Abstract
Drug therapy is a powerful tool to improve outcome, but there is an urgent need to improve pharmacotherapy in neonates through tailored prevention and management of adverse drug reactions (ADRs). At present, infants commonly receive off-label drugs, at dosages extrapolated from those in children or adults. Besides the lack of labelling, inappropriate formulations, (poly)pharmacy, immature organ function and multiple illnesses further raise the risk for ADRs in neonates and infants. Pharmacovigilance to improve the prevention and management of ADRs needs to be tailored to neonates and infants. We illustrate this using prevention strategies for drug prescription and administration errors (e.g. formulation, bedside manipulation, access), detection through laboratory signalling or clinical outlier data (e.g. reference laboratory values, overall high morbidity), assessment through algorithm scoring (e.g. Naranjo or population specific), as well as understanding of the developmental toxicology (e.g. covariates, developmental pharmacology) to avoid re-occurrence and for development of guidelines. Such tailored strategies need collaborative initiatives to combine the knowledge and expertise of different disciplines, but hold promise to become a very effective tool to improve pharmacotherapy and reduce ADRs in infants.
Keywords: adverse drug reaction, developmental pharmacology, infant, newborn, pharmacovigilance
Introduction
An adverse drug reaction (ADR) has been defined by the World Health Organization (WHO) as ‘any noxious or unintended drug response at doses commonly used for prophylaxis, diagnosis or treatment of a disease or condition’. Some ADRs are considered to be predictable based on the known pharmacology and frequently relate to the dose (e.g. side-effects, secondary effects, interactions or toxicity), while others remain unpredictable based on currently available knowledge and are less dose related (e.g. idiosyncratic reactions, (pseudo)allergic reactions or intolerance) 1. Despite the fact that this kind of ADR definition already includes a wide range of drug-related complications, its application in the specific subpopulation of neonates and infants remains difficult because different ADR definition-related assumptions (pharmacology and dose are known, interactions can be quantified, side-effects or secondary effects anticipated, active compound only or formulation) cannot be taken for granted.
It may be more reasonable to use ‘an unintended and harmful effect resulting from the use of medications intended for diagnostic or therapeutic reasons (irrespective of the dose)’ as a broader definition, because of the common practice of prescribing drugs off label and unlicensed and the absence of dose guidance in this population 2–6. Obviously, this definition results in overlap with errors related to inappropriate drug administration, commonly referred to as medication errors (e.g. wrong route, dose or patient). Finally, adverse drug reactions and medication errors relate to the broader and growing field of research on iatrogenesis, i.e. any adverse condition occurring as a result of a diagnostic procedure or treatment by a medical care provider (Figure 1) 2–6. Although the research related to iatrogenesis is of obvious relevance to improve outcome and, despite the fact that some of the methods can also be applied to generate more insights into the incidence and prevention of ADRs in neonates or infants, this review aims to focus on the broader ADR definition including medication errors. Such an integrated approach and broader view on patient safety is in line with the trend of the authorities not only to focus on the medicinal product itself, but to integrate human factors (how is this medicine used and how do errors/ADR happen?) resulting in, e.g. an action plan to reduce the burdern of medication errors and ADR events supported by the European Medicines Agency, and of relevance for all stakeholders involved 7.
Figure 1.
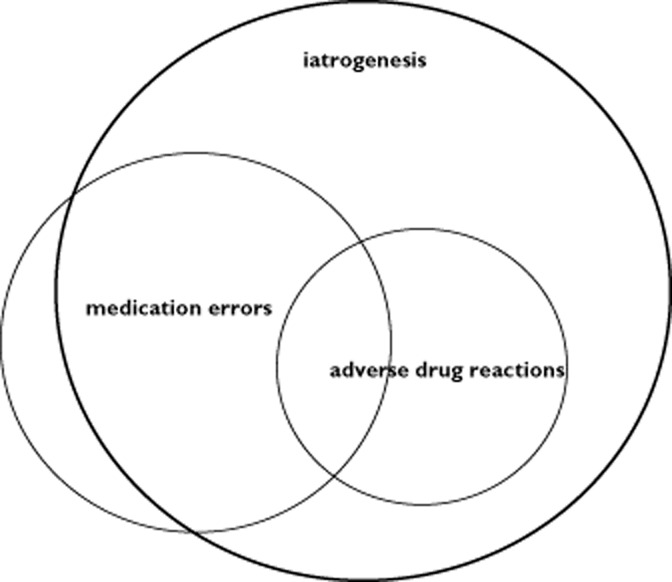
Scheme showingthe relationship and overlap between adverse drug reactions, medication errors and iatrogenic errors
First, we focus on several important aspects of neonatal clinical pharmacology. We refer to compound-specific major ADRs to illustrate the impact of pharmacological, physiological and toxicological particularities on specific major ADRs in infancy. Thereafter, the burden of pharmaco-epidemiology and ADRs in infancy is discussed, followed by suggestions for a population-tailored approach to improve pharmacotherapy and reduce this ADR burden (prevention, detection, assessment and understanding) in neonates.
