Abstract
Aims
Fatal adverse drug reactions (ADRs) are important causes of death, but data from resource-limited settings are scarce. We determined the proportion of deaths in South African medical inpatients attributable to ADRs, and their preventability, stratified by human immunodeficiency virus (HIV) status.
Methods
We reviewed the folders of all patients who died over a 30 day period in the medical wards of four hospitals. We identified ADR-related deaths (deaths where an ADR was ‘possible’, ‘probable’ or ‘certain’ using WHO-UMC criteria and where the ADR contributed to death). We determined preventability according to previously published criteria.
Results
ADRs contributed to the death of 2.9% of medical admissions and 56 of 357 deaths (16%) were ADR-related. Tenofovir, rifampicin and co-trimoxazole were the most commonly implicated drugs. 43% of ADRs were considered preventable. The following factors were independently associated with ADR-related death: HIV-infected patients on antiretroviral therapy (adjusted odds ratio (aOR) 4.4, 95% confidence interval (CI) 1.6, 12), exposure to more than seven drugs (aOR 2.5, 95% CI 1.3, 4.8) and increasing comorbidity score (aOR 1.3, 95% CI 1.1, 1.7).
Conclusions
In our setting, where HIV and tuberculosis are highly prevalent, fatal in-hospital ADRs were more common than reported in high income settings. Most deaths were attributed to drugs used in managing HIV and tuberculosis. A large proportion of the ADRs were preventable, highlighting the need to strengthen systems for health care worker training and support.
Keywords: adverse drug reaction, antiretroviral therapy, anti-tuberculosis therapy, in-hospital death, preventability, South Africa
What Is Already Known about This Subject
Adverse drug reactions (ADRs) are implicated in 2.5–18% of deaths of hospitalized patients.
Fatal ADRs are frequently preventable.
There is a lack of evidence regarding the burden of ADR-related deaths in low- and middle-income countries, and in settings of high human immunodeficiency virus (HIV) and tuberculosis prevalence.
What This Study Adds
In South African medical wards, a larger proportion of in-hospital deaths are ADR-related than previously described.
Unlike the pattern in high income countries, the drugs implicated in ADR-related deaths were mostly drugs used in the treatment of HIV and tuberculosis, reflecting the high burden of these diseases in our setting.
Introduction
Adverse drug reactions (ADRs) that result in death, particularly those considered to have been preventable, are of major public health interest. A population-based study in three Swedish counties found that 3% of all deaths were suspected to be due to ADRs, making ADRs the seventh most common cause of death 1. This figure agrees with a systematic review 2 which ranked ADRs among the top six causes of death in the USA.
Most epidemiological data on ADR-related deaths come from hospital-based studies. Depending on the definitions and methods used, ADRs have been implicated in 2.5–18% of in-hospital deaths 3–7. However, there are limited data on mortality associated with ADRs from resource-limited settings, in particular from settings with high human immunodeficiency virus (HIV) and tuberculosis prevalence. South Africa has implemented the world’s largest roll-out of antiretroviral therapy (ART), with just over 2 million people on ART in 2012 8, and has among the highest tuberculosis incidence globally, estimated to be 1000 new cases per 100 000 persons per year in 2012 9.
A previous survey performed by our group at a secondary level hospital in South Africa in 2005 7, showed that HIV-infected persons were twice as likely to be admitted with an ADR compared with persons who were HIV negative or whose HIV status was unknown. In that survey, antiretroviral drugs (ARVs) and drugs used to treat opportunistic infections were frequently implicated as causing ADRs, albeit that the survey was performed early in the ART era, when more toxic ARVs were in use. Thus, there is a need for larger studies to determine the current impact of ADRs on morbidity and mortality in our setting.
We performed a cross-sectional survey in the medical wards of four hospitals in South Africa to determine the mortality attributable to ADRs, to identify the drugs implicated in these ADR-related deaths, to determine the proportion of fatal ADRs that were preventable and to explore the effect of patients’ HIV status on mortality attributed to ADRs.
Methods
Study design and setting
Between April and September 2013, we identified all patients who died in the medical wards of Groote Schuur Hospital, Edendale Hospital, Cecilia Makiwane Hospital and Frere Hospital, over a 30 day period at each site, by daily scrutiny of ward and/or mortuary administrative records. A description of the sites is included in Table S1. We included all patients where a decision to admit had been made by the medical team, including those who died in the emergency unit while awaiting transfer to the ward.
Identification of suspected ADRs and causality assessment
We defined an ADR according to the definition of Aronson & Ferner 10, but we did not regard intentional overdoses or cases of immune reconstitution syndrome as ADRs. The assessment process flow is shown in Figure 1. Between October 2013 and April 2014, one author (JPM) reviewed each patient’s medical notes, medication exposure over the 30 days prior to admission, prescription charts related to the admission, as well as the patient’s laboratory data, using a trigger tool 11 to flag the deaths suspected to be related to ADRs, and additionally flagging all cases where the cause of death was unclear. We did not attempt to augment incomplete data from other sources. For each flagged case, JPM prepared a narrative summary of events, as well as a detailed summary of drug use (codified to five level ATC codes), investigative results and clinical problems (codified to ICD-10 codes).
