screening instrument. Concerning continuous predictors in meta-regression analyses, the number of drugs at baseline, the time until outcome assessment (follow-up duration), the mean age of the patients and the number of omitted drugs at baseline did not significantly influence the outcome of pharmaceutical care interventions targeting medication underuse (Figure4). However, a trend of increased effectiveness of the interventions could be assumed for patients with fewer baseline omissions (Figure4A) and younger age (Figure4B).
Figure 3.
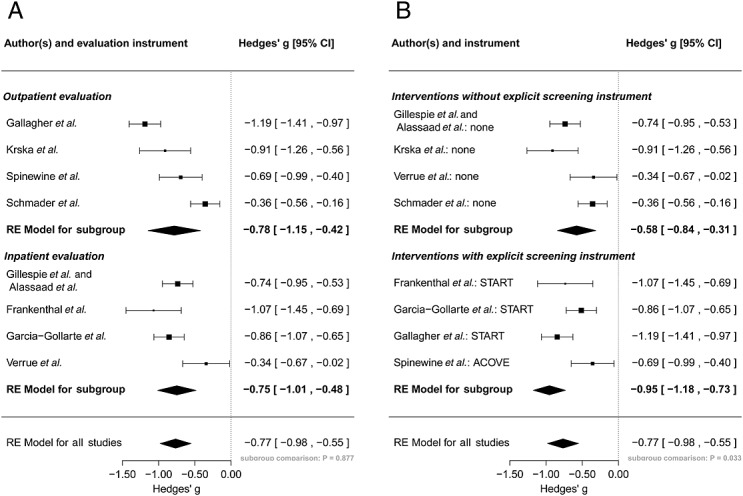
Subgroup meta-analysis of different outcomes converted into a common effect size (Hedges’ g). Studies are stratified according to the setting of outcome assessment (i.e. inpatient setting, including long-term care and outpatient setting) (A) and the application of explicit screening instruments (B). ACOVE, Assessing Care of Vulnerable Elderly; CI, confidence interval; START, Screening Tool to Alert Doctors to Right Treatments; RE, random-effects
Figure 4.
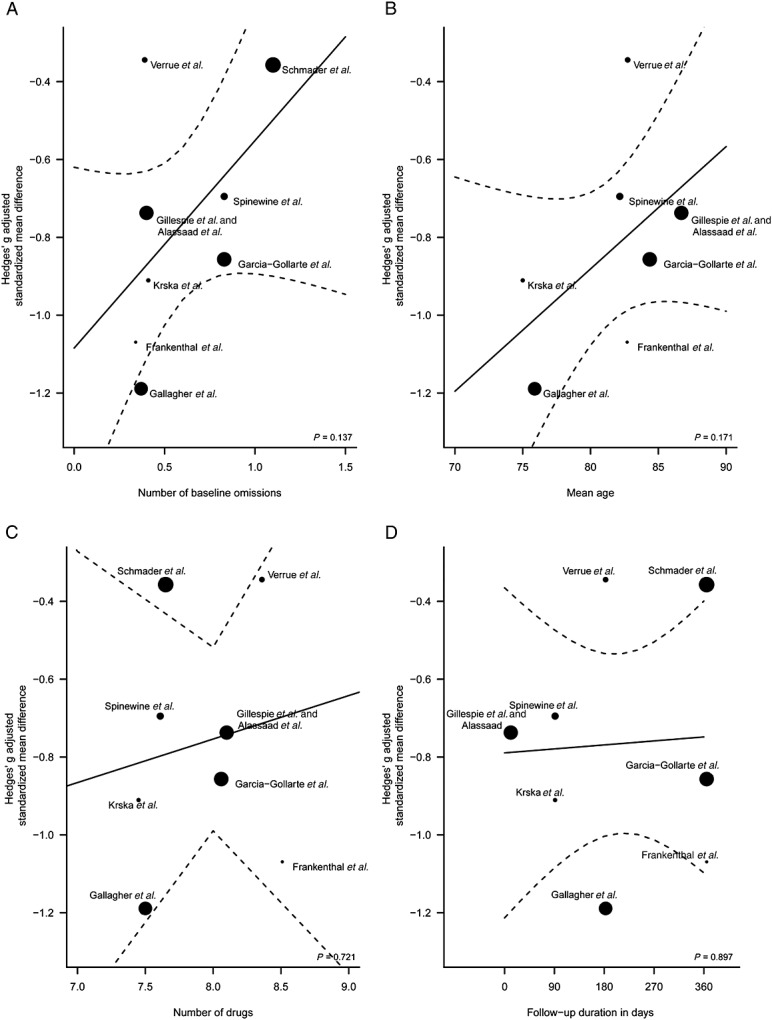
Meta-regression of different outcomes converted into a common effect size (Hedges’ g). Study-level covariates include the mean number of baseline omission per patient (A), patients’ mean age (B), the mean number of drugs per patient (C) and the follow-up duration (D). Regression lines are visualized as solid lines with 95% confidence regions (dotted lines). Studies were weighted according to their sample sizes as indicated by different spot sizes.
