Abstract
Aims
A systematic review of the literature published in English over 10 years was undertaken in order to describe the use of electronic healthcare data in the identification of potential adverse drug reactions (ADRs) in children.
Methods
MEDLINE and EMBASE were searched using MESH headings and text words. Titles, keywords and abstracts were checked for age <18 years, potential ADRs and electronic healthcare data. Information extracted included age, data source, pharmacovigilance method, medicines and ADRs. Studies were quality assessed.
Results
From 14 804 titles, 314 had a full text review and 71 were included in the final review. Fifty were published in North America, 10 in Scandinavia. Study size ranged from less than 1000 children to more than 10 million. Sixty per cent of studies used data from one source. Comparative observational studies were most commonly reported (66.2%) with 15% using passive surveillance. Electronic healthcare data set linkage and the quality of the data source were poorly reported. ADRs were classified using the International Classification of Disease (ICD10). Multi-system reactions were most commonly studied, followed by central nervous system and mental and behavioural disorders. Vaccines were most frequently prescribed followed by corticosteroids, general anaesthetics and antidepressants.
Conclusions
Routine electronic healthcare records were increasingly reported to be used for pharmacovigilance in children. This growing and important health protection activity could be enhanced by consistent reporting of studies to improve the identification, interpretation and generalizability of the evidence base.
Keywords: adverse drug reactions, children, electronic healthcare data, systematic review
Introduction
The therapeutic use of medicines is one of the most significant contributors to adverse events associated with healthcare 1,2. The potential for adverse drug reactions (ADRs) in children is high 3 with a range of factors contributing to this vulnerability including the physiological changes which take place from birth to late adolescence, lack of evidence-based information regarding the safety and/or efficacy of medicines for paediatric use and high volume of off-label and unlicensed prescribing 4–6.
The overall incidence of ADRs in hospitalized children has been reported in two systematic reviews (2001 and 2009) to be 9.5% and 10.9%, respectively. Admissions to hospital due to ADRs were estimated to be 1.8% to 2.1%, of which up to 39.3% were considered life threatening. The overall incidence in children attending out-patient clinics was 1.0% to 1.5% 7,8.
A qualitative review in 2010 of ADRs in children highlighted the potential of data collected in national databases for detecting information about previously unknown ADRs 9 but a systematic literature review in 2010 10 noted that a mixture of methods was used in the majority of studies (58/102) including drawing on case records, computerized records, attendance at ward rounds and interviewing patients. A large proportion (31/102) relied only on case note review. Despite the recognized limitation of under-reporting, spontaneous reports of ADRs continue to play an important role 11.
Given the high numbers of ADRs reported in children, some of which are life-threatening and many of which are preventable, efficient methods of identifying ADRs as part of routine practice are a critical part of improving patient care 12,13. There are ’no gold standards’ for identifying ADRs in health systems and a range of approaches has been developed 6. The use of electronic healthcare records in the detection of ADRs has increasingly appeared to have potential and the use for ADR detection in adults has been reported 14. Electronic healthcare records include a wide range of data source types, from administrative data systems, dispensing data sets, disease registries and spontaneous reports where collated routinely.
In order to describe the use of routinely collected electronic healthcare data in the identification of potential ADRs in children we undertook a systematic review of the literature published in English over 10 years.
Methods
Literature search
Literature published in English was identified in EMBASE and MEDLINE databases between 1999 and 2010. The search was supplemented by searching reference lists of retrieved reviews. The initial search was conducted in September 2009 and updated in January 2010.
Inclusion and exclusion criteria
Papers were considered eligible for inclusion if they referred to ADRs in children (aged 0–18 years). A broad definition of ADR was used, accepting papers reporting the investigation of any potentially adverse clinical event (e.g. specific clinical signs, symptoms or diagnoses, or a clinical event such as an admission to hospital or a visit to a physician) associated with a medicinal product, including vaccines. Only papers reporting the use of routinely collected electronic healthcare data were included. ’Routine’ was defined as either a) systems that were part of the day to day recording of clinical care (e.g. medical records, prescribing, administrative data and complaints) or b) special data collections where information collection was a well established part of clinical practice (e.g. specialist registries, incident reporting systems, post-marketing surveillance).