Figure 1.
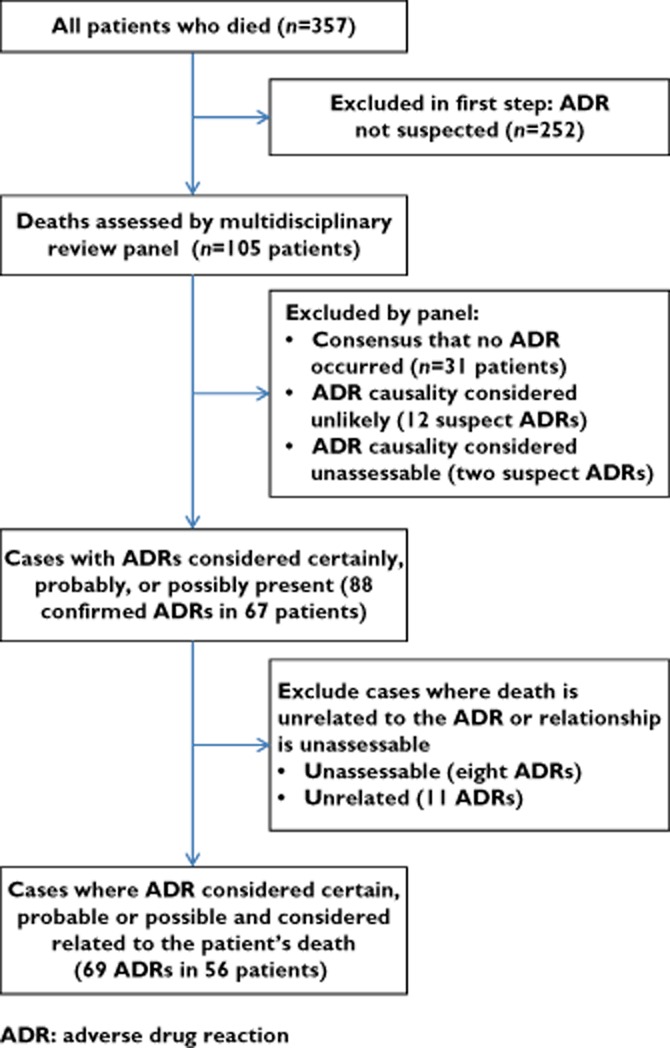
Flowchart describing the assessment process
A multidisciplinary review panel consisting of a clinical pharmacologist, a clinical pharmacist, and at least one but up to three physicians/internists met to assess each of the flagged cases for the presence of an ADR and its preventability. Each panellist was provided with the prepared case summaries and an electronic, anonymized copy of the hospital records. For each suspected ADR that was identified by one or more members of the review panel, the panel discussed its causality using the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) system for standardized case causality assessment 12, until consensus was reached. For ADRs categorized as ‘possible’, ‘probable’ or ‘certain’, the panel assessed the relationship between the ADR and the death of the patient. We categorized the relationship between ADR and death into one of four categories: (i) the ADR was a major contributor to the death of the patient, (ii) the ADR contributed to the patient’s death, but was not the major contributor, (iii) the ADR was unrelated to the patient’s death or (iv) the relationship between ADR and death could not be determined. The panel also decided on the preventability of the ADR using the criteria of Schumock & Thornton 13. Where the panel could not reach consensus on the causality or preventability of an ADR, or on its relationship to the patient’s death, the majority decision was recorded. In one case, where the panel consisted of four members who were evenly split in opinion over the presence/absence of an ADR, the third physician’s opinion was sought following the panel meeting, to obtain a majority decision.
We defined an ADR-related death as a death where the panel held (either in consensus, or in majority) that, firstly, an ADR was ‘possible’, ‘probable’ or ‘certain’, according to the WHO-UMC methodology and that, secondly, the ADR was a major contributor or a contributor to the patient’s death. An ADR was considered preventable if any one or more of the Schumock & Thornton criteria was answered in the affirmative.
For every patient who died, we calculated a comorbidity score modified from the Charlson comorbidity score 14. As the original Charlson comorbidity score assigns a score for HIV infection that is probably inappropriately high in the era of ART 15 and as we wanted to explore the independent effect of HIV-infected status with and without antiretroviral therapy, we excluded a diagnosis of HIV/AIDS from the calculation of the modified Charlson comorbidity score. We also calculated a drug count for every patient by counting each drug to which the patient was documented to have been exposed in the period starting 3 weeks prior to the date of admission and ending on the day of death, excluding vitamin and mineral supplements. For fixed drug combinations, we counted each component drug individually.
Data entry and analysis
Data were entered into a Microsoft Access 2010 database and analyzed using Stata 13.1 (Stata Corporation, College Station, Texas, USA). Continuous variables were summarized using medians and interquartile ranges, as they were non-normally distributed. We explored univariate associations for binary and categorical variables by cross-tabulation, and calculated chi-square statistics or performed Fisher’s exact test as appropriate. As data were non-normally distributed, we used the Wilcoxon rank-sum test for between group comparisons of continuous and ordinal variables.