Neonatal clinical pharmacology
When a drug is prescribed, the overall aim is to attain specific, targeted effects (e.g. bactericidal, analgesic, blood-pressure normalizing), preferably without disproportional side-effects (e.g. drug toxicity, hypotension, tachycardia). Neonatal clinical pharmacology aims to predict and estimate these (side)-effects at the level of the population or, preferably, the individual infant through integration of the covariates that explain the inter- and intra-individual variability 8,9. The most obvious covariates in neonates relate to growth and maturation, reflected and quantified by birth weight, current weight, or age (postnatal, gestational or postmenstrual age). There is already at least one order of variability in weight (<0.5 up to 5 kg) at birth, while both the height velocity rate (10–20 cm year−1) and the increase in bodyweight (50% increase in the first 6 weeks) reflect the dynamics of a rapidly evolving biological system. This maturation-related variability is further aggravated by interfering disease characteristics (e.g. renal failure, sepsis, growth restriction) or treatment modalities (e.g. co-medication, extracorporeal membrane oxygenation, whole-body cooling). All these covariates will affect pharmacokinetics 8,9. Moreover, maturation (e.g. receptor expression, receptor activity, cellular metabolism, enzyme activity) interrelates with growth. Some tissues may be more sensitive to specific compounds in early life, irrespective of a given concentration or exposure, whereas others will be less sensitive. This will affect population-specific pharmacodynamics. In fact, the most crucial factor in neonates is their rapidly evolving physiology 8,9.
Unfortunately, this also predisposes to population-specific drug toxicity, as highlighted in Table 1 10–17. This table provides some illustrations of major adverse drug reactions as reported (1951–2009) in neonates. Some of these ADRs can be explained by developmental pharmacokinetics (e.g. competitive albumin binding with bilirubin 11, deficient glucuronidation capacity 12, deficient alcohol dehydrogenase capacity 13), while others relate to developmental pharmacodynamics (e.g. oxygen toxicity on retinal and alveolar microvascular structures 10,15, neuronal apoptosis following dexamethasone exposure 14). Obviously, if one understands the mechanisms associated with drug toxicity, one can minimize drug toxicity in the future for the same but also for similar compounds. Unfortunately, the recent Kaletra® event (deficient alcohol dehydrogenase capacity resulted in alcohol accumulation-related toxicity in neonates) illustrates that we still fail to translate the available information to prevent similar events related to new compounds 16.
Table 1.
Illustrative compound- or formulation-specific serious adverse drug reactions in neonates as reported in the literature (illustrations are provided chronologically to illustrate that this remains an ongoing issue 10–17)
| Compound/formulation | Clinical syndrome | Developmental pharmacology/toxicology |
|---|---|---|
| Oxygen, preterm (1951) [10] | ‘Retinopathy of prematurity’ | Early neonatal overexposure to oxygen results in microvascular retinal overgrowth |
| Sulfonamides (1956) [11] | ‘Kernicterus’ | Highly albumin-bound antibiotics, competitive with endogenous compounds, including bilirubin. This results in higher free bilirubin concentrations and subsequent kernicterus |
| Chloramphenicol (1959) [12] | ‘Grey baby syndrome’ | Impaired glucuronidation capacity, which results in accumulation of chloramphenicol and subsequent mitochrondial dysfunction, circulatory collapse and death |
| Benzyl alcohol (1982) [13] | ‘Gasping syndrome’ | Benzyl alcohol, coadministered as a preservative in parenteral formulations, results in accumulation in preterm neonates because of their limited metabolic (alcohol dehydrogenase) clearance capacity. Accumulation results in metabolic acidosis, followed by seizures, bradycardia, gasping respiration and hypotension preceding cardiovascular collapse and, ultimately, death |
| Dexamethasone (2000) [14] | ‘Cerebral palsy’ | High-dose dexamethasone exposure in neonatal life results in an increased risk of subsequently displaying cerebral palsy during infancy, probably due to increased neuronal apoptosis |
| Oxygen, preterm (2000) [15] | ‘Bronchopulmonary dysplasia’ | Early neonatal overexposure to oxygen results in an increased incidence of bronchopulmonary dysplasia (chronic lung disease of prematurity) |
| Lopinavir/ritonavir syrup (2005) [16] | ‘Alcohol accumulation’ | Kaletra® syrup contains both ethanol and propylene glycol. Impaired metabolic clearance results in accumulation and subsequent hyperosmolality, lactic acidosis, renal toxicity, central nervous system impairment, cardiac arrhythmia, haemolysis and collapse |