Discussion
In the present meta-analysis, we identified, assessed and summarized evidence supporting the effectiveness of pharmaceutical care interventions such as medication reviews in combination with screening instruments to reduce medication underuse in older patients. Our results emphasize the value of pharmaceutical care interventions for reducing inappropriate prescribing, particularly if explicit screening tools are used. In explicit screening tools, medication underuse mainly relates to regularly prescribed prescription medicines (e.g. beta-blockers with ischaemic heart disease). Nonprescription medicines are also included as far as sufficient evidence exists for their use (e.g., vitamin D and calcium supplements in patients with known osteoporosis).
Considering the diversity of clinical practice, we included studies conducted in older outpatients, hospitalized older people and nursing home residents. The corresponding interventions consisted of unstructured medication reviews (i.e. not using a screening instrument such as START), medication reviews based on screening instruments or an educational intervention that included exemplary use of screening instruments. A successful intervention may also depend on the clinical setting. Educational interventions can be useful in settings such as nursing homes, but can be less effective if decision makers do not consider them to be important, or when the change is complex and requires the coordinated interaction of many individuals 35. Medication reviews are usually conducted by physicians or (clinical) pharmacists. The role of pharmacists can vary from (passively) recommending changes to active involvement in multidisciplinary teams of healthcare professionals. Similarly, the responsibility of the person conducting the medication review and the acceptance rate of recommendations may differ in primary care, hospital care or long-term care settings of older inpatients.
Our results confirm the expected benefit of pharmaceutical care interventions targeting medication underuse. We did not restrict inclusion to a specific clinical setting but rather included all, and even diverse, populations as they are encountered in clinical practice and accounted for the heterogeneity in the random-effects meta-analyses. Clinical implications can easily be deduced; given our pooled odds ratio of 0.29 and prevalence of medication underuse between 0.55 and 0.73 36, the number needed to treat (NNT) is approximately 3. A similar NNT of between 2 and 3 is obtained by the pooled estimate of –0.44 in the mean reduction in omitted drugs per patient. Because explicit screening instruments such as the START criteria are fast and cheap to apply 26,37, this is a reasonable number to justify the implementation of such instruments into clinical practice.
In all subgroup analyses, the only significant influential factor for improving success was the utilization of explicit screening instruments when conducting a medication review, although other factors, such as the patient’s age or the number of already omitted drugs, might become significant when larger data sets are analysed. A prescriber may be more inclined to prescribe an indicated preventive medicine to a younger patient with fewer comorbidities and longer life expectancy. Likewise, it is probably easier to achieve a reduction in omitted drugs if fewer drugs at baseline are indicated for a patient.