Papers were excluded if they reported a mix of adults and children but did not separate the results. Adverse reactions or complications occurring as a result of surgical or other physical procedures, medicine withdrawal, dietary treatment and supplementation and other non-drug therapy interventions were excluded. We did not include intended or accidental poisoning/overdose or papers concerned with adverse reactions following in utero drug exposure. Papers containing insufficient information about the data sources or definition of ADRs were also excluded.
A search strategy was developed, piloted and refined in collaboration with an experienced clinical librarian. Subject headings and subheadings from the MeSH vocabulary for MEDLINE were combined using Boolean terminology with a wide range of free text terms covering four domains: adverse reactions, drug therapy, observational studies and paediatric populations (Supplemental Appendix 1). The text term ’randomized’ and MeSH term ’pregnancy’ were used to remove randomized controlled studies and reports regarding drugs prescribed during pregnancy. The results were limited to ’all children (0 to 18 years)’. A similar search strategy was applied in EMBASE.
Duplicate publications were removed. Titles and abstracts of the remaining papers were examined against the inclusion/exclusion criteria by three reviewers. This initial screening was conducted using a conservative approach. Full-text papers were retrieved if titles/abstracts appeared to meet the eligibility criteria or if the decision could not be made based on the titles and abstracts alone. Assessment of the full texts of each retrieved paper was undertaken independently by two reviewers using the same criteria (percentage agreement 81%). Any disagreements about inclusion were resolved through discussion (19% of papers). Assessment by a third reviewer to resolve disagreements was not required.
Data extraction
Data extraction was carried out by two reviewers independently using a specifically designed extraction form. Information extracted included age, data source, pharmacovigilance method, medicines and ADRs. Particular attention was paid to the quality of reporting the data source and ADRs. A simple checklist was adapted from guidelines for reporting data linkage studies and selection of databases for pharmacoepidemiology 15,16. It included the following key quality issues: ethics review, data entry procedures, data quality assurance, data linkage methods and quality assurance, denominator information and completeness of exposure and outcome data.
Box 1.
Pharmacovigilance methods: WHO classification (adapted) 6
Passive surveillance
Spontaneous reports*
’an unsolicited communication that describes one or more adverse drug reactions (ADRs) in a patient who was given one or more medicinal products and that does not derive from a study or any organized data collection scheme’
Case series
Stimulated reporting
’methods have been used to encourage and facilitate reporting by health professionals in specific situations (e.g. in hospital settings) for new products or for limited time periods’Active surveillance’seeks to ascertain the exact number of adverse events via a continuous pre-organized process’
Sentinel site
’reviewing medical records or interviewing patients and/or physicians in a sample of sentinel sites to ensure that complete and accurate data on reported adverse events are collected’
Medicine event monitoring
’cohort-based and prospective and observational’ using active follow up through regular surveys.
Registries – based on disease or medicine exposure
Comparative observational studies’There are a number of observational study designs that are useful in validating signals’
Cross sectional surveys
Case-control
Cohort
’patients for case–control studies and cohort studies can be identified from large automated databases or from data collected specifically for the study at hand’.*Data-mining, proportional reporting ratios, Bayesian techniques included under spontaneous reports as methods for signal detection
The findings of the review were summarized narratively and key characteristics of the studies tabulated. Pharmacovigilance methods were categorized as passive surveillance, active surveillance or comparative observational studies 6 as shown in Box 1. The classification of data sources is summarized in Supplementary Table E1. Information about the size and population coverage was tabulated and summarized graphically. Medicines used in the studies were classified according to the British National Formulary (BNF) categories. If more than three classes of medication were reported in a single study, ’various drug groups’ was recorded. ADRs were classified using the International Classification of Diseases (ICD-10). If more than three ICD classes were reported, the ADRs were classified as ’multisystem’.