| Ceftriaxone + calcium (2009) [17] | ‘Cardiovascular collapse’ | Simultaneous administration of calcium-containing infusions and ceftriaxone results in intravascular precipitate, as observed during autopsy |
Drug use, safety and adverse drug reactions in neonates and infants
Compared with the available information on benefits/risks that enables decisions to be made on pharmacotherapy in individual adults, the currently existing information to enable such informed decisions to be made and to identify and quantify ADRs in neonates and infants is much more limited. Off-label or unlicensed use of drugs still remains most prevalent in neonates and infants (up to 80–90%) 2,18. Moreover, there is anecdotal evidence (aminoglycosides, morphine) that dosing regimens suggested in reference textbooks differ and are only rarely supported by studies that were prospectively validated 19,20.
Unfortunately, the analysis of Benjamin et al. provides evidence that, even after the introduction of the paediatric exclusivity act, only 12% of the paediatric studies included newborns, and a large proportion of these studies included fewer than 30 patients 21. Although such studies may provide preliminary data on pharmacokinetics, they are clearly insufficient to support any claim about safety. Despite this, neonates are still commonly exposed to (poly)pharmacy 22. Based on 208 459 patient files, 1 425 992 individual prescriptions and 409 unique medications were retrieved, showing exposure to antibiotics (ampicillin, gentamicin, vancomycin, cefotaxim), caffeine, diuretics, vitamin supplements and surfactant in at least 10% of the admissions 22. We commonly use formulations that were not initially developed for this population (e.g. excipients, dilution and manipulation), further increasing the risks for ADRs 23.
Given that drugs are commonly used in neonates, despite the absence of robust data on pharmacotherapy in a population at the extreme of age and with an overall high morbidity, it seems likely that the incidence and severity of ADRs is higher in neonates and infants. Unfortunately, data on ADR incidence are relatively scarce when compared with adults. Approximately 5% of hospitalized adults and 13% of adult patients in ambulatory care are reported to experience adverse drug events (ADEs), and 11–90% are estimated to be preventable 24. A population-based study in 4970 adults documented a prevalence for ADE and ADR of 12 and 6%, respectively, during a 3 month time interval 24. In children, the overall incidence of ADR in hospitalized and outpatient settings is 9.53 (95% confidence interval 6.81–12.26) and 1.46% (95% confidence interval 0.7–3.03), respectively 25. A recent systematic review on observational studies of ADR in children suggested a pooled estimate of 2.9 (0.4–10.3)% as the cause for hospital admission, while the incidence of ADR during admission was estimated to occur in 0.6–16.8% of children 26. Compared with these data, we have some observations that support the claim that the incidence and severity of ADRs is higher in neonates and infants.
In a prospective study (1977) on the epidemiology of ADRs in 200 consecutively admitted neonates, 136 ADRs occurred in 60 neonates (30%), 20 were life threatening, 24 were moderate (prolonged hospital stay) and ∼50% of patients had multiple ADRs 5. However, it is doubtful to what extent these observations can still be applied to contemporary neonatal intensive care units (NICUs). More recently, Le et al. analysed 1087 (1995–2004) reported events and documented an overall ADR incidence of 1.6% in hospitalized children in one paediatric hospital, but ADRs were significantly more common in the NICU 27. Finally, in a focused analysis on adverse drug events as reported in the MedWatch adverse event reports system, Moore et al. identified 7111 reports (1.5% of the total data set) in infants and children under 2 years of age, with a high mortality (100 of 243 cases = 40%) in the first month of life 28. Interestingly, drugs administered to the mother in the perinatal period constituted an important route of exposure to major ADRs in newborns.
Population-tailored pharmacovigilance to improve pharmacotherapy in neonates
It is essential that new and established treatments are monitored for their effectiveness and safety in real-life conditions, including information in specific population groups. Pharmacovigilance to improve the management of ADRs is based on prevention, detection, assessment and understanding, but all these aspects need to be tailored to neonates and infants in order to turn these into effective tools 29–34.