We constructed a multivariate logistic regression model exploring independent associations with ADR-related death. Variables were included in the model based on an a priori decision. We included the following variables in the model: age, sex, comorbidity score, drug count (using a binary variable by cutting the data at the median), HIV infection and antiretroviral treatment status (using a three category variable: HIV-negative or unknown status, HIV-infected and not on ART, HIV-infected on ART), and whether or not patients were being treated for tuberculosis. Assumptions of linearity were checked in the final model. We constructed a similar model, with the addition of the most recent CD4 count, in an analysis restricted to patients known to be HIV-infected. We used a significance level of 0.05.
Ethics
This study was approved by the Human Research Ethics Committee of the Faculty of Health Sciences at the University of Cape Town (Reference no. 576/2011). Permission to conduct the research was granted by the respective hospitals’ management and/or provincial Departments of Health.
Results
There were 1951 admissions and 357 deaths during the survey period, for a crude mortality rate of 18 deaths per 100 admissions. At the first level of review, 252 cases were assessed as having no suspected ADRs (Figure 1). The multidisciplinary panel reviewed the remaining 105 cases, and concluded that ADR-related causes contributed to the deaths of 56 of the 357 (16%) patients, which represents 2.9% of the 1951 patients admitted over the study period.
Sixty-nine different ADRs (four ‘certain’, 10 ‘probable’ and 55 ‘possible’, according to the WHO-UMC method) were identified to have contributed to the death of these 56 patients. Of the 56 patients, four had at least one ‘certain’ ADR, eight at least one ‘probable’ ADR and the remaining 44 had ‘possible’ ADRs contributing to death. In 51 cases, the panel reached consensus over the ADRs1 and contribution to death, while in the remaining five cases the majority of the panel agreed on the ADR, with some dissent. Patient characteristics are summarized in Table 1. Table S2 lists individual patients’ characteristics and details of the ADRs.
Table 1.
Characteristics of patients whose deaths were considered ADR-related, vs. patients whose deaths were not considered ADR-related
| All deaths | ADR-related deaths | Other deaths | P value | |
|---|---|---|---|---|
| All patients: n | 357 | 56 | 301 | |
| Females: n (%) | 184 (52%) | 30 (54%) | 154 (51%) | P = 0.741 |
| Age (years): median [IQR] | 53 [38, 70] | 52.5 [33, 65.5] | 53 [39, 72] | P = 0.2209 |
| Known HIV infected (%) | 135 (38%) | 31 (55%) | 104 (35%) | P = 0.003 |
| On treatment for TB (%) | 55 (15%) | 14 (25%) | 41 (14%) | P = 0.030 |
| Number of drugs exposed to: median [IQR] | 7 [5, 11] | 9 [7, 11] | 7 [4, 11] | P = 0.0008 |
| Modified Charlson co-morbidity score: median [IQR] | 2 [0, 3] | 2 [1, 3] | 2 [0, 3] | P = 0.0246 |
| Time from admission to death (days): median [IQR] | 5 [3, 10] | 6.5 [3, 11.5] | 5 [3, 10] | P = 0.3478 |
| HIV-infected patients only: n | 135 | 31 | 104 | |
| Females: n (%) | 61 (45%) | 15 (48%) | 46 (44%) | P = 0.683 |
| Age (years): median [IQR] | 37 [30, 44] | 37 [31, 52] | 37 [30, 43] | P = 0.3393 |
| CD4-count (cells mm−3): median [IQR]* | 52 [19, 167] | 126 [32, 299] | 41 [19, 109] | P = 0.0509 |
| On ART (%) | 66 (49%) | 20 (65%) | 46 (44%) | P = 0.047 |
| On treatment for TB (%) | 45 (33%) | 12 (39%) | 33 (32%) | P = 0.469 |
| Number of drugs exposed to: median [IQR] | 9 [6, 12] | 9 [7, 10] | 9 [6, 12] | P = 0.8053 |
| Modified Charlson co-morbidity score: median [IQR] | 0 [0, 2] | 2 [0, 2] | 0 [0, 2] | P = 0.0579 |
| Time from admission to death (days): median [IQR] | 5 [3, 8] | 4 [2, 7] | 5.5 [3, 8.5] | P = 0.2082 |
20 patients missing data. ADR, adverse drug reaction; ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
Thirty of the 69 ADRs (43%) were considered preventable according to the Schumock & Thornton criteria 13. Most often, this was because the drug was not considered appropriate for the patient, or because laboratory monitoring was inadequate (Table 2). In 28 of the 56 deaths (50%), at least one ADR was considered preventable.
Table 2.
