Abstract
In order to reduce the numbers of medication errors (MEs) that cause adverse reactions (ARs) many authors have tried to identify patient-related risk factors. However, the evidence remains controversial. The aim was to review systematically the evidence on the relationship between patient-related risk factors and the risk of serious ARs. A systematic search in Pubmed, Embase, Cochrane Systematic Reviews, Psychinfo and SweMed+ was performed. Included full text articles were hand searched for further references. Peer reviewed papers including adults from primary and secondary healthcare were included if they clearly defined seriousness of the ARs and described correlations to risk factors by statistical analysis. A total of 28 studies were identified including 85 212 patients with 3385 serious ARs, resulting in an overall frequency of serious ARs in 4% of patients. Age, gender and number of drugs were by far the most frequently investigated risk factors. The total number of drugs was the most consistent correlated risk factor found in both univariate and multivariate analyses. The number of drugs is the most frequently documented independent patient-related risk factor for serious ARs in both the general adult population as well as in the elderly. The existing evidence is however conflicting due to heterogeneity of populations and study methods. The knowledge of patient-related risk factors for experiencing ARs could be used for electronic risk stratification of patients and thereby allocation of healthcare resources to high risk patients.
Keywords: drug-related side effects and adverse reactions, medication errors, risk factors
Introduction
An adverse reaction (AR) is a clinical response and is a response to a medicinal product which is noxious and unintended and the term includes medication errors (MEs), non-compliance and intentional overdose (Table1) 1. The definition of an AR was updated by the World Health Organization (WHO) and adopted by the European Authorities in 2012. In order to accumulate knowledge about drug safety and communication across countries a consistent terminology is essential. In order to facilitate and support a consistency in terminology this paper will use the new official term 1,2.
Table 1.
The characteristics of the included studies that investigated risk factors of ARs
| Adverse reaction | |
|---|---|
| An adverse reaction is a response to a medicinal product which is noxious and unintended. This includes adverse reactions which arise from: | |
| • Use of a medicinal product within the terms of the marketing authorization | |
| • Use outside the terms of the marketing authorization, including overdose, misuse, abuse and medication errors | |
| • Occupational exposure 1,2 | |
The overall rate of ARs in hospitalized patients reported in meta-analyses varies between 6.1–9.2 % 31–37. An estimate of prolonged hospitalization of 1.2–8.5 days per AR per patient has been suggested 35,38,39. Preventing ARs causing these stays is therefore important for both patients and the healthcare systems.
In order to reduce the numbers of ARs many authors have searched for a relationship between patient-related risk factors and ARs 40–42. However the evidence remains controversial, probably due to differences in definitions and the use of statistical methods. Some authors have reported that more than 50% of patients admitted with an AR were females and likewise that patients over 65 years of age accounted for more than 50% of all hospitalizations caused by ARs 43,44. However, adjusting for confounding factors can ameliorate both age and gender differences. In a meta-analysis from 2007 11 studies estimated risk factors for ARs of which the most important were polypharmacy, female gender, use of drugs with a narrow therapeutic range, age > 65 years and reduced renal function 35. However, other studies did not verify a correlation to some of these risk factors but revealed correlation to other risk factors such as length of hospitalization, ischaemic heart disease, depression and cognitive problems 11,13,42,45. Reviews of ARs causing hospitalizations are available as well as reviews from hospitalizations 31–37. To the best of our knowledge reviews of risk factors for serious ARs are currently not available.
If patient-related risk factors for serious ARs are known it might be possible to intervene prior to their occurrence, provided that some degree of certainty of the risk factors has been established. Therefore, the aim of this study was to perform a systematic review in order to establish the most important patient-related risk factors for serious ARs.
Methods
Search strategy
The following databases were searched in October 2011: Pubmed, Embase, Cochrane Systematic Reviews, Psychinfo and SweMed+ using the terms (medication errors OR pharmaceutical preparations/adverse effects OR drug toxicity OR drug therapy/adverse effects) AND risk factors and epidemiologic studies AND drug therapy/adverse effects (see appendix for a full search strategy). The reference lists of all included full text articles were hand searched for further references. An updated search was performed in December 2014 revealing no new studies.
Inclusion criteria
Peer reviewed papers were included that reported on either prospective or retrospective detection of ARs that were serious and clearly defined seriousness according to WHO and the European Medical Authorities as any untoward medical occurrence that at any dose results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity and is a congenital anomaly/birth defect 1,2,46. When seriousness could be interpreted from the article e.g. hospitalizations due to ARs the studies were also included. Further to this, papers presenting statistical correlation methods in order to distinguish serious ARs separately from non-serious, to patient-related risk factors, were included. Papers in the following languages were included: Danish, Swedish, Norwegian, German and English. Finally, papers reporting older definitions of ARs, which are ADRs and ADEs, were also included, provided that seriousness according to the WHO could be evaluated.
Exclusion criteria
Papers were excluded if they 1) investigated ARs that could potentially harm patients, but had not resulted in harm according to the above mentioned seriousness criteria, 2) included non-compliance and intentional drug overdose unless they were handled separately in the statistical analysis and thereby be excluded from the analysis and 3) included children at age < 18 years if mentioned in the paper. Children were excluded as drug use is different from adult use. One major difference is the off-label use which might result in different safety problems from those seen in adults.