Results
Included studies
From a total of 14 804 titles retrieved by the initial electronic search strategy, 314 studies were identified for full text review. Of these, 243 papers were excluded because they did not meet the inclusion criteria or provide adequate information about data sources (n = 23) and ADRs. Seventy-one papers were included in the final review (Figure1).
Figure 1.
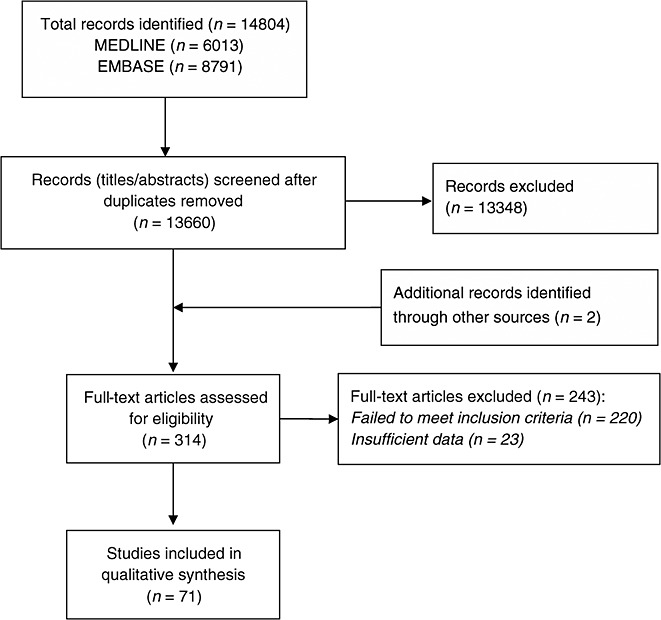
PRISMA flow diagram summarizing study selection process
Characteristics of included studies
The main characteristics of the papers are summarized in Supplemental Digital Content Table E2 17–87. The number of published studies grew rapidly since 1999 with one third of the papers (n = 23, 32.4%) being published within the last 2 years of the review. Research was dominated by North America with 46 (64.8%) of the studies carried out in the USA, four in Canada and one based in both countries. Scandinavia contributed 10 (14.1%) and the UK, four papers (5.6%). Age ranged from birth to 18 years, with five papers focusing on neonates (first 28 days of life) exclusively (7.0%).
Pharmacovigilance methods
A range of pharmacovigilance methods was observed with six studies adopting more than one methodological approach. Comparative observational methods were the most commonly reported (n = 47, 66.2%), with 15 (21.1%) reporting passive surveillance methods and only three (4.0%) reporting active surveillance using routine healthcare data.
The predominant study design within comparative observational methodology was the cohort study (n = 38, 53.5%). The remaining studies used case–control (n = 5, 7%), cross-sectional (n = 1) or a combination of designs (n = 3, 4.0%).
Passive surveillance methods, in many cases, used national adverse event reporting schemes including some specific to the medication type, such as vaccines. Most studies adopted descriptive epidemiological methods, reporting the frequency of various potential ADRs. Some used information from prescribing or dispensing data to estimate the size of the " at risk" population thereby allowing event rates to be approximated. Data mining methods were applied to identify potential ADR signals.
In the studies reporting active surveillance methods, registers, as part of routine care, were kept for all patients taking specific medicines and ADR information was sought proactively by linkage to other healthcare data or by proactive follow-up and recording of ADRs in the register.
Data sources
A total of 68 different data sources were identified in the 71 studies which met the inclusion criteria (Supplemental Table E3). The majority of studies (n = 42, 59.1%) used data from a single data source, such as a financial reimbursement system (n = 14, 19.7%), hospital database (n = 11, 15.5%), or spontaneous reporting system (n = 12, 16.9%).
Studies based on more than one data source (n = 29, 40.9%) often included the use of registries, financial reimbursement systems and spontaneous reporting systems.
More than half of the studies which used multiple data sources used data linkage (n = 15, 51.2%), 10 (n = 10, 34.5%) studies used unlinked data and in the remaining four (n = 4, 13.8%) studies it could not be ascertained from the reported methods whether the data sources were linked. Where no formal linkage was undertaken, the multiple datasets were used to describe potential ecological associations or to provide estimates of the exposed population to accompany ADR reports in another data source.