Prevention: how to avoid the avoidable?
Neonates are highly vulnerable to medication errors because of polypharmacy, the absence of evidence on pharmacotherapy and the lack of neonate-specific formulations 32,33. To illustrate this, Kaushal et al., in a prospective study of 1120 paediatric patients, documented 616 medication errors in two different medical centres 34. This rate was significantly higher in the NICU setting and involved drug ordering, dosing and intravenous formulations. Suggested efforts to improve this were computerized physician order entry or ward-based clinical pharmacists. This has been further confirmed in a systematic review on medication errors in neonates that identified dose errors as the most common type of error 35. Computerized physician order entry and interventions by clinical pharmacists were the most common interventions suggested to improve drug safety in neonates. However, at that time (2007) only limited data were available on the effects of such interventions.
In two more recently performed studies on iatrogenesis in NICUs, about one-third of the events were preventable, of which about one-quarter related to medical errors 3,6. For these drug errors, ordering and prescription (one-half), as well as administration (one-half, including the well-known 10-fold administration errors due to the lack of neonate-specific formulations) emerged as causes of errors. A barcode medication administration system (verification of drug and patient), in combination with a double check when further manipulation is needed in order to reduce this burden of targeted, preventable medication errors effectively in the NICU setting 36.
Despite the fact that intravenous formulations are commonly used in neonates, this population should be treated with tailored formulations. Volume overload should be avoided, while too low volumes or too concentrated formulations may result in dose inaccuracy due to either inaccurate volumes or inaccurate serial dilutions in order to achieve the required dose. There are observations on the impact of serial dilutions of inotropics, opioids or antibiotics to illustrate the extent of dosing inaccuracy 37–39. Nunn et al. recently quantified the practice of medicines manipulation to provide accurate doses for children, including neonates in one regional children’s hospital [40]. Based on 5375 drug administration events recorded in neonatal and paediatric patients, ∼10% were judged to require manipulation or needed a small dosing volume (<0.2 ml). Measure doses <0.1 ml accounted for 25% of the manipulations, and this was most common in the NICU (60%) 40.
Detection: how to detect the signal in the large background noise?
It is easier to recognize a signal when you know what to search for. Compared with other populations, sources of ADR information such as pre- and postmarketing studies are much more limited in neonates and infants. This in itself will affect passive surveillance or spontaneous reporting, or adverse event registries. Signal detection is further obscured by the overall high morbidity related to extreme preterm birth and the absence of standardization of care. Collaborative efforts in quality improvement are emerging, and uniform registration of clinical characteristics, management and outcome variables in merged databases (e.g. EuroNeoStat, Vermont-Oxford database, Australian Neonatal Database) may provide a powerful tool to compare between-unit differences in outcome as a screening tool for potential ADRs. At least, such an evidence-based practice for improving quality initiative has improved surfactant use 41 and pain management 42.
We would like to stress two relevant issues for this specific population when data sets are merged. Firstly, the already-mentioned example of dexamethasone taught us that long-term outcome variables are crucial. Dexamethasone does reduce the need for oxygen in neonatal life, but is associated with cerebral palsy 14. Cerebral palsy is only detected very rarely in the first 6 months of life, but has a major impact on the neurodevelopmental outcome throughout childhood and beyond. Secondly, we should be aware that ‘simple’ computerized rule-based signal detection based on outliers in biochemical values (e.g. liver enzymes or creatinine) necessitates the validation of reference values 30,43,44. Another key barrier is the lack of a common terminology that provides uniform definitions and descriptions of clinical observations and data; this is urgently needed to build upon previous findings, to reuse data-collection tools and data-management processes 45. Such strategies have been illustrated to be effective, e.g. for signal detection of potentially drug-induced acute liver injury in children, applying a multicountry healthcare database network 46.
The need to improve pharmacovigilance methods concerns not only new compounds, but also currently administered drugs that are commonly administered in an off-label setting 32. The above-mentioned data sets can also be applied as part of more active pharmacovigilance and surveillance 34. As illustrated by the Genotype-specific Approaches to Therapy in Children (GATC) study, active surveillance, drug- or ADR-targeted pharmacovigilance, trained surveillance clinicians, case–control methodology and standardized procedures turned out to be a promising strategy for generation of knowledge about ADRs in children 29. Similar efforts in neonatal care, based on valid databases, are likely to be an effective tool to make clinically relevant progress.