Data extraction
All citations were uploaded to Refworks, an online reference management tool 47. First, all duplicates were removed. Second, each title and abstract was screened to determine whether the full text research paper should be retrieved or whether it was evident that it did not fulfill the inclusion criteria.
The following data were extracted from the full text papers that met the inclusion criteria: reference identification number, first author, title and year, country of origin, study design, number of patients, patient category, age group, type of ARs investigated, statistical methods used, correlation investigated and results. Authors were not contacted for further information.
Risk factors of individual drugs and their correlation with ARs are not presented in this review.
Results
After removing duplicates 2859 references were identified (Figure1). Titles and abstracts were screened and full text was retrieved for 144 references. Of these, 122 papers were excluded based on the exclusion criteria. From the reference lists a further six references were identified and included.
Figure 1.
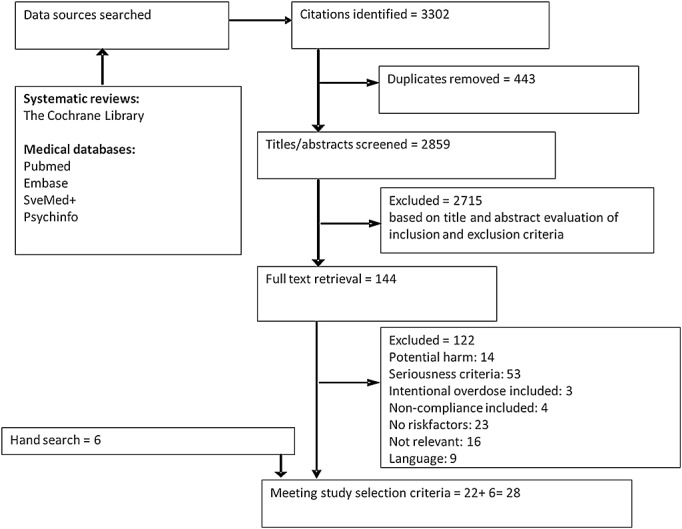
Flow chart of the literature search
The 28 full text papers are shown in Table2. Thirteen countries were represented and they were mainly from the western part of the world. Study designs were primarily observational, and most of them were prospective. One case-control study was included and one study included psychiatric patients. Two studies had been published or partly published twice. Thus the total number of studies included was 26. Patients included in the studies varied from being all ages (19 studies) to include only the elderly (seven studies), most frequently past the age of 65 years. All studies investigated risk factors in hospital settings except for one study that was performed in a nursing home. Most studies (20 in total) investigated hospitalizations caused by ARs. Four studies investigated ARs during hospitalization, one study investigated both and finally one study investigated ARs causing transfer to an intensive care unit.
Definition of terms
| Reference | Year | Country | Patients (5) | Age (years) | Study design | Seriousness criteria |
|---|---|---|---|---|---|---|
| ARs during hospitalization | ||||||
| Cabello et al. 3 | 2009 | Spain | 289 | >18 | Retrospective analysis | Fatality |
| Buajordet et al. | 2001 | Norway | 732 | All | Prospective observational | Fatality |
| Ebbesen et al. 4,5* | ||||||
| Fattinger et al. 6 | 2000 | Switzerland | 3624 | ? | Prospective observational | Hospitalization |
| Darchy et al. 7 | 1999 | France | 623 | ? | Retrospective study | Life-threatening (ICU) |
| ARs causing and during hospitalization | ||||||
| Bordet et al. 8 | 2001 | France | 16916 | Adults | Prospective observational | Hospitalization |
| Moore et al. 9 | 1998 | France | 329 | ? | Prospective observational | Hospitalization, fatality, life-threatening, prolongation of hospitalization |
| ARs causing hospitalization | ||||||
| Santamaria-Pablos et al. 10 | 2009 | Spain | 163 | >18 | Prospective cohort | Hospitalization |
| Wawruch et al. 11 | 2009 | Slovakia | 600 | ≥65 | Retrospective cohort | Hospitalization |
| Helldén et al. 12 | 2009 | Sweden | 153 | ≥65 | Retrospective observational | Hospitalization |
| Leendertse et al. 13 | 2008 | Holland | 12793 | ≥18 | Prospective case-control | Hospitalization |
| Mjörndal et al. 14 | 2002 | Sweden | 681 | ? | Prospective observational | Hospitalization |
| Onder et al. 15 | 2002 | Italy | 28411 | All | Prospective observational | Hospitalization |
| Malhotra et al. 16 | 2001 | India | 578 | ≥65 | Prospective observational | Hospitalization |
| Mannesse et al. 17 | 2000 | Holland | 106 | ≥70 | Prospective observational | Hospitalization |
| Pouyanne et al. 18 | 2000 | France | 3137 | ? | Prospective cross-sectional | Hospitalization |
| Cooper et al. 19 | 1999 | USA | 332 | Nursing home | Prospective observational | Hospitalization |
| Hallas et al. 20 | 1992 | Denmark | 1999 | ? | Prospective observational | Hospitalization |
| Hallas et al. 21 | 1991 | Denmark | 294 | Geriatric patients | Prospective observational | Hospitalization |
| Col et al. 22 | 1990 | USA | 315 | ≥65 | Prospective observational | Hospitalization |
| Hallas et al. 23 | 1990 | Denmark | 366 | <36–85 | Prospective observational | Hospitalization |
| Colt et al. 24 | 1989 | USA | 244 | All | Retrospective analysis | Hospitalization |
| Davidsen et al. 25 | 1988 | Denmark | 426 | ? | Prospective observational | Hospitalization |
| Hermesh et al. 26 | 1985 | Israel | 321 | ≥18 | Prospective follow-up | Hospitalization |
| Bergman et al. 27 | 1981 | Sweden | 285 | 16–97 | Prospective observational | Hospitalization |
| Levy et al. | 1980 | Israel + Germany | I: 2499 | >20–81 | Prospective observational | Hospitalization |
| Levy et al. 28,29* | 1979 | G: 2933 | ||||
| Caranasos et al. 30 | 1974 | USA | 6063 | 11–100 | Prospective observational | Hospitalization |
The study has been published or partly published twice. The paper mentioned first was included. ICU, intensive care unit.