Most of the 68 different data sources reported in the included studies were representative at a single country level (n = 46, 67.6%). Eleven (n = 11, 16.2%) were representative at regional level or above. With regard to the size of the data sources, information on 32 out of 68 (47.1%) was not reported within the included published paper and had to be obtained through extra searching. Data sources were reported either based on the population covered (n = 54; 79.4%) or the number of events reported per year/within the study period (n = 15, 22.1%) (Figure2 and Supplemental Table E3).
Figure 2.
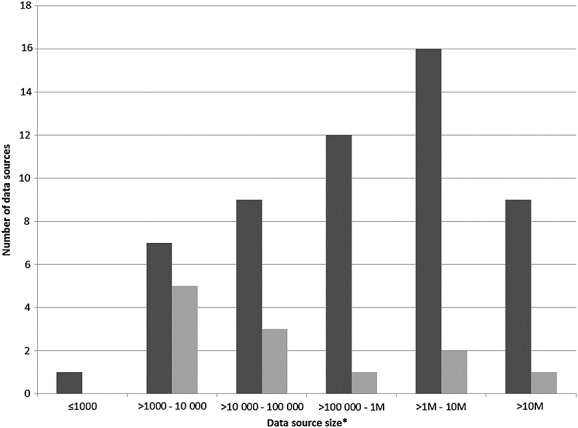
Size of the 68 data sources reported by population covered or the number of events reported per year/within the study period. M, Millions *two data sources did not report size.  , Population;
, Population;  , Records/Reports
, Records/Reports
Quality of reporting data sources
The amount of information and level of detail reported for the data source varied greatly across studies (Figure3). Ethics permissions were well reported. Most studies used unconsented data (e.g. without individual patient consent for the data to be used in research in general or specifically in a given research project). Data entry methods were poorly reported in most of the studies with many reliant on data collection as part of routine clinical care but little information about who entered the data. Very few commented on the completeness or quality assurance of the source data. Validation against clinical registries or other sources were noted by some authors, in the main relating to well established administrative and healthcare databases used regularly for research.
Figure 3.
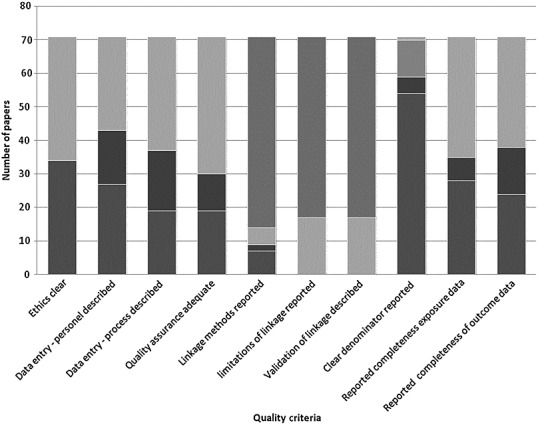
Summary of the quality assessment.  , Not applicable;
, Not applicable;  , Not reported;
, Not reported;  , Did not meet criteria;
, Did not meet criteria;  , Partially met criteria;
, Partially met criteria;  , Met criteria
, Met criteria
Linkage methods were poorly described and the limitations were rarely quantified. None of the studies reported on whether deterministic or probabilistic matching was undertaken. The completeness and accuracy of the linkage identifiers or the validation checks undertaken to ensure robust linkage were also poorly reported.
Some important limitations were noted by the reviewers, particularly with the passive surveillance methods reliant on potential ADRs being reported by various professional groups and patients to a central registry. The relationship between reporting and various factors including publicity in relation to an ADR were acknowledged. The lack of a robust denominator (how many were exposed) was recognized but largely complete pharmacy dispensing data in some countries allowed this limitation to be overcome. ADR recording in routine data was noted, by many authors, to be poor. The application of disease or ADR definitions and coding of conditions was not uniform within and between studies. The impact this had on generalizability was recognized. The need for high quality information about the date of onset of symptoms in relation to the timing of medicine use was also noted as a limitation by some. However, there was a consistent recognition of the importance of electronic healthcare data as a mechanism to follow up large numbers of medicine users over long periods of time in a real life care setting. This was considered to be critical for both good governance of the introduction of new medicines for long term ADR monitoring and for rare ADR detection.