Although we conducted our literature search in conformity with the criteria for a systematic review, we cannot rule out the possibility of missing relevant studies owing to the intrinsic weaknesses of any search strategy. We based our search on the most relevant databases, but did not search grey literature. Publication bias was not assessed owing to the small number of studies and the fact that underuse was most often not the primary study outcome. Conceptually, we addressed the problem of medication underuse from a pharmacological perspective. Looking at the process of a successful drug therapy, it should be evident that therapeutic failure is not solely caused by the mere omission of indicated pharmacotherapy, but may also be due to poor prescribing (e.g. under-dosing), patient non-adherence or drug–drug interactions interfering with the exposure to or effectiveness of the drug of interest 38–40. It thus remains the question of appropriateness: good prescribing is a multifaceted task that accounts for the benefits and the risks of a specific medication and also considers treatment goals, quality of life, patient preferences, life expectancy and time until benefit 41,42. Therefore, the evaluation of appropriateness as part of the prescription process requires widespread evaluation not only focused on pharmacological appropriateness, but also considering the requirements and needs of the individual patient. Whereas explicit screening instruments are efficient, the use of combinations of implicit and explicit instruments may be even more powerful and probably the only strategy to tailor treatments comprehensively to patients’ needs 43. Hence, explicit prescribing criteria are not substitutes for careful clinical decision making but can focus the attention of the healthcare professional on the potential flaws of a current medication. The caring physician or pharmacist will still have to assess whether a certain intervention is feasible in the given situation and whether all clinically important aspects are considered.
Regarding the implications for further research 44, several suggestions can be made based on our results. The current evidence for the efficacy of pharmaceutical care interventions targeting medication underuse is mainly limited to medication appropriateness as opposed to the clinical value of preventing underuse in a specific population. While it appears intuitive that the absence of indicated medicines may be detrimental, indiscriminate addition of medicines may also pose risks due to (unconsidered) drug–drug interactions or drug–disease interactions.Therefore, the value of an intervention targeting medication underuse has to be properly evaluated, and ultimately such studies should provide evidence on clinical outcomes. As there will be considerable heterogeneity in the effects based on clinical or social differences between different patients, a precise definition of the study population is equally as important as the definition of the intervention in terms of type, frequency and duration, among others. Because contextual factors 19 may influence the effectiveness of an intervention, a detailed description of the settings is strongly recommended. Our findings indicate that: (i) explicit criteria should be applied as an integral part of any intervention addressing medication underuse; (ii) their combination with implicit criteria should be examined to assess whether patient requirements are better met; and (iii) most importantly, outcomes should include patient-relevant endpoints (e.g. clinical endpoints, quality of life and patient satisfaction) rather than omitted medicines only.
Conclusion
Although medication underuse is a frequent finding in older people, studies aimed at reducing it are sparse, heterogeneous and of variable quality. The limited body of published evidence suggests that pharmaceutical care interventions such as medication reviews are effective in reducing medication underuse, and substantially more effective if explicit screening instruments are applied. The magnitude of pooled-effect estimates on prescribing outcomes is considerable, suggesting a potentially relevant impact on clinical endpoints.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: AL received a personal scholarship from the ‘Dr. August und Dr. Anni Lesmüller Stiftung’; ADM, AB, HMS and WEH had no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This work was supported in part by the German Federal Ministry of Education and Research (BMBF, Berlin, Germany) under Grant Number 01GY1320B. The authors would like to thank Dr Sylwia I. Bujkiewicz for support in Bayesian multivariate meta-analysis and Professor Wolfgang Viechtbauer for support in frequentist multivariate meta-analysis.
Contributors
ADM was involved in preparation of the review protocol and development of the search strategy. ADM and AB were involved in assessing abstracts and all full-text copies, assessing the risk of bias of all included papers. ADM was responsible for data synthesis. ADM and WEH drafted and revised the manuscript. AL was involved in the preparation of the review protocol, development of the search strategy, assessment of the risk of bias and in revision of the final manuscript, and acted as the third person for consultancy in disagreements between two reviewers. AB and HMS were involved in revision of the manuscript. All authors read and approved the final manuscript.