A total of 85 212 patients were included in the 26 studies, with a total of 3385 serious ARs resulting in an overall frequency of 4% of patients experiencing an AR. The frequency of serious ARs varied from 0.5–23.6 % of patients (Tables3 and 4) with the smaller studies finding larger frequencies (Figure2). In the studies exclusively investigating the elderly population the frequency of serious ARs was 11.9 %. However, they only included 2387/85 662 (2.8 %) of patients. The most frequently investigated risk factors were gender, age, co-morbidity, number of drugs and impaired renal function and are presented in Tables3 and 4. Some of the less frequent risk factors identified that are not presented here were ischaemic heart disease, heart failure, depression, cognitive problems, dependent living situation, length of hospital stay, previous history of AR, geriatric ward, general internal medicine ward and smaller hospital (not university), cardiovascular complications and coagulation disorders 8,9,11,13,29,48.
Table 3.
Risk factors correlated with adverse reactions in adult patients
| Reference | Demography | Correlation with risk factors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | Serious ARs or hospitalizations (n) | ARs (%) | Gender | Age | Number of drugs | Co-morbidities | Impaired renal function | |||||||
| ARs during and/or causing hospitalization: | ||||||||||||||
| Bordet et al. 8 | 16 916 | 86 | 0.5 | O | ||||||||||
| Fattinger et al. 6 | 3624 | 144 | 4 | F | No | No | ||||||||
| Cabello et al. 3 | 289 | 17 | 5.9 | No | No | F | No | |||||||
| Darchy et al. 7 | 623 | 41 | 6.6 | F | O | Yes | ||||||||
| Moore et al. 9 | 329 | 31 | 9.4 | F | O | Yes | ||||||||
| Buajordet et al. 4 | 732 | 133 | 18.2 | No | M | Yes | Yes | |||||||
| ARs causing hospitalization: | ||||||||||||||
| Caranasos et al. 30 | 6063 | 177 | 2.9 | F | O | |||||||||
| Pouyanne et al. 18 | 3137 | 100 | 3.2 | F | O | |||||||||
| Onder et al. 15 | 28 411 | 964 | 3.4 | O | Yes | |||||||||
| Hallas et al. 23 | 366 | 15 | 4.1 | No | O | No | ||||||||
| Levy et al. 29 | 5432 | 270 | 5 | F | No | Yes | Yes | |||||||
| Leendertse et al. 13 | 12 793 | 714 | 5.6 | No | O | Yes | Yes | Yes | ||||||
| Hermesh et al. 26 | 321 | 24 | 7.5 | O | ||||||||||
| Hallas et al. 20 | 1999 | 157 | 7.9 | F | O | Yes | ||||||||
| Colt et al. 24 | 244 | 23 | 9.4 | No | Yes | No | ||||||||
| Bergman et al. 27 | 285 | 31 | 10.9 | F | No | Yes | ||||||||
| Davidsen et al. 25 | 426 | 49 | 11.5 | Yes | ||||||||||
| Mjörndal et al. 14 | 681 | 99 | 14.5 | No | No | Yes | ||||||||
| Santamaria-Pablos et al. 10 | 163 | 27 | 16.6 | No | Y | No | No | |||||||
| Mean | 4360 | 163 | 3.7 | |||||||||||
| Risk factor (yes/no) | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | ||||
| Univariate analysis (number of studies) | 7 | 8 (F) | 5 | 10 (O) | 3 | 10 | 3 | 3 | 0 | 2 | ||||
| 1 (M) | 1 (F) | |||||||||||||
| 1 (Y) | 1 | |||||||||||||
| Multivariate analysis (number of studies) | 2 | 1 (F) | 3 | 1 (O) | 1 | 3 | ||||||||
| 1 (Y) | ||||||||||||||
F, Female; M, Male; O, correlation with older patients; Y, correlation with younger patients.
Table 4.