Frequency at which various preventability factors were observed amongst 69 ADRs considered to have contributed to the death of 56 patients
| Schumock & Thornton criteria | Number of occurrences |
|---|---|
| Was the drug involved in the ADR not considered appropriate for the patient’s clinical condition based on published guidelines, clinical studies, abstracts, case reports, case series? | 16 |
| Was the dose, route and frequency of administration not appropriate for the patient’s age, weight, or disease state (e.g. renal/hepatic function) based on published literature (e.g. South African Medicines Formulary, MIMS) | 5 |
| Was required therapeutic drug monitoring or other laboratory test not performed? | 11 |
| Was there a history of allergy or previous reaction to the drug? | 0 |
| Was a drug interaction involved in the reaction? | 4 |
| Was the serum drug concentration above the therapeutic range documented in adults? | 0 |
| Did compliance possibly contribute to the reaction? | 2 |
ADR, adverse drug reaction.
Of the 357 deaths, 135 patients (38%) were known to be HIV-infected, 39 (11%) were known to be HIV-negative and 183 (51%) had no HIV serology result. In the 135 HIV-infected patients, a higher proportion of patients who died from ADR-related causes were taking ARV therapy: 20/31 (65%) vs. 46/104 (44%). Of 55 patients on treatment for tuberculosis, 45 (82%) were co-infected with HIV.
Amongst the 56 patients with ADR-related deaths, 31 were HIV-infected, four were HIV-seronegative and the HIV status of 21 patients was unknown. When comparing the 31 HIV-infected patients to the 25 patients with negative or unknown HIV serology status, we found that ADR-related deaths in HIV-infected persons occurred at a significantly younger age (median [IQR] years 37 [31, 52] vs. 66 [55, 79], P < 0.00005), and in persons with a lower comorbidity score (median [IQR] score 2 [0, 2] vs. 3 [2, 4], P = 0.0010). There was no significant difference in the drug count (median [IQR] count 9 [7, 10] vs. 9 [7, 13], P = 0.5071), proportion female (48% vs. 60%, P = 0.386) or in the proportion of cases with at least one preventable ADR (48% vs. 52%, P = 0.788).
We constructed a multivariate logistic regression model to identify independent associations with ADR-related death (Table 3). HIV-infected patients on ART had increased odds of ADR-related death (adjusted odds ratio (aOR) 4.4, 95% confidence interval (CI) 1.6, 12). However, the odds of ADR-related death in HIV-infected patients not on ART was not significantly different from the reference group (adjusted OR 2.4, 95% CI 0.78, 7.1). Exposure to more than seven medicines prior to death was independently associated with increased odds of ADR-related death (aOR 2.5, 95% CI 1.3, 4.8). Increasing modified Charlson comorbidity score was associated with increased odds of ADR-related death (aOR 1.3, 95% CI 1.1, 1.7, for a one unit increase in score). Treatment for tuberculosis was associated with ADR-related death on univariate, but not on multivariate analysis. Age and sex were not associated with ADR-related death.
Table 3.
Multivariate logistic regression model for the association between risk factors and ADR-related death (n = 357)
| Variable | Patients, n (%) | Crude odds ratio (95% confidence interval) | Adjusted odds ratio (95% confidence interval) | Wald test P value |
|---|---|---|---|---|
| Sex | 357 | |||
| Male | 173 (48%) | 1 | 1 | |
| Female | 184 (52%) | 1.1 (0.62, 2.0) | 1.2 (0.64, 2.2) | 0.581 |
| Age* | 357 | 0.91 (0.78, 1.1) | 1.1 (0.83, 1.3) | 0.644 |
| HIV group | 357 | |||
| HIV serology negative or unknown | 222 (62%) | 1 | 1 | |
| HIV-infected and not on ART | 69 (19%) | 1.5 (0.69, 3.2) | 2.4 (0.78, 7.1) | 0.127 |
| HIV-infected and on ART | 66 (18%) | 3.4 (1.8, 6.7) | 4.4 (1.6, 12) | 0.003 |
| TB infection status | 357 | |||
| Not on treatment for TB | 302 (85%) | 1 | 1 | |
| On treatment for TB | 55 (15%) | 2.1 (1.1, 4.2) | 1.2 (0.53, 2.6) | 0.689 |
| Drug count | 357 | |||
| ≤7 drugs | 181 (51%) | 1 | 1 | |
| >7 drugs | 176 (49%) | 3.4 (1.8, 6.3) | 2.5 (1.3, 4.8) | 0.009 |
| Modified Charlson comorbidity score† | 357 | 1.2 (1.0, 1.5) | 1.3 (1.1, 1.7) | 0.009 |
Included in the model as a continuous variable. The odds ratio is for a 10 year increase in age. †The odds ratio is for a one unit increase in the comorbidity score. ADR, adverse drug reaction; ART, antiretroviral therapy; TB, tuberculosis.
To explore independent predictors of ADR-related death in HIV-infected patients, we constructed a second multivariate model restricted to the 115 patients known to be HIV-infected with complete data for all variables in the model (Table 4), including the variables above and CD4 count. After adjustment for age, sex, CD4 count, ART, tuberculosis treatment and modified Charlson score, only exposure to more than seven medicines prior to death remained significantly associated with increased odds of ADR-related death (aOR 3.6, 95% CI 1.1, 12). ART and CD4 count were associated with ADR-related death on univariate, but not on multivariate analysis (which may be due to lack of power).