Supporting Information
TableS1 Detailed search strategy for MEDLINE and subsequent adaption to EMBASE
TableS2 Study characteristics of retrieved studies
TableS3 Risk of bias assessment of retrieved studies
Supporting info item
Supporting info item
Supporting info item
References
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Morike K, Klotz U. The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol. 2008;64:183–99. doi: 10.1007/s00228-007-0422-1. [DOI] [PubMed] [Google Scholar]
- Laroche ML, Charmes JP, Bouthier F, Merle L. Inappropriate medications in the elderly. Clin Pharmacol Ther. 2009;85:94–7. doi: 10.1038/clpt.2008.214. [DOI] [PubMed] [Google Scholar]
- Hanlon JT, Schmader KE, Ruby CM, Weinberger M. Suboptimal prescribing in older inpatients and outpatients. J Am Geriatr Soc. 2001;49:200–9. doi: 10.1046/j.1532-5415.2001.49042.x. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Corsonello A, Lattanzio F. Underprescription of beneficial medicines in older people: causes, consequences and prevention. Drugs Aging. 2012;29:463–75. doi: 10.2165/11631750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Simonson W, Feinberg JL. Medication-related problems in the elderly: defining the issues and identifying solutions. Drugs Aging. 2005;22:559–69. doi: 10.2165/00002512-200522070-00002. [DOI] [PubMed] [Google Scholar]
- Hill-Taylor B, Sketris I, Hayden J, Byrne S, O’Sullivan D, Christie R. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360–72. doi: 10.1111/jcpt.12059. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Bero LA, Bird JA, McPhee SJ. Undermedication among geriatric outpatients: results of a randomized controlled trial. Ann Rev Gerontol Ger. 1992;12:95–108. [Google Scholar]
- Beer C, Hyde Z, Almeida OP, Norman P, Hankey GJ, Yeap BB, Flicker L. Quality use of medicines and health outcomes among a cohort of community dwelling older men: an observational study. Br J Clin Pharmacol. 2011;71:592–9. doi: 10.1111/j.1365-2125.2010.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelino RL, Bajorek BV, Chen TF. Targeting suboptimal prescribing in the elderly: a review of the impact of pharmacy services. Ann Pharmacother. 2009;43:1096–106. doi: 10.1345/aph.1L700. [DOI] [PubMed] [Google Scholar]
- Topinkova E, Baeyens JP, Michel JP, Lang PO. Evidence-based strategies for the optimization of pharmacotherapy in older people. Drugs Aging. 2012;29:477–94. doi: 10.2165/11632400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lowe CJ, Petty DR, Zermansky AG, Raynor DK. Development of a method for clinical medication review by a pharmacist in general practice. Pharm World Sci. 2000;22:121–6. doi: 10.1023/a:1008758823788. [DOI] [PubMed] [Google Scholar]
- Greenwald JL, Halasyamani L, Greene J, LaCivita C, Stucky E, Benjamin B, Reid W, Griffin FA, Vaida AJ, Williams MV. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477–85. doi: 10.1002/jhm.849. [DOI] [PubMed] [Google Scholar]
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2014;44:213–8. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46:72–83. doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- Wenger NS, Roth CP, Shekelle P ACOVE Investigators. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55(Suppl. 2):S247–52. doi: 10.1111/j.1532-5415.2007.01328.x. [DOI] [PubMed] [Google Scholar]
- Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135:642–6. doi: 10.7326/0003-4819-135-8_part_2-200110161-00002. [DOI] [PubMed] [Google Scholar]
- Shekelle PG, MacLean CH, Morton SC, Wenger NS. Acove quality indicators. Ann Intern Med. 2001;135:653–67. doi: 10.7326/0003-4819-135-8_part_2-200110161-00004. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration.
- Freigofas J, Haefeli WE, Schottker B, Brenner H, Quinzler R. Indirect evidence for proton pump inhibitor failure in patients taking them independent of meals. Pharmacoepidemiol Drug Saf. 2014;23:768–72. doi: 10.1002/pds.3620. [DOI] [PubMed] [Google Scholar]
- Wei Y, Higgins JP. Estimating within-study covariances in multivariate meta-analysis with multiple outcomes. Stat Med. 2013;32:1191–205. doi: 10.1002/sim.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey CS, Hoaglin DC, Antczak-Bouckoms A, Mosteller F, Colditz GA. Meta-analysis of multiple outcomes by regression with random effects. Stat Med. 1998;17:2537–50. doi: 10.1002/(sici)1097-0258(19981130)17:22<2537::aid-sim953>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- Riley RD, Thompson JR, Abrams KR. An alternative model for bivariate random-effects meta-analysis when the within-study correlations are unknown. Biostatistics. 2008;9:172–86. doi: 10.1093/biostatistics/kxm023. [DOI] [PubMed] [Google Scholar]