Risk factors correlated with adverse reactions in elderly patients only
| Reference | Demography | Correlation to risk factors | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | Age (years) (mean) | Serious ARs or hospitalizations (n) | ARs (%) | Gender | Age | Number of drugs | Co-morbidities | Impaired renal function | |
| Malhotra et al. 16 | 578 | 72.5 | 39 | 6.7 | No | ||||
| Wawruch et al. 11 | 600 | 76.6 | 47 | 7.8 | No | ≥75 | ≥6 | ≥4 | No |
| Hallas et al. 21 | 294 | 81 | 33 | 11.2 | No | No | No | ||
| Helldén et al. 12 | 153 | 82.1 | 22 | 14.4 | No | F | No | F | |
| Col et al. 22 | 315 | 76.6 | 53 | 16.8 | Yes | ||||
| Cooper et al. 19 | 332 | 82 | 64 | 19.3 | No | No | Yes | ||
| Mannesse et al. 17 | 106 | 78 | 25 | 23.6 | No | ≥3 | ≥5 | ||
| Mean | 340 | 78.4 | 40.4 | 11.9 | |||||
| Multivariate analysis | |||||||||
| Reference | Gender | Age | Number of drugs | Co-morbidities | Impaired renal function | ||||
| Wawruch et al. 11 | |||||||||
| Malhotra et al. 16 | ≥3 | ||||||||
| Mannesse et al. 17 | ≥3 | No | |||||||
| Hallas et al. 21 | No | No | No | ||||||
| Col et al. 22 | >3 | ||||||||
Figure 2.
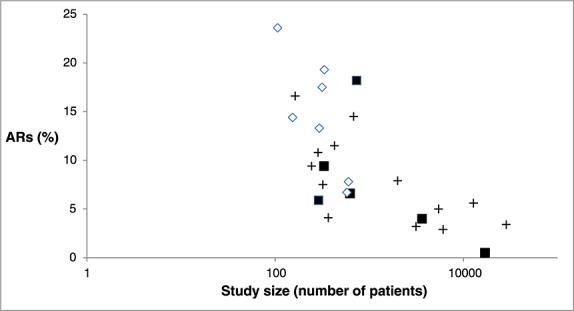
The relationship between study size and percentage of patients with ARs in the studies.  Studies including only the elderly and ARs as cause of hospitalization.
Studies including only the elderly and ARs as cause of hospitalization.  Studies including all adults and ARs as cause of hospitalization.
Studies including all adults and ARs as cause of hospitalization.  Studies including ARs during hospitalization (all adults).
Studies including ARs during hospitalization (all adults).  AR, Adverse reaction.
AR, Adverse reaction.
The importance of study size
When analyzing studies with a study size below 2000 patients (17 studies), a total of 7815 patients were included of whom 10 % experienced an AR. Ten of these studies included all adults and seven studies only the elderly. Studies comprising more than 2000 patients showed a mean frequency of ARs of 3.2 % and none of these studies included only the elderly. Figure2 illustrates that studies including only the elderly population seem to find larger frequencies of ARs, but are of smaller size.
Risk factors in studies including all adults
Table3 shows studies with statistically significant correlations (univariate analysis) between ARs in adult patients and risk factors. The studies were ranked according to frequency of ARs. The top of the table shows the studies investigating ARs during hospitalizations and the bottom shows the studies investigating ARs causing hospitalization. The mean frequency of ARs was 3.7 % (0.5–18.2) and the mean study size was 4360 (163–28 411) patients. The types of risk factors were equally distributed among the smaller and larger studies and no differences were seen among these. Age, gender and number of drugs were most frequently investigated.
Univariate analysis showed age, number of drugs and co-morbidity to be risk factors in 10 of 17, 11 of 14 and three of six studies, respectively. Multivariate analysis was only performed in four of those studies and number of drugs was confirmed as a risk factor in three out of four. Co-morbidity and age as risk factors were not confirmed. No studies investigated impaired renal function in multivariate analysis. Considering the studies of ARs at admission and ARs during admission separately did not change the overall picture.
Risk factors in studies including only the elderly
Studies including the elderly population exclusively are shown in Table4. The definition of elderly was ≥ 65 years of age (four studies), ≥ 70 years (one study), geriatric patients (one study) or nursing home residents (one study). The mean rate of ARs was higher in these studies, namely 11.9 % (6.7–23.6), while the studies were smaller, with an average of 340 patients in each. In univariate analysis the most convincing risk factor was number of drugs with four out of six studies and co-morbidity with two out of two studies showing an association. In multivariate analysis the number of drugs was still a risk factor in three out of four studies, while co-morbidity was not a risk factor in one out of one study. Impaired renal function was found to be a risk factor in women in one of two studies and was not investigated in multivariate analysis. Finally, gender was not found to be a risk factor in univariate analysis in this population.
Discussion
We found that 85 212 patients had a total of 3385 serious ARs resulting in an overall frequency of serious ARs in 4% of patients. The primary risk factor correlated with the risk of ARs was the total number of drugs when considering studies using multivariate analysis.
Is study size of importance?
In the studies exclusively investigating the elderly population the frequency of serious ARs was 11.9 %. However, they represented only a minority of the patients. Considering studies with less than 2000 patients, there were 10 studies of all adults and seven of the elderly. This simple subdivision points in the direction of small study size rather than age being the reason for increased frequencies of ARs. The tendency of smaller studies uncovering more ARs could be related to practical issues such as scrutiny of hospital records, which is manageable when only few patients are investigated in comparison with studies of larger populations. Other possible explanations are publication bias, as studies displaying large frequencies are more interesting to publish, or it could be a question of dilution as larger populations might include less ill patients. In a meta-analysis from 2002 a total of 68 studies were analyzed with the purpose of estimating the number of patients hospitalized due to ARs 32. Eleven of those studies were also included in the present review as they examined patient-related risk factors. Beijer et al. found a total rate of 4.9 % of hospitalizations due to ARs, 4.1 % in the non-elderly population and 16.6 % in the elderly population and a tendency towards larger studies to display a lower percentage of ARs were identical.
Is the number of drugs a risk factor for ARs?