Table 4.
Multivariate logistic regression model for the association between risk factors and ADR-related death in HIV-infected persons (n = 115)
| Variable | Patients, n (%) | Crude odds ratio (95% confidence interval) | Adjusted odds ratio (95% confidence interval) | Wald test P value |
|---|---|---|---|---|
| Sex | 115 | |||
| Male | 61 (53%) | 1 | 1 | |
| Female | 54 (47%) | 1.4 (0.59, 3.4) | 1.2 (0.47, 3.2) | 0.664 |
| Age* | 115 | 1.3 (0.88, 2.0) | 1.3 (0.80, 2.0) | 0.305 |
| CD4-count† | 115 | 1.1 (1.0, 1.3) | 1.1 (0.98, 1.3) | 0.088 |
| Antiretroviral therapy | 115 | |||
| Not on ART | 58 (50%) | 1 | 1 | |
| On ART | 57 (50%) | 2.9 (1.1, 7.3) | 2.4 (0.86, 6.6) | 0.096 |
| TB infection status | 115 | |||
| Not on treatment for TB | 75 (65%) | 1 | 1 | |
| On treatment for TB | 40 (35%) | 1.5 (0.62, 3.7) | 1.1 (0.42, 3.1) | 0.802 |
| Drug count | 115 | |||
| ≤7 drugs | 41 (36%) | 1 | 1 | |
| >7 drugs | 74 (64%) | 3.9 (1.2, 12) | 3.6 (1.1, 12) | 0.041 |
| Modified Charlson comorbidity score‡ | 115 | 1.3 (0.95, 1.9) | 1.2 (0.84, 1.8) | 0.281 |
Included in the model as a continuous variable. The odds ratio is for a 10 year increase in age. †Included in the model as a continuous variable. The odds ratio is for an increase of 50 cells mm–3. ‡The odds ratio is for a one unit increase in the comorbidity score. ADR, adverse drug reaction; ART, antiretroviral therapy; TB, tuberculosis.
The drugs implicated in ADR-related deaths are listed in Table 5. The two most common ADRs contributing to death were renal failure and drug-induced liver injury. Drug-induced renal failure occurred in 23 patients, with tenofovir implicated as the cause in 14 (61%) of these. In all 14 of these cases, renal failure was considered ‘possibly’ related to tenofovir, mostly because of concomitant drug-related causes (four cases) or concomitant disease-related causes (eight cases). Other causal drugs identified (alone or in combination) were co-trimoxazole (three cases), rifampicin (two cases), furosemide (two cases), co-amoxiclavulanic acid (two cases) and ibuprofen, enalapril, spironolactone, ciprofloxacin, aciclovir and indomethacin (one case each). In 12 of these 23 cases (52%), the ADR was considered preventable, because of inadequate laboratory monitoring (seven cases), inappropriate use of the drug (five cases) and/or inappropriate dosing (two cases). Drug-induced liver injury occurred in 10 patients. In seven cases the causal drug was assessed to be an antituberculosis agent (rifampicin, isoniazid and/or pyrazinamide), and in three cases co-trimoxazole was implicated (alone or in combination with other drugs). Fluconazole, erythromycin and sodium valproate were each implicated once. In two of these 10 cases the ADR was considered to have been preventable. On both occasions use of the drug was not considered appropriate.
Table 5.
Drugs implicated in ADR-related deaths, grouped according to ATC drug classes
| Second-level ATC drug class* | Drugs* |
|---|---|
| A06 Drugs for constipation (1) | sodium sulfate/polyethylene glycol (1) |
| A10 Drugs used in diabetes (5) | insulin (4); metformin (1) |
| B01 Antithrombotic agents (7) | warfarin (2); heparin (1); enoxaparin (1); aspirin (3) |
| B05 Blood substitutes and perfusion solutions (1) | sodium chloride (1) |
| C02 Antihypertensives (2) | prazosin (1); hydralazine (1) |
| C03 Diuretics (9) | hydrochlorothiazide (2); furosemide (5); spironolactone (2) |
| C07 Beta blocking agents (2) | atenolol (2) |
| C08 Calcium channel blockers (2) | amlodipine (2) |
| C09 Agents acting on the renin-angiotensin system (3) | enalapril (2); perindopril (1) |
| H02 Corticosteroids for systemic use (3) | prednisone (3) |
| J01 Antibacterials for systemic use (11) | co-amoxiclavulanic acid (2); co-trimoxazole (7); erythromycin (1); ciprofloxacin (1) |
| J02 Antimycotics for systemic use (1) | fluconazole (1) |
| J04 Antimycobacterials (15) | rifampicin (9); isoniazid (3); pyrazinamide (3) |
| J05 Antivirals for systemic use (19) | aciclovir (1); lopinavir/ritonavir (1); stavudine (3); tenofovir (14) |
| L01 Antineoplastic agents (1) | chlorambucil (1) |
| L04 Immunosuppressants (1) | tacrolimus (1) |
| M01 Anti-inflammatory and antirheumatic products (3) | indomethacin (1); ibuprofen (2) |
| M04 Antigout preparations (2) | allopurinol (1); colchicine (1) |
| N02 Analgesics (3) | codeine (2); tramadol (1) |
| N03 Anti-epileptics (2) | clonazepam (1); valproate (1) |
| N05 Psycholeptics (1) | haloperidol (1) |
| N06 Psychoanaleptics (1) | amitriptyline (1) |
| R03 Drugs for obstructive airway diseases (1) | theophylline (1) |
| V03 Other therapeutic products (1) | polystyrene sulfonate (1) |
The number of times each drug and drug class was implicated in an ADR-related death appears in brackets. The three most commonly implicated drugs appear in bold. ADR, adverse drug reaction; ATC, anatomical therapeutic chemical.