ADRs and therapeutic groups of medicines studied in routine healthcare data
The definition of ADR varied between studies with some including all ADRs and adverse events, and others restricted to serious, life threatening ADRs or specific clinical outcomes. The studies reported the investigation of a spectrum of potential ADRs involving different organ systems. In 23 (32.4%) studies, electronic healthcare data were used to identify potential ADRs across multiple organ systems (Supplementary Table E2). Where studies focused on three or less ICD classes, the most commonly studied were mental/behavioural disorders (n = 10, 14.1%), central nervous system (n = 10, 14.1%) and digestive system (n = 8, 11.3%). One study reported using laboratory results.
Almost 40% of included studies (n = 27) were concerned with investigation of potential ADRs to vaccines (Figure4). Antidepressants, antipsychotics and other central nervous system (CNS) drugs were the second most commonly studied therapeutic class (n = 13, 18.3%), followed by corticosteroids (n = 7, 9.9%), antibiotics and antivirals (n = 7, 9.9%) and general anaesthetics (n = 7; 9.9%).
Figure 4.
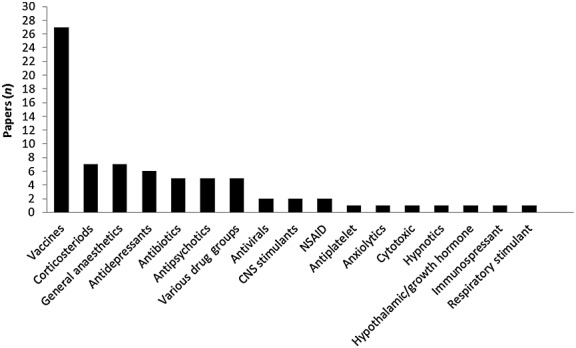
Therapeutic groups of medicines studied for potential ADRs in routine electronic healthcare data
Discussion
In this systematic review, we identified many pharmacovigilance studies in children using routine electronic healthcare data. The number of studies increased over the period of the review and reflected pharmacovigilance activity in many countries in particular North America. A wide variety of routine electronic healthcare data sets were used. Traditional, passive ADR reporting databases were used in 17% of the studies but there was also substantial evidence of the use of single and linked administrative datasets and specialist registries to detect ADRs. Methods such as data mining and comparative observational studies were applied to a wide range of data sources but signal generation, as an early alert to potential ADRs, still very much relied on passive reporting to ADR registries such as the UK Yellow Card Scheme or the US Vaccine Adverse Event reporting System.
This review, by focusing on ADR studies in children using electronic healthcare data, highlights the potential for proactive surveillance for ADRs utilizing the growth in digital medication data and the ability to link to health event data. This approach is complimentary to passive surveillance programmes. Our review also highlights the need for better quality reporting ADR literature.
Proactive ADR surveillance
The Erice Manifesto for Global Reform of the Safety of Medicines in Patient Care (2006) documented challenges in developing pharmacovigilance from a largely reactive activity to proactive study in routine clinical practice. It highlighted the need to develop new ways of collecting, analyzing and communicating information in relation to drug safety and the importance of quality assured research in databases and registries. Despite the WHO making the case for integrated pharmacovigilance as an essential component of public health programmes, we found little reporting of the integration of active surveillance using routine administrative, healthcare or laboratory data to generate potential signals 88.