The most consistent variable in both the general population and in the elderly population was the total number of drugs. An exponential relationship between the number of concurrently used drugs and the likelihood of an AR is described in a paper by Smith et al. from 1966, and the paper concludes that the absolute drug number probably is the only truly independent variable 49. Today an exponential curve might not be entirely true, as drugs have become safer, and the morbidity from drugs has likely decreased. Instead of an exponential curve, it might rather be flat or even regress at first as morbidity due to drug treatment will lessen until a certain level, where too many drugs exceed the advantages of drug treatment.
Could knowledge of the total number of drugs as a patient-related risk factor be used in relation to drug treatment? A reduction of the number of prescribed drugs would be the natural consequence. However, knowledge about the plateau of decreased morbidity due to disease and increased morbidity due to drug treatment is necessary. The total number of drugs along with the individual drug’s risk could be incorporated into an electronic algorithm and thereby capture patients with expected higher risk of serious ARs. In this way allocation of healthcare resources could be focused on high risk patients.
Is age a risk factor for ARs?
Age does not seem to be a risk factor for ARs based on this review. Polypharmacy and polymorbidity often go hand in hand which might hamper distinction between symptoms of the disease and symptoms of the ARs. Consequently, this might lead to both over- and under-interpretation of ARs when hospital records are available and studied in detail. Studies have found that polymorbidity and polypharmacy were more frequently present in the group of patients with ARs resulting in hospitalization 11,50–53. In addition, elderly use more drugs than younger individuals 51,54–57 and an exponential increase between number of ARs and drugs taken has been described 49,58. These might serve as confounding factors.
Some studies investigated the impact of reduced cognition and found it to be negatively associated with the number of ARs, both serious and non-serious 50,53. Impaired cognition is expected to cause more mistakes in drug consumption. However, patient notification might be significantly lower and the patients might have difficulty in verbalizing or recalling symptoms resulting in underdiagnosing of ARs. Similarly, patients older than 80 years of age were significantly less likely to experience ARs than younger patients in some studies 59,60. This could be explained by the under-reporting of ARs by both healthcare professionals and patients or simply reflect the survival of the fittest.
Studies investigating the correlation with both serious and non-serious ARs found that age was not an independent risk factor for ARs. However, they revealed that the elderly patients had more severe ARs and the numbers of non-serious ARs were reduced 51,61. Carbonin et al. analyzed the data in a multivariate logistic model, which confirmed that age was not a real risk factor. The elderly population is most likely more fragile to the impact of medication and at greater need of it but, it is not evident that age per se is a risk factor for ARs.
Is female gender a risk factor for ARs?
Seven studies found females to be at higher risk, while eight studies did not find gender to be a risk factor. For the elderly population six out of six studies did not find gender to be a risk factor for ARs. Several studies found that women more often had non-serious ARs, while men had, at least, as often serious ARs. In fact, some studies demonstrated that men more frequently had serious ARs than women 41,55,62. This is in line with the perception that women seek medical attention more often than men, whereas men tend not to accept their disease, and therefore, do not resort to medical services as often 63.
Further to this, several studies have shown that women in general take a higher number of drugs than men 16,22,30,55–57 and their co-morbidity was higher 53. In the present review we chose to exclude papers that included non-compliance if impossible to distinguish ARs, that were caused or not caused by non-compliance. It might partly explain why studies including non-compliance as ARs find female gender to be a risk factor, as a higher number of drugs are found to cause less compliance 64,65.
Strengths and limitations
Karch & Lasagna investigated the difficulties in evaluating ARs by having three clinical pharmacologists independently evaluate 60 selected cases and found that they agreed on only 50% 66. Many studies have found the same problem with imprecise judgments and it is an important reason for the heterogeneity of these studies. Even though we tried to narrow the inclusion criteria it is not possible to judge if the evaluation of ARs across studies or even within studies is consistent.
A number of other factors may explain the discrepancy in results between studies, like the heterogeneity of the populations being studied with regard to age, size and co-morbidities. Another problem is the descriptive nature of most reports and thereby a lack of control group. Most studies included in this review were cross sectional studies and as such investigated the immediate relation between drug exposure and ARs. This method will provide information concerning prevalence of ARs and determine possible risk factors for ARs. However it cannot provide information about a causal relation, and the studies should be interpreted carefully. Control patients should ideally be a representative sample from those at risk of an AR that would necessitate hospital admission. The only included study aiming at this was the case-control study by Leendertse et al. 13.
Thus, it was not possible to evaluate adequately the influence of independent variables, the confounding nature of these and the possible interactions among them. Likewise the data extracted were not amenable to meta-analysis due to the heterogeneity of study methods and definitions and we did not formally assess the risk of bias.
It is possible that the reason for the marked variability in results is the lack of control for, or consideration of, many factors that may influence development of drug-related illnesses separately.
Conclusion
Based on this systematic review the number of drugs is the most frequently documented independent patient-related risk factor for serious ARs. Further, we found an overall frequency of serious ARs in 4% of patients based on 26 studies including 85 212 patients. Interestingly, the size of studies was important for the interpretation of reliable frequencies of ARs, consistent with results from review of hospitalizations.