Stavudine was assessed as causing lactic acidosis contributing to the death of two patients and as causing neutropaenia contributing to the death of another. Lopinavir-ritonavir and rifampicin caused diarrhoea in two patients, thereby contributing to their death, and co-trimoxazole contributed to the death of two patients through bone marrow suppression.
Seven cases of drug-associated haemorrhage resulting in death occurred, five intracranial bleeds and two gastrointestinal bleeds. Drugs implicated in the intracranial haemorrhages were warfarin (two cases), aspirin (two cases) and heparin (one case), whereas one gastrointestinal bleed was attributed to a combination of colchicine, enoxaparin and aspirin, and the other to prednisone. Cardiovascular agents were implicated in 11 deaths, with diuretics the most common subclass of agents amongst these, implicated in eight deaths.
Table 6 lists the causes of death and the frequency of each cause among the 301 deaths where an ADR was not considered to contribute to the death. Mortality in this group of patients was mostly attributed to tuberculosis, respiratory infections and cerebrovascular disease.
Table 6.
Causes of death in cases where an ADR did not contribute to death
| Cause of death | Frequency |
|---|---|
| Tuberculosis (confirmed or suspected) | 56 (19%) |
| Cerebrovascular disease | 44 (15%) |
| Respiratory infections | 43 (14%) |
| Malignancies | 25 (8%) |
| Infections (other*) | 19 (6%) |
| Chronic obstructive airways disease | 17 (6%) |
| Renal failure | 13 (4%) |
| Cardiac failure and cardiomyopathy | 11 (4%) |
| Meningo-encephalitis | 11 (4%) |
| Liver failure | 5 (2%) |
| Myocardial infarction | 2 (1%) |
| Other† and unclear | 55 (18%) |
| Total | 301 (100%) |
Infections other than tuberculosis, respiratory infections and meningo-encephalitis. †Causes of death identified in one patient each: diabetic ketoacidosis, pulmonary embolism, asbestosis, obstructive hydrocephalus, myelitis, aplastic anaemia and pulmonary interstitial fibrosis.
Discussion
We found that ADRs contributed to the death of 2.9% of patients admitted to the adult medical wards of four geographically diverse hospitals in South Africa. The overall mortality rate in our study wards was 18 per 100 admissions and 16% of these deaths were ADR-related. Factors independently associated with ADR-related deaths were exposure to more than seven drugs, HIV-infected patients on ART and higher non-HIV co-morbidity score. ARVs, antituberculosis drugs and co-trimoxazole (which is virtually exclusively prescribed for the prevention and treatment of opportunistic infections in HIV-infected patients) were the most commonly implicated drugs in ADR-related deaths.
Our ADR-related inpatient mortality rate is considerably higher than has been reported in studies from high income countries. Norwegian researchers who used a broad definition of adverse drug events (including intoxications and medication errors) and conducted extensive pre- and post-mortem serum drug concentration measurements and autopsies, reported mortality attributable to drugs at 0.95% of all admissions in medical patients (18.2% of in-hospital deaths) 3,4. In a Finnish university hospital, deaths in 0.05% of admissions (5% of in-hospital deaths) were attributed to ADRs 5. In a large study from the UK, 0.15% of patients admitted to two hospitals died from an ADR present on admission 16. In another UK study, an ADR that developed in hospital was considered to have contributed to the deaths of 0.4% of the patients admitted (8.2% of in-hospital deaths) 6. A study in three Swiss teaching hospitals reported an ADR-related mortality rate of 0.05% of admissions 17.
There are scanty data on the mortality rate of ADRs in low- to middle-income countries. A recent study at a tertiary hospital in China reported no deaths amongst 2739 patients who experienced an ADR during their admission 18, while two studies from hospitals in Brazil 19,20 report zero and one death, respectively, amongst 81 and 212 patients with identified ADRs. Zargarzadeh et al. 21 found the mortality rate attributable to drug-related problems (including ADRs and therapeutic failures) to be 0.9% of all the admissions to three Iranian teaching hospitals. An Indian study reported an ADR-related mortality rate of 0.24% of all admissions 22, while another Indian study reported no ADR-related deaths amongst 1221 medical admissions 23. In the previous ADR survey by our group, we found two ADR-related deaths out of 80 deaths occurring over a 3 month period in 2005 (i.e. 2.5%) in the medical wards of a secondary hospital in Cape Town 7. There were 665 admissions included in the analysis, giving a mortality rate of 0.3% of admissions.