Assessment: towards a tailored ‘Naranjo’ algorithm to assess ADRs in infants
Following detection, the differentiation of ‘true’ ADRs from confounding reactions associated with organ dysfunction, immaturity and underlying diseases remains difficult. As a result of these confounding variables, the commonly applied ADR algorithm scoring systems do not reliably document causality, and none of them has been validated in infants 47. Using a stepwise, systematic approach, an algorithm was developed and subsequently validated. This new algorithm is based on 13 questions (Table 2) and turned out to be more valid and reliable when compared with the Naranjo algorithm. Although further prospective testing is needed, this seems to be a promising approach for surveillance and assessment in this specific population 47.
Table 2.
New adverse drug reactions algorithm for infants in neonatal intensive care units (similar to Table 3 of Du et al. 47)
| Adverse drug reactions, assessment criteria | Yes | No | Not applicable/unknown |
|---|---|---|---|
| (1) Was the timing of AE consistent with an ADR to the suspected drug? | 6 | −7 | 0 |
| (2) Is the AE a well-documented ADR to the suspected drug? | 0 | −6 | 0 |
| (3) Are there published resports on this AE that are related to the suspected drug in newborns? | 4 | −4 | 0 |
| (4) Was the AE likely to be a change (excerbation, recurence, complication or new manifestation) in a pre-existing clinical condition? | −3 | 7 | 0 |
| (5) Are there any alternative aetiological candidates other than the pre-existing condition (e.g. concomitant drugs) that are a common cause of the AE? | −3 | 2 | 0 |
| (6) Was an alternative aetiological candidate confirmed by any objective evidence? | −3 | 3 | 0 |
| (7) Did the AE improve after the suspected drug was discontinued? | 4 | −1 | 0 |
| (8) Was the AE less severe when the dose was reduced? | 4 | −2 | 0 |
| (9) Did the AE improve after a specific antagonist was administered? | 3 | −1 | 0 |
| (10) Did the AE significantly diminish or disappear while the patient was still taking the suspected drug? | −2 | 1 | 0 |
| (11) Did the AE reappear/worsen when the suspected drug was reintroduced? | 9 | −1 | 0 |
| (12) Was the suspected drug detected in blood or other fluids in concentrations known to be toxic? | 4 | −2 | 0 |
| (13) Is there unequivocal evidence that the amount of the suspected drug received was an overdose for this patient? | 4 | −4 | 0 |
| If total score ≥14 → definite | |||
| If 7 ≤ total score ≤ 13 → probable | |||
| If 3 ≤ total score ≤ 6 → possible | Tocal score = _______ | ||
| If total score ≤2 → unlikely | Category = ___________ | ||
Abbreviations are as follows: ADR, adverse drug reaction; AE, adverse event.
Understanding: developmental pharmacology should facilitate secondary prevention
The earlier mentioned Kaletra® case illustrates the need to integrate the emerging knowledge on developmental pharmacology 16. Despite the fact that it was known that alcohols accumulate in neonates because of limited clearance, this was not considered sufficiently during the drug-development process. The same holds true for perinatal ADRs following maternal drug use. Moore et al. already highlighted that drugs administered to the mother in the perinatal period constituted an important route of exposure to major ADRs in newborns 28. The risks for neonatal sedation following exposure to opioids (codeine, oxycodone) through breastfeeding were linked to maternal pharmacogenetics (cytochrome P450 2D6 polymorphisms) 48,49. Likewise, the risks for neonatal opioid abstinence syndrome were linked to neonatal pharmacogenetics and breastfeeding 49,50.