The knowledge of patient-related risk factors for experiencing ARs could be used for electronic risk stratification of patients and thereby allocation of healthcare resources to high risk patients.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Supporting info item
References
- Guideline on good pharmacovigilance practices - Module VI. Management and reporting of adverse reactions to medicinal products. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000570.jsp (last accessed 18 December 2013)
- 2013. E2AClinical safety data management: Definitions and standards for expedited reporting. Available at www.ich.org (last accessed 4 July 2013)
- Pardo Cabello AJ, Gonzalez Contreras LG, Manzano Gamero MV, Gomez Jimenez FJ, Puche Canas E. Prevalence of fatal adverse drug reactions in hospitalized patients. Int J Clin Pharmacol Ther. 2009;47:596–602. doi: 10.5414/cpp47596. [DOI] [PubMed] [Google Scholar]
- Buajordet I, Ebbesen J, Erikssen J, Brors O, Hilberg T. Fatal adverse drug events: the paradox of drug treatment. J Intern Med. 2001;250:327–41. doi: 10.1046/j.1365-2796.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- Ebbesen J, Buajordet I, Erikssen J, Brors O, Hilberg T, Svaar H, Sandvik L. Drug-related deaths in a Department of Internal Medicine. Arch Intern Med. 2001;161:2317–23. doi: 10.1001/archinte.161.19.2317. [DOI] [PubMed] [Google Scholar]
- Fattinger K, Roos M, Vergeres P, Holenstein C, Kind B, Masche U, Stocker DN, Braunschweig S, Kullak-Ublick GA, Galeazzi RL, Follath F, Gasser T, Meier PJ. Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol. 2000;49:158–67. doi: 10.1046/j.1365-2125.2000.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darchy B, Le Miere E, Figueredo B, Bavoux E, Domart Y. Iatrogenic diseases as a reason for admission to the intensive care unit: incidence, causes and consequences. Arch Intern Med. 1999;159:71–8. doi: 10.1001/archinte.159.1.71. [DOI] [PubMed] [Google Scholar]
- Bordet R, Gautier S, Le Louet H, Dupuis B, Caron J. Analysis of the direct cost of adverse drug reactions in hospitalised patients. Eur J Clin Pharmacol. 2001;56:935–41. doi: 10.1007/s002280000260. [DOI] [PubMed] [Google Scholar]
- Moore N, Lecointre D, Noblet C, Mabille M. Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol. 1998;45:301–8. doi: 10.1046/j.1365-2125.1998.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria-Pablos A, Redondo-Figuero C, Baena MI, Faus MJ, Tejido R, Acha O, Novo FJ. Negative results related to drugs required in hospitalisation. Farm Hosp. 2009;33:12–25. [PubMed] [Google Scholar]
- Wawruch M, Zikavska M, Wsolova L, Kuzelova M, Kahayova K, Strateny K, Kristova V. Adverse drug reactions related to hospital admission in Slovak elderly patients. Arch Gerontol Geriatr. 2009;48:186–90. doi: 10.1016/j.archger.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Hellden A, Bergman U, von Euler M, Hentschke M, Odar-Cederlof I, Ohlen G. Adverse drug reactions and impaired renal function in elderly patients admitted to the emergency department: a retrospective study. Drugs Aging. 2009;26:595–606. doi: 10.2165/11315790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM HARM Study Group. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168:1890–6. doi: 10.1001/archinternmed.2008.3. [DOI] [PubMed] [Google Scholar]
- Mjorndal T, Boman MD, Hagg S, Backstrom M, Wiholm BE, Wahlin A, Dahlqvist R. Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf. 2002;11:65–72. doi: 10.1002/pds.667. [DOI] [PubMed] [Google Scholar]
- Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R, Gambassi G. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA) J Am Geriatr Soc. 2002;50:1962–8. doi: 10.1046/j.1532-5415.2002.50607.x. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Karan RS, Pandhi P, Jain S. Drug related medical emergencies in the elderly: role of adverse drug reactions and non-compliance. Postgrad Med J. 2001;77:703–7. doi: 10.1136/pmj.77.913.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannesse CK, Derkx FH, de Ridder MA, Man in ’t Veld AJ, van der Cammen TJ. Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing. 2000;29:35–9. doi: 10.1093/ageing/29.1.35. [DOI] [PubMed] [Google Scholar]
- Pouyanne P, Haramburu F, Imbs JL, Begaud B. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. French Pharmacovigilance Centres. BMJ. 2000;320:1036. doi: 10.1136/bmj.320.7241.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JW. Adverse drug reaction-related hospitalizations of nursing facility patients: a 4-year study. South Med J. 1999;92:485–90. doi: 10.1097/00007611-199905000-00007. [DOI] [PubMed] [Google Scholar]
- Hallas J, Gram LF, Grodum E, Damsbo N, Brosen K, Haghfelt T, Harvald B, Beck-Nielsen J, Worm J, Jensen KB. Drug related admissions to medical wards: a population based survey. Br J Clin Pharmacol. 1992;33:61–8. doi: 10.1111/j.1365-2125.1992.tb04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallas J, Worm J, Beck-Nielsen J, Gram LF, Grodum E, Damsbo N, Brosen K. Drug related events and drug utilization in patients admitted to a geriatric hospital department. Dan Med Bull. 1991;38:417–20. [PubMed] [Google Scholar]