As already alluded to, the ADR definition used, and specifically the question whether ADRs considered ‘possible’ should be counted as ADRs or not, will influence the reported rate of ADRs. We decided a priori to include ‘possible’ ADRs in our analysis, but if we had limited our analyses only to those cases where an ADR was considered ‘certain’ or ‘probable’, we would have shown a mortality rate of 12/1951 admissions (0.61%), which is still in the upper range of rates previously reported (0.05% to 0.95%) or 12/357 deaths (3.4%).
Studies conducted in high income countries 1,3–5,16,17 report that the most frequent fatal ADRs are gastrointestinal and intracranial haemorrhages attributed to warfarin, non-steroidal anti-inflammatory drugs, heparins, antiplatelet agents, corticosteroids and/or selective serotonin re-uptake inhibitors. We found that antiretrovirals, antituberculosis agents and/or co-trimoxazole were implicated in 27 ADR-related deaths, nearly half of all the ADR-related deaths identified, reflecting the very high burden of HIV and tuberculosis in our region.
Our multivariate regression analysis identified HIV-infection and treatment with ART as an independent risk factor for ADR-related death. While stavudine is no longer recommended as a first line drug due to its toxicity 24, it was still associated with the death of three patients in our series. In our survey, tenofovir, which has replaced stavudine due to its lower toxicity, was the single drug most commonly implicated in ADR-related deaths. Tenofovir can cause acute or chronic renal failure, but both are uncommon 25. An additional insult, e.g. dehydration due to acute gastroenteritis, was present in many of the fatal cases of tenofovir-associated renal failure we identified. Dialysis facilities are limited in our region, even for acute renal failure, and many cases of severe renal failure have a fatal outcome.
Co-trimoxazole and/or anti-tuberculosis therapy was implicated in most cases of drug-induced liver injury (DILI) in our survey. In keeping with the idiosyncratic nature of most DILIs, we only identified a preventable ADR in 20% of the DILI cases, lower than the overall figure of 50% among all the deaths.
Known risk factors for the development of ADRs include multi-morbidity and polypharmacy 3,4,6,19, and, in some but not all studies, advancing age 2,6,16,21. In our survey, more severe co-morbidity as measured by the modified Charlson comorbidity score, and exposure to a higher number of drugs before and during hospitalization, but not age, were associated with ADR-related death. This did not, however, hold true for the subgroup of HIV-infected persons, in whom the only independent predictor of ADR-related death was exposure to a higher number of drugs.
Forty-three per cent of the ADRs contributing to death in our survey were considered preventable. While methodological differences make it difficult to compare this figure with other studies, our proportion is in a similar range to figures previously described in South Africa (53% of community acquired ADRs and 33% of hospital acquired ADRs) 7 and in the UK (72% of community acquired 16 and 53% of hospital acquired 6 ADRs) and did not differ in the HIV-infected subgroup. Considering the preventability factors identified, training of health care workers should focus on avoiding inappropriate drug prescribing and on the importance of routine laboratory monitoring, particularly monitoring renal function.
Our study was limited by its design as a retrospective chart review of hospital data. Moreover, assessing ADR causation is always subjective, as is ascertaining the cause of death by means of folder review. However, the two-step assessment process, including the use of a trigger tool followed by the discussion of each case by a multi-disciplinary panel of experts offset the inherent subjectivity of the process to some extent. A strength of our study, increasing its generalizability, is that we included hospitals from three of South Africa’s nine provinces, providing a mixture of secondary and tertiary level care, thereby increasing the survey’s representativeness.
In conclusion, a larger proportion of in-hospital deaths were ADR-related in our survey than has previously been described in high income settings, Moreover, unlike the pattern in high-income countries, the drugs implicated in ADR-related deaths in our survey were mostly drugs used in the treatment of HIV and tuberculosis. The major contribution of antiretrovirals to ADR-related mortality in our setting reflects our high HIV disease burden rather than a high incidence of toxicity due to the ARV drugs used. It is concerning to note that more than half of the HIV-infected patients who died during our survey were not on antiretroviral therapy, despite having advanced HIV disease. There is a need for more studies in low- to middle-income countries characterizing ADR-related deaths and exploring appropriate systems to improve healthcare worker prescribing in order to minimize preventable drug-related harm.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare JPM, AS, CWN, NK, GM and KC had support for the submitted work from the Centers for Disease Control and Prevention (CDC), no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under terms of Cooperative Agreement Number GGH000371. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
The authors gratefully acknowledge the clinical, nursing, administrative and managerial staff at the four hospitals for their assistance and support in this study.
Contributors
The study was conceptualized and designed by KC, GM, MB, UM, AGP, DPKW and AS. Data were collected by JPM, CWN and NK, while AS managed data. KC, GM, UM, AGP and DPKW constituted the panel of experts who performed causality assessments. JPM and KC analyzed the data. JPM, KC and GM wrote the first draft of this article. All authors contributed to the final paper.