Conclusions
Improved ADR management is based on prevention, detection, assessment and understanding of secondary prevention, but all these aspects need to be tailored to neonates and infants to turn these into effective tools. We have illustrated that computerized physician order entry, ward-based clinical pharmacists or bedside barcode scanning have proved to be effective tools to reduce medication and administration errors. Signal detection should be improved through collaborative efforts and database building. However, such databases should include long-term outcome data and will necessitate validation of biochemical reference values. In order to assess causality, it seems appropriate that ADRs should be based on population-tailored algorithms. Finally, understanding developmental toxicology (e.g. covariates, developmental pharmacology) is the most effective approach for secondary prevention. Such a tailored strategy needs collaborative initiatives to combine the knowledge and expertise of different disciplines, but holds the promise to become a very effective tool to improve pharmacotherapy and reduce ADRs in infants.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: the clinical research of KA is supported by the Fund for Scientific Research, Flanders (Fundamental Clinical Investigatorship 1800214N), and JNvdA is supported by NIH grants (R01HD048689, K24DA027992 and U54HD071601) and FP7 grants TINN (223614), TINN2 (260908) and GRIP (261060); no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- World Health Organization. 2002. The importance of pharmacovigilance; safety monitoring of medicinal products. Geneva, World Health Organization,. Available at www.who.int/medicinedocs/en/d/Js4893e (last accessed 22 March 2014)
- Fabiano V, Mameli C, Zuccotti GV. Adverse drug reactions in newborns, infants and toddlers: pediatric pharmacovigilance between present and future. Expert Opin Drug Saf. 2012;11:95–105. doi: 10.1517/14740338.2011.584531. [DOI] [PubMed] [Google Scholar]
- Ligi I, Arnaud F, Jouve E, Tardieu S, Sambuc R, Simeoni U. Iatrogenic events in admitted neonates: a prospective cohort study. Lancet. 2008;371:404–410. doi: 10.1016/S0140-6736(08)60204-4. [DOI] [PubMed] [Google Scholar]
- Sharek PJ, Horbar JD, Mason W, Bisarya H, Thurm CW, Suresh G, Gray JE, Edwards WH, Goldmann D, Classen D. Adverse events in the neonatal intensive care unit: development, testing, and findings of a NICU-focused trigger tool to identify harm in North American NICUs. Pediatrics. 2006;118:1332–1340. doi: 10.1542/peds.2006-0565. [DOI] [PubMed] [Google Scholar]
- Aranda JV, Portuguez-Malavasi A, Collinge JM, Germanson T, Outerbrigde EW. Epidemiology of adverse drug reactions in the newborn. Dev Pharmacol Ther. 1982;5:173–184. [PubMed] [Google Scholar]
- Kugelman A, Inbar-Sanado E, Shinwell ES, Makhoul IR, Leshem M, Zangen S, Wattenberg O, Kaplan T, Riskin A, Bader D. Iatrogenesis in neonatal intensive care units: observational and interventional, prospective, multicenter study. Pediatrics. 2008;122:550–555. doi: 10.1542/peds.2007-2729. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. 2014. Medication errors, follow up actions from workshop. Implementation plan 2014–2015, 15 April 2014. Human Medicines Research and development support. London, European Medicines Agency,. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Other/2014/04/WC500165496.pdf (last accessed 20 June 2014)
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology – drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- Allegaert K, Langhendries JP, van den Anker JN. Educational paper: do we need neonatal clinical pharmacologists? Eur J Pediatr. 2013;172:429–435. doi: 10.1007/s00431-012-1734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia; a clinical approach. Med J Aust. 1951;14:48–50. [PubMed] [Google Scholar]
- Andersen DH, Blanc WA, Crozier DN, Silverman WA. A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic antibacterial regimens. Pediatrics. 1956;18:614–625. [PubMed] [Google Scholar]
- Sutherland JM. Fatal cardiovascular collapse of infants receiving large amounts of chloramphenicol. Am J Dis Child. 1959;97:761–767. doi: 10.1001/archpedi.1959.02070010763001. [DOI] [PubMed] [Google Scholar]
- Gershanik J, Boeclerr B, Ensley H, McCloskey S, George W. The gasping syndrome and benzyl alcohol poisoning. N Engl J Med. 1982;307:1384–1388. doi: 10.1056/NEJM198211253072206. [DOI] [PubMed] [Google Scholar]
- Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader S, Bader D, Yurman S, Dolfin T, Kogan A, Dolberg S, Arbel E, Goldberg M, Gur I, Naor N, Sirota L, Mogilner S, Zaritsky A, Barak M, Gottfried E. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2000;83:F177–181. doi: 10.1136/fn.83.3.F177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. 2014;105:55–63. doi: 10.1159/000356561. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. 2011. FDA drug safety communication: serious health problems seen in premature babies given Kaletra (lopinavir/ritonavir) oral solution http://www.fda.gov/Drugs/DrugSafety/ucm246002.htm (last accessed 8 March 2011)
- FDA. 2008. Information for healthcare professionals: ceftriaxone (marketed as Rocephin and generics) http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm084263.htm (last accessed 24 December 2008)
- Kemper EM, Merkus M, Wierenga PC, Van Rijn PC, Van der Werff D, Lie-A-Huen L, Offringa M. Towards evidence-based pharmacotherapy in children. Paediatr Anaesth. 2011;21:183–189. doi: 10.1111/j.1460-9592.2010.03493.x. [DOI] [PubMed] [Google Scholar]
- De Cock RF, Allegaert K, Schreuder MF, Sherwin CM, de Hoog M, van den Anker JN, Danhof M, Knibbe CA. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin Pharmacokinet. 2012;51:105–117. doi: 10.2165/11595640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Krekels EH, Tibboel D, de Wildt SN, Ceelie I, Dahan A, van Dijk M, Danhof M, Knibbe CA. Evidence-based morphine dosing for postoperative neonates and infants. Clin Pharmacokinet. 2014;53:553–563. doi: 10.1007/s40262-014-0135-4. [DOI] [PubMed] [Google Scholar]
- Benjamin DK, Jr, Smith PB, Murphy MD, Roberts R, Mathis L, Avant D, Califf RM, Li JS. Peer-reviewed publication of clinical trials completed for pediatric exclusivity. JAMA. 2006;296:1266–1273. doi: 10.1001/jama.296.10.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- Turner MA, Duncan JC, Shah U, Metsvaht T, Varendi H, Nellis G, Lutsar I, Yakkundi S, McElnay JC, Pandya H, Mulla H, Vaconsin P, Storme T, Rieutord A, Nunn AJ. Risk assessment of neonatal excipient exposure: lessons from food safety and other areas. Adv Drug Deliv Rev. 2013 doi: 10.1016/j.addr.2013.11.003. 10.1016/j.addr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Hakkarainen KM, Gyllensten H, Jönsson AK, Andersson Sundell K, Petzold M, Hägg S. Prevalence, nature and potential preventability of adverse drug events – A population-based medical record study in 4970 adults. Br J Clin Pharmacol. 2014 doi: 10.1111/bcp.12314. 10.1111/bcp.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard L, Christensen A, Holme-Hansen E. Information about adverse drug reactions reported in children: a qualitative review of empirical studies. Br J Clin Pharmacol. 2010;70:481–491. doi: 10.1111/j.1365-2125.2010.03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth RM, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R, Williamson P. Adverse drug reactions in children – a systematic review. PLoS ONE. 2012;7:e24061. doi: 10.1371/journal.pone.0024061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Nguyen T, Law AV, Hodding J. Adverse drug reactions among children over a 10 year period. Pediatrics. 2006;118:555–562. doi: 10.1542/peds.2005-2429. [DOI] [PubMed] [Google Scholar]
- Moore TJ, Weiss SR, Kaplan S, Blaisdell CJ. Reported adverse drug events in infants and children under 2 years of age. Pediatrics. 2002;110:e53. doi: 10.1542/peds.110.5.e53. [DOI] [PubMed] [Google Scholar]
- Castro-Pastrana LI, Carleton BC. Improving pediatric drug safety: need for more efficient clinical translation of pharmacovigilance knowledge. J Popul Ther Clin Pharmacol. 2011;18:e76–e88. [PubMed] [Google Scholar]
- Lazou K, Farini M, Koutkias V, Drossou V, Maglaveras N, Bassiliades N. Adverse drug event prevention in neonatal care: a rule-based approach. Stud Health Technol Inform. 2013;186:170–174. [PubMed] [Google Scholar]
- Ward RM, Kern SE. Clinical trials in neonates: a therapeutic imperative. Clin Pharmacol Ther. 2009;86:585–587. doi: 10.1038/clpt.2009.207. [DOI] [PubMed] [Google Scholar]
- Choonara I. Educational paper: aspects of clinical pharmacology in children – pharmacovigilance and safety. Eur J Pediatr. 2013;172:577–580. doi: 10.1007/s00431-012-1871-9. [DOI] [PubMed] [Google Scholar]