Recognizing ADR literature
In this review we used a sensitive search strategy and systematically reviewed a large number of titles and abstracts seeking relevant studies, approaches similar to other reviews in this area 10,89. Using search terms, either MeSH headings or as free text, proved of limited value in focusing a search strategy without losing key references. In general, studies did not clearly identify that they were studying ADRs. The methodological approach of the study was also rarely reported clearly in the title, keywords or abstract. Guidance for the reporting of other study designs now clearly states the importance of including a statement about study design and aim in the title to improve the ability to retrieve relevant evidence from bibliographic databases 90 and similar guidance would benefit the reporting of studies of ADRs.
ADR detection methods
The WHO classifies ADR detection methods based on data collection procedures as well as study methodology (Box 1) 6. Here, we found a more mixed picture, particularly with the growth in data linkage of passive spontaneous reports and active registries to administrative data. The range of analytical methodologies where then determined not by the approach to gathering the data, but by the research question with descriptive epidemiology through to data mining for signal generation being applied as appropriate. The WHO classification no longer well categorizes the way researchers are approaching ADR studies.
Quality of reporting
Despite restricting our review to studies with sufficient information about the data sources, we still found substantial variation in the detail and quality of reporting. There was particularly limited information recorded about the robustness and validity of the datasets. Michel et al. 91, reviewing methods for assessing the nature and scale of harm caused by the health system, previously drew attention to the importance of the reliability of healthcare data and emphasized in particular the need for information about the completeness of data in medical records.
Bohensky et al. 16 recently published guidelines for the reporting of data linkage studies. Here, the included studies performed poorly against such criteria. Box 2 summarizes recommendations for authors reporting pharmacovigilance studies using routine electronic healthcare data.
Box 2.
Recommendations for authors reporting pharmacovigilance studies using routine electronic healthcare data.Title and abstractIdentify study as pharmacovigilance or ADR detection using electronic healthcare data.
Data source detailsIn the methods, describe the data source(s) including details of:
Purpose of the data collection
Who entered the data
Data entry process
Quality checks on data entry
Linkage
In the methods, describe linkage process including:
Methods for linkage
Quality of linkage and checks made to ensure validity
ADR detection method
In the methods, report whether
Active or passive surveillance approach used to gather ADR data
Signal detection, data mining, observational reporting or comparative observational analysis to identify ADRs
Exposure details
In the methods, describe the exposure:
Definition
Completeness of recording
ADR details:
In the methods, describe the ADR:
Definition
Completeness of recording
Denominator definition
In the methods, describe who was included in the dataset and any baises
Ethical review
The ethical review process should be reported
Strengths and limitations of the review
We report a large systematic review of the methods and electronic healthcare data sources used for ADR detection in children but there were a number of limitations to our review. We undertook a sensitive search but this resulted in a large number of titles and abstracts for review. As a result, only one researcher reviewed each title. To minimize inconsistencies, we used detailed inclusion and exclusion criteria and adopted a conservative approach of including studies for full text review where uncertainty existed. We know that other studies of ADRs in children using electronic routine healthcare data have been published but were not identified in our review often because they were reported as the association between a specific medicine and a disease or symptom and as a result were not clearly identifiable as a study of ADRs.
Post-marketing surveillance using electronic healthcare data
Surveillance of drugs in the post-marketing phase since the thalidomide disaster in the 1960s 92 has depended largely on analyses of spontaneous reports to identify new adverse drug events and of observational healthcare studies to confirm or refute suspected adverse events. The withdrawal of rofecoxib in 2004 reinforced again the importance of adverse drug event monitoring to identify as early as possible serious unwanted adverse effects of drugs 93.
The potential of using routine electronic healthcare data to identify adverse events has increasingly been recognised and during the period of this review significant progress has been made in North America and Europe 94,95.
Conclusion
This systematic literature review identified a large number of routine electronic healthcare datasets worldwide used to study a wide range of medicines and potential ADRs in children. The increasing utility of routine electronic healthcare datasets for pharmacovigilance in children was evident and this growing and important health protection activity could be enhanced by consistent reporting of studies to improve the identification, interpretation and generalizability of the evidence base. Titles, key words and abstracts rarely identified the methodology. A clear classification system should be developed to aid consistent definition of ADR detection methods. Published guidelines should be used for reporting data linkage studies. Reporting of key quality issues should be improved. There is a wealth of electronic healthcare data and realisation of its potential could contribute significantly to pharmacovigilance.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare CB, PH, NTM had support (as part of the CHIMES study) from the Scottish Government, Chief Scientist Office [Grant Number: ARPG/07/4] and Lily Charlton Trust for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
We would like to acknowledge the methodological and clinical contribution of the Child Medical Records for Safer Medicines (CHIMES) study team to the design of the review protocol.