Supporting Information
Table S1
Description of sites
Table S2
Characteristics of the 56 patients considered to have died from an ADR-related cause and details of their ADRs
References
- Wester K, Jönsson AK, Spigset O, Druid H, Hägg S. Incidence of fatal adverse drug reactions: a population based study. Br J Clin Pharmacol. 2008;65:573–579. doi: 10.1111/j.1365-2125.2007.03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- Buajordet I, Ebbesen J, Erikssen J, Brørs O, Hilberg T. Fatal adverse drug events: the paradox of drug treatment. J Intern Med. 2001;250:327–341. doi: 10.1046/j.1365-2796.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- Ebbesen J, Buajordet I, Erikssen J, Brørs O, Hilberg T, Svaar H, Sandvik L. Drug-related deaths in a department of internal medicine. Arch Intern Med. 2001;161:2317–2323. doi: 10.1001/archinte.161.19.2317. [DOI] [PubMed] [Google Scholar]
- Juntti-Patinen L, Neuvonen PJ. Drug-related deaths in a university central hospital. Eur J Clin Pharmacol. 2002;58:479–482. doi: 10.1007/s00228-002-0501-2. [DOI] [PubMed] [Google Scholar]
- Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS ONE. 2009;4:e4439. doi: 10.1371/journal.pone.0004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta U, Durrheim DN, Blockman M, Kredo T, Gounden R, Barnes KI. Adverse drug reactions in adult medical inpatients in a South African hospital serving a community with a high HIV/AIDS prevalence: prospective observational study. Br J Clin Pharmacol. 2008;65:396–406. doi: 10.1111/j.1365-2125.2007.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. Geneva: UNAIDS; 2013. [Google Scholar]
- World Health Organisation. Global Tuberculosis Report. Geneva: World Health Organisation; 2013. [Google Scholar]
- Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf. 2005;28:851–870. doi: 10.2165/00002018-200528100-00003. [DOI] [PubMed] [Google Scholar]
- Rozich JD, Haraden CR, Resar RK. Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care. 2003;12:194–200. doi: 10.1136/qhc.12.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. The use of the WHO-UMC system for standardized case causality assessment [Internet]. Uppsala: The Uppsala Monitoring Centre. Available at http://who-umc.org/Graphics/24734.pdf (last accessed 17 March 2014)
- Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Zavascki AP, Fuchs SC. The need for reappraisal of AIDS score weight of Charlson comorbidity index. J Clin Epidemiol. 2007;60:867–868. doi: 10.1016/j.jclinepi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppi M, Braunschweig S, Kuenzi UP, Maibach R, Hoigné R. Incidence of lethal adverse drug reactions in the comprehensive hospital drug monitoring, a 20-year survey, 1974–1993, based on the data of Berne/St. Gallen. Eur J Clin Pharmacol. 2000;56:427–430. doi: 10.1007/s002280000158. [DOI] [PubMed] [Google Scholar]
- Qing-ping S, Xiao-dong J, Feng D, Yan L, Mei-ling Y, Jin-xiu Z, Shu-qiang Z. Consequences, measurement, and evaluation of the costs associated with adverse drug reactions among hospitalized patients in China. BMC Health Serv Res. 2014;14:73. doi: 10.1186/1472-6963-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MGADA, Pinheiro SMB, Castro JGD, Momenté VG, Pranchevicius M-CS. Adverse drug reaction monitoring: support for pharmacovigilance at a tertiary care hospital in Northern Brazil. BMC Pharmacol Toxicol. 2013;14:5. doi: 10.1186/2050-6511-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noblat ACB, Noblat LACB, Toledo LAKde, Santos P de M, Oliveira MGde, Tanajura GM, Spinola SU, Almeida JRMde. Prevalence of hospital admission due to adverse drug reaction in Salvador, Bahia. Rev Assoc Med Bras. 2011;57:42–45. [PubMed] [Google Scholar]
- Zargarzadeh AH, Emami MH, Hosseini F. Drug-related hospital admissions in a generic pharmaceutical system. Clin Exp Pharmacol Physiol. 2007;34:494–498. doi: 10.1111/j.1440-1681.2007.04600.x. [DOI] [PubMed] [Google Scholar]
- Vora MB, Trivedi HR, Shah BK, Tripathi CB. Adverse drug reactions in inpatients of internal medicine wards at a tertiary care hospital: a prospective cohort study. J Pharmacol Pharmacother. 2011;2:21–25. doi: 10.4103/0976-500X.77102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut A, Diwan A, Patel C, Patel P, Pawar A. Incidence, severity and financial burden associated with adverse drug reactions in medicine inpatients. Asian J Pharm Clin Res. 2011;4:103–111. [Google Scholar]
- World Health Organisation. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for A Public Health Approach. Geneva: World Health Organisation; 2013. [PubMed] [Google Scholar]
- Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Description of sites
Table S2
Characteristics of the 56 patients considered to have died from an ADR-related cause and details of their ADRs