- Rieder M. New ways to detect adverse drug reactions in pediatrics. Pediatr Clin North Am. 2012;59:1071–1092. doi: 10.1016/j.pcl.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, Goldmann DA. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285:2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- Chedoe I, Molendijk HA, Dittrich TA, Jansman FG, Harting JW, Brouwers JR, Taxis K. Incidence and nature of medication errors in neonatal intensive care with strategies to improve safety. Drug Saf. 2007;30:503–513. doi: 10.2165/00002018-200730060-00004. [DOI] [PubMed] [Google Scholar]
- Morriss FH, Abramowitz PW, Nelson SP, Milavetz G, Michael SL, Gordon SN, Pendergast JF, Cook EF. Effectiveness of a barcode medication administration system in reducing preventable adverse drug events in a neonatal intensive care unit: a prospective cohort study. J Pediatr. 2009;154:363–368. doi: 10.1016/j.jpeds.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Rosenbluth G, Wilson SD. Pediatric and neonatal patients are particularly vulnerable to epinephrine dosing errors. Ann Emerg Med. 2010;56:704–705. doi: 10.1016/j.annemergmed.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Aguado-Lorenzo V, Weeks K, Tunstall P, Turnock K, Watts T, Arenas-Lopez S. Accuracy of the concentration of morphine infusions prepared for patients in a neonatal intensive care unit. Arch Dis Child. 2013;98:975–979. doi: 10.1136/archdischild-2013-304522. [DOI] [PubMed] [Google Scholar]
- Allegaert K, Anderson BJ, Vrancken M, Debeer A, Desmet K, Cosaert K, Tibboel D, Devlieger H. Impact of a paediatric vial on the magnitude of systematic medication errors in preterm neonates: amikacin as an example. Paed Perinatal. Drug Ther. 2006;7:59–63. [Google Scholar]
- Nunn A, Craig JV, Shah UU, Barker C, Craig J, Peak M, Ford J, Turner M. Estimating the requirement for manipulation of medicines to provide accurate doses for children. Eur J Hosp Pharm. 2013;20:3–7. [Google Scholar]
- Horbar JD, Carpenter JH, Buzas J, Soll RF, Suresh G, Bracken MB, Leviton LC, Plsek PE, Sinclair JC. Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial. BMJ. 2004;329:1004. doi: 10.1136/bmj.329.7473.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin CM, Baker GR, Lee SK, Ohlsson A, McMillan DD, Seshia MM Canadian Neonatal Network EPIQ Study Group. Reflections on knowledge translation in Canadian NICUs using the EPIQ method. Healthc Q. 2011;14:8–16. doi: 10.12927/hcq.2011.22539. [DOI] [PubMed] [Google Scholar]
- Victor S, Dickinson H, Turner MA. Plasma aminotransferase concentrations in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2011;96:F144–145. doi: 10.1136/adc.2008.152454. [DOI] [PubMed] [Google Scholar]
- George I, Mekahli D, Rayyan M, Levtchenko E, Allegaert K. Postnatal trends in creatininemia and its covariates in extremely low birth weight (ELBW) neonates. Pediatr Nephrol. 2011;26:1843–1849. doi: 10.1007/s00467-011-1883-0. [DOI] [PubMed] [Google Scholar]
- Kahn MG, Bailey LC, Forrest CB, Padula MA, Hirschfeld S. Building a common pediatric research terminology for accelerating child health research. Pediatrics. 2014;133:516–525. doi: 10.1542/peds.2013-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrajolo C, Coloma PM, Verhamme KM, Schuemie MJ, de Bie S, Gini R, Herings R, Mazzaglia G, Picelli G, Giaquinto C, Scotti L, Avillach P, Pedersen L, Rossi F, Capuano A, van der Lei J, Trifiró G, Sturkenboom MC EU-ADR consortium. Signal detection of potentially drug-induced acute liver injury in children using a multi-country healthcare database network. Drug Saf. 2014;37:99–108. doi: 10.1007/s40264-013-0132-9. [DOI] [PubMed] [Google Scholar]
- Du W, Lehr VT, Leih-Lai M, Koo W, Ward RM, Rieder MJ, van den Anker JN, Reeves JH, Mathew M, Lulic-Botica M, Aranda JV. An algorithm to detect adverse drug reactions in the neonatal intensive care unit: a new approach. J Clin Pharmacol. 2012;53:87–95. doi: 10.1177/0091270011433327. [DOI] [PubMed] [Google Scholar]
- Kelly LE, Chaudry SA, Rieder MJ, ’t Jong G, Moretti ME, Lausman A, Ross C, Berger H, Carleton B, Hayden MR, Madadi P, Koren G. A clinical tool for reducing central nervous system depression among neonates exposed to codeine through breast milk. PLoS ONE. 2013;8:e70073. doi: 10.1371/journal.pone.0070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Anker JN. Is it safe to use opioids for obstetric pain while breastfeeding? J Pediatr. 2012;160:4–6. doi: 10.1016/j.jpeds.2011.08.066. [DOI] [PubMed] [Google Scholar]
- Wachman EM, Hayes MJ, Brown MS, Paul J, Harvey-Wilkes K, Terrin N, Huggins GS, Aranda JV, Davis JM. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA. 2013;309:1821–1827. doi: 10.1001/jama.2013.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]