- Col N, Fanale JE, Kronholm P. The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly. Arch Intern Med. 1990;150:841–5. [PubMed] [Google Scholar]
- Hallas J, Haghfelt T, Gram LF, Grodum E, Damsbo N. Drug related admissions to a cardiology department; frequency and avoidability. J Intern Med. 1990;228:379–84. doi: 10.1111/j.1365-2796.1990.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Colt HG, Shapiro AP. Drug-induced illness as a cause for admission to a community hospital. J Am Geriatr Soc. 1989;37:323–6. doi: 10.1111/j.1532-5415.1989.tb05498.x. [DOI] [PubMed] [Google Scholar]
- Davidsen F, Haghfelt T, Gram LF, Brosen K. Adverse drug reactions and drug non-compliance as primary causes of admission to a cardiology department. Eur J Clin Pharmacol. 1988;34:83–6. doi: 10.1007/BF01061423. [DOI] [PubMed] [Google Scholar]
- Hermesh H, Shalev A, Munitz H. Contribution of adverse drug reaction to admission rates in an acute psychiatric ward. Acta Psychiatr Scand. 1985;72:104–10. doi: 10.1111/j.1600-0447.1985.tb02578.x. [DOI] [PubMed] [Google Scholar]
- Bergman U, Wiholm BE. Drug-related problems causing admission to a medical clinic. Eur J Clin Pharmacol. 1981;20:193–200. doi: 10.1007/BF00544597. [DOI] [PubMed] [Google Scholar]
- Levy M, Lipshitz M, Eliakim M. Hospital admissions due to adverse drug reactions. Am J Med Sci. 1979;277:49–56. doi: 10.1097/00000441-197901000-00006. [DOI] [PubMed] [Google Scholar]
- Levy M, Kewitz H, Altwein W, Hillebrand J, Eliakim M. Hospital admissions due to adverse drug reactions: a comparative study from Jerusalem and Berlin. Eur J Clin Pharmacol. 1980;17:25–31. doi: 10.1007/BF00561673. [DOI] [PubMed] [Google Scholar]
- Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalization. JAMA. 1974;228:713–7. [PubMed] [Google Scholar]
- Hakkarainen KM, Hedna K, Petzold M, Hagg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions - a meta-analysis. PLoS One. 2012;7:e33236. doi: 10.1371/journal.pone.0033236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 2002;24:46–54. doi: 10.1023/a:1015570104121. [DOI] [PubMed] [Google Scholar]
- de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Health Care. 2008;17:216–23. doi: 10.1136/qshc.2007.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42:1017–25. doi: 10.1345/aph.1L037. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30:379–407. doi: 10.2165/00002018-200730050-00003. [DOI] [PubMed] [Google Scholar]
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- Muehlberger N, Schneeweiss S, Hasford J. Adverse drug reaction monitoring--cost and benefit considerations. Part I: frequency of adverse drug reactions causing hospital admissions. Pharmacoepidemiol Drug Saf. 1997;6:S71–7. doi: 10.1002/(sici)1099-1557(199710)6:3+<s71::aid-pds282>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Monguio R, Otero MJ, Rovira J. Assessing the economic impact of adverse drug effects. Pharmacoeconomics. 2003;21:623–50. doi: 10.2165/00019053-200321090-00002. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Levy M, Muth-Selbach U, Oelkers R, Neumann F, Dormann H, Azaz-Livshits T, Criegee-Rieck M, Schneider HT, Hahn E, Brune K, Geisslinger G. Retrospective analysis of the frequency and recognition of adverse drug reactions by means of automatically recorded laboratory signals. Br J Clin Pharmacol. 1999;47:557–64. doi: 10.1046/j.1365-2125.1999.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DW, Miller EB, Cullen DJ, Burdick L, Williams L, Laird N, Petersen LA, Small SD, Sweitzer BJ, Vander Vliet M, Leape LL. Patient risk factors for adverse drug events in hospitalized patients. Arch Intern Med. 1999;159:2553–60. doi: 10.1001/archinte.159.21.2553. [DOI] [PubMed] [Google Scholar]
- Macedo AF, Alves C, Craveiro N, Marques FB. Multiple drug exposure as a risk factor for the seriousness of adverse drug reactions. J Nurs Manag. 2011;19:395–9. doi: 10.1111/j.1365-2834.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- Ben-Yehuda A, Bitton Y, Sharon P, Rotfeld E, Armon T, Muszkat M. Risk factors for prescribing and transcribing medication errors among elderly patients during acute hospitalization: A cohort, case-control study. Drugs Aging. 2011;28:491–500. doi: 10.2165/11590610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Patel H, Bell D, Molokhia M, Srishanmuganathan J, Patel M, Car J, Majeed A. Trends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998-2005. BMC Clin Pharmacol. 2007;7:9. doi: 10.1186/1472-6904-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Munoz-Torrero JF, Barquilla P, Velasco R, Fernandez Capitan Mdel C, Pacheco Y, Vicente L, Chicon JL, Trejo S, Zamorano J, Lorenzo Hernandez A. Adverse drug reactions in internal medicine units and associated risk factors. Eur J Clin Pharmacol. 2010;66:1257–64. doi: 10.1007/s00228-010-0866-6. [DOI] [PubMed] [Google Scholar]