- Bujkiewicz S, Thompson JR, Sutton AJ, Cooper NJ, Harrison MJ, Symmons DP, Abrams KR. Multivariate meta-analysis of mixed outcomes: a Bayesian approach. Stat Med. 2013;32:3926–43. doi: 10.1002/sim.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89:845–54. doi: 10.1038/clpt.2011.44. [DOI] [PubMed] [Google Scholar]
- Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc. 2014;62:1658–65. doi: 10.1111/jgs.12993. [DOI] [PubMed] [Google Scholar]
- Garcia-Gollarte F, Baleriola-Julvez J, Ferrero-Lopez I, Cuenllas-Diaz A, Cruz-Jentoft AJ. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. J Am Med Dir Assoc. 2014;15:885–91. doi: 10.1016/j.jamda.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Gillespie U, Alassaad A, Hammarlund-Udenaes M, Morlin C, Henrohn D, Bertilsson M, Melhus H. Effects of pharmacists’ interventions on appropriateness of prescribing and evaluation of the instruments’ (MAI, STOPP and STARTs’) ability to predict hospitalization – analyses from a randomized controlled trial. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062401. : e62401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alassaad A, Bertilsson M, Gillespie U, Sundstrom J, Hammarlund-Udenaes M, Melhus H. The effects of pharmacist intervention on emergency department visits in patients 80 years and older: subgroup analyses by number of prescribed drugs and appropriate prescribing. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111797. : e111797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, Downie G, Seymour DG. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing. 2001;30:205–11. doi: 10.1093/ageing/30.3.205. [DOI] [PubMed] [Google Scholar]
- Schmader KE, Hanlon JT, Pieper CF, Sloane R, Ruby CM, Twersky J, Francis SD, Branch LG, Lindblad CI, Artz M, Weinberger M, Feussner JR, Cohen HJ. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med. 2004;116:394–401. doi: 10.1016/j.amjmed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, Tulkens PM. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc. 2007;55:658–65. doi: 10.1111/j.1532-5415.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- Verrue C, Mehuys E, Boussery K, Adriaens E, Remon JP, Petrovic M. A pharmacist-conducted medication review in nursing home residents: impact on the appropriateness of prescribing. Acta Clin Belg. 2012;67:423–9. doi: 10.2143/ACB.67.6.2062707. [DOI] [PubMed] [Google Scholar]
- Forsetlund L, Eike MC, Gjerberg E, Vist GE. Effect of interventions to reduce potentially inappropriate use of drugs in nursing homes: a systematic review of randomised controlled trials. BMC Geriatr. 2011;11:16. doi: 10.1186/1471-2318-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P, Lang PO, Cherubini A, Topinkova E, Cruz-Jentoft A, Montero Errasquin B, Madlova P, Gasperini B, Baeyens H, Baeyens JP, Michel JP, O’Mahony D. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol. 2011;67:1175–88. doi: 10.1007/s00228-011-1061-0. [DOI] [PubMed] [Google Scholar]
- Ryan C, O’Mahony D, Kennedy J, Weedle P, Byrne S. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol. 2009;68:936–47. doi: 10.1111/j.1365-2125.2009.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- MacLaughlin EJ, Raehl CL, Treadway AK, Sterling TL, Zoller DP, Bond CA. Assessing medication adherence in the elderly: which tools to use in clinical practice? Drugs Aging. 2005;22:231–55. doi: 10.2165/00002512-200522030-00005. [DOI] [PubMed] [Google Scholar]
- Kaiser RM, Schmader KE, Pieper CF, Lindblad CI, Ruby CM, Hanlon JT. Therapeutic failure-related hospitalisations in the frail elderly. Drugs Aging. 2006;23:579–86. doi: 10.2165/00002512-200623070-00004. [DOI] [PubMed] [Google Scholar]
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605–9. doi: 10.1001/archinte.166.6.605. [DOI] [PubMed] [Google Scholar]
- Barber N. What constitutes good prescribing? BMJ. 1995;310:923–5. doi: 10.1136/bmj.310.6984.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8–14. doi: 10.14283/jfa.2013.2. [DOI] [PubMed] [Google Scholar]
- Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, Forbes C, Glanville J, Hicks NJ, Moody J, Twaddle S, Timimi H, Young P. How to formulate research recommendations. BMJ. 2006;333:804–6. doi: 10.1136/bmj.38987.492014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item
Supporting info item