- 2013. Glossary of Terms in Pharmacovigilance. Available at http://www.who-umc.org (last accessed 23 December 2013)
- 2014. Refworks Web Based Bibliographic Management Software. Available at www.refworks.com (last accessed 9 April 2014)
- Zaal RJ, van Doormaal JE, Lenderink AW, Mol PG, Kosterink JG, Egberts TC, Haaijer-Ruskamp FM, van den Bemt PM. Comparison of potential risk factors for medication errors with and without patient harm. Pharmacoepidemiol Drug Saf. 2010;19:825–33. doi: 10.1002/pds.1977. [DOI] [PubMed] [Google Scholar]
- Smith JW, Seidl LG, Cluff LE. Studies on the epidemiology of adverse drug reactions. V. Clinical factors influencing susceptibility. Ann Intern Med. 1966;65:629–40. doi: 10.7326/0003-4819-65-4-629. [DOI] [PubMed] [Google Scholar]
- Onder G, Gambassi G, Scales CJ, Cesari M, Vedova CD, Landi F, Bernabei R. Adverse drug reactions and cognitive function among hospitalized older adults. Eur J Clin Pharmacol. 2002;58:371–7. doi: 10.1007/s00228-002-0493-y. [DOI] [PubMed] [Google Scholar]
- Carbonin P, Pahor M, Bernabei R, Sgadari A. Is age an independent risk factor of adverse drug reactions in hospitalized medical patients? J Am Geriatr Soc. 1991;39:1093–9. doi: 10.1111/j.1532-5415.1991.tb02875.x. [DOI] [PubMed] [Google Scholar]
- Field TS, Gurwitz JH, Harrold LR, Rothschild J, DeBellis KR, Seger AC, Auger JC, Garber LA, Cadoret C, Fish LS, Garber LD, Kelleher M, Bates DW. Risk factors for adverse drug events among older adults in the ambulatory setting. J Am Geriatr Soc. 2004;52:1349–54. doi: 10.1111/j.1532-5415.2004.52367.x. [DOI] [PubMed] [Google Scholar]
- Caamano F, Pedone C, Zuccala G, Carbonin P. Socio-demographic factors related to the prevalence of adverse drug reaction at hospital admission in an elderly population. Arch Gerontol Geriatr. 2005;40:45–52. doi: 10.1016/j.archger.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Huic M, Mucolic V, Vrhovac B, Francetic I, Bakran I, Giljanovic S. Adverse drug reactions resulting in hospital admission. Int J Clin Pharmacol Ther. 1994;32:675–82. [PubMed] [Google Scholar]
- Trifiro G, Calogero G, Ippolito FM, Cosentino M, Giuliani R, Conforti A, Venegoni M, Mazzaglia G, Caputi AP. Adverse drug events in emergency department population: a prospective Italian study. Pharmacoepidemiol Drug Saf. 2005;14:333–40. doi: 10.1002/pds.1074. [DOI] [PubMed] [Google Scholar]
- Raschetti R, Morgutti M, Menniti-Ippolito F, Belisari A, Rossignoli A, Longhini P, La Guidara C. Suspected adverse drug events requiring emergency department visits or hospital admissions. Eur J Clin Pharmacol. 1999;54:959–63. doi: 10.1007/s002280050582. [DOI] [PubMed] [Google Scholar]
- Stanton LA, Peterson GM, Rumble RH, Cooper GM, Polack AE. Drug-related admissions to an Australian hospital. J Clin Pharm Ther. 1994;19:341–7. doi: 10.1111/j.1365-2710.1994.tb00691.x. [DOI] [PubMed] [Google Scholar]
- Routledge PA, O’Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. Br J Clin Pharmacol. 2004;57:121–6. doi: 10.1046/j.1365-2125.2003.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bemt PM, Robertz R, de Jong AL, van Roon EN, Leufkens HG. Drug administration errors in an institution for individuals with intellectual disability: an observational study. J Intellect Disabil Res. 2007;51:528–36. doi: 10.1111/j.1365-2788.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- Ganeva M, Gancheva T, Lazarova R, Tzvetanova Y, Hristakieva E. A prospective study of adverse drug reactions in a dermatology department. Methods Find Exp Clin Pharmacol. 2007;29:107–12. doi: 10.1358/mf.2007.29.2.1075348. [DOI] [PubMed] [Google Scholar]
- Bowman L, Carlstedt BC, Hancock EF, Black CD. Adverse drug reaction (ADR) occurrence and evaluation in elderly inpatients. Pharmacoepidemiol Drug Saf. 1996;5:9–18. doi: 10.1002/(SICI)1099-1557(199601)5:1<9::AID-PDS192>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Tran C, Knowles SR, Liu BA, Shear NH. Gender differences in adverse drug reactions. J Clin Pharmacol. 1998;38:1003–9. doi: 10.1177/009127009803801103. [DOI] [PubMed] [Google Scholar]
- Spiers N, Jagger C, Clarke M, Arthur A. Are gender differences in the relationship between self-rated health and mortality enduring? Results from three birth cohorts in Melton Mowbray. United Kingdom Gerontologist. 2003;43:406,11. doi: 10.1093/geront/43.3.406. ; discussion 372-5. [DOI] [PubMed] [Google Scholar]
- Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother. 2011;9:11–23. doi: 10.1016/j.amjopharm.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303–12. doi: 10.1345/aph.1D252. [DOI] [PubMed] [Google Scholar]
- Karch FE, Smith CL, Kerzner B, Mazzullo JM, Weintraub M, Lasagna L. Adverse drug reactions-a matter of opinion. Clin Pharmacol Ther. 1976;19:489–92. doi: 10.1002/cpt1976195part1489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