Supporting Information
Supporting info item
Supporting info item
Supporting info item
References
- Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, Hebert L, Newhouse JP, Weiler PC, Hiatt H. The nature of adverse events in hospitalized patients-results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- Thomas EJ, Studdert DM, Burstin HR, Orav EJ, Zeena T, Williams EJ, Howard KM, Weiler PC, Brennan TA. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261–71. doi: 10.1097/00005650-200003000-00003. [DOI] [PubMed] [Google Scholar]
- WHO. 2002. Safety of Medicines - A Guide to Detecting and Reporting Adverse Drug Reactions - Why Health Professionals Need to Take Action. WHO/EDM/QSM/2002.2.. Geneva, World Health Organization.
- Horen B, Montastruc JL, Lapeyre-Mestre M. Adverse drug reactions and off-label drug use in paediatric outpatients. Br J Clin Pharmacol. 2002;54:665–70. doi: 10.1046/j.1365-2125.2002.t01-3-01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impicciatore P, Mohn A, Chiarelli F, Pandolfini C, Bonati M. Adverse drug reactions to off-label drugs on a paediatric ward: an Italian prospective pilot study. Paediatric and Perinatal Drug Therapy. 2002;5:19–24. [Google Scholar]
- WHO. Promoting safety of medicines for children. Geneva, Switzerland: WHO Press, World Health Organization, 2007.
- Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52:77–83. doi: 10.1046/j.0306-5251.2001.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavenna A, Bonati M. Adverse drug reactions in childhood: a review of prospective studies and safety alerts. Arch Dis Child. 2009;94:724–8. doi: 10.1136/adc.2008.154377. [DOI] [PubMed] [Google Scholar]
- Aagaard L, Christensen A, Hansen EH. Information about adverse drug reactions reported in children: a qualitative review of empirical studies. Br J Clin Pharmacol. 2010;70:481–91. doi: 10.1111/j.1365-2125.2010.03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth RMD, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R, Williamson P. Adverse drug reactions in children - a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0024061. : e24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32:19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- Al Tajir GK, Kelly WN. Epidemiology, comparative methods of detection, and preventability of adverse drug events. Ann Pharmacother. 2005;39:1169–74. doi: 10.1345/aph.1E559. [DOI] [PubMed] [Google Scholar]
- Kunac DL, Kennedy J, Austin N, Reith D. Incidence, preventability, and impact of adverse drug events (ADEs) and potential ADEs in hospitalized children in New Zealand: A prospective observational cohort study. Pediatric Drugs. 2009;11:153–60. doi: 10.2165/00148581-200911020-00005. [DOI] [PubMed] [Google Scholar]
- Suling M, Pigeot I. Signal detection and monitoring based on longitudinal healthcare data. Pharmaceutics. 2012;4:607–40. doi: 10.3390/pharmaceutics4040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GC, Sauer B, Bourke A, Brown JS, Reynolds MW, Casale RL. Guidelines for good database selection and use in pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2012;21:1–10. doi: 10.1002/pds.2229. [DOI] [PubMed] [Google Scholar]
- Bohensky MA, Jolley D, Sundararajan V, Evans S, Ibrahim J, Brand C. Development and validation of reporting guidelines for studies involving data linkage. Aust N Z J Public Health. 2011;35:486–9. doi: 10.1111/j.1753-6405.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- Andrews N, Miller E, Grant A, Stowe J, Osborne V, Taylor B. Thimerosal exposure in infants and developmental disorders: a retrospective cohort study in the United kingdom does not support a causal association. Pediatrics. 2004;114:584–91. doi: 10.1542/peds.2003-1177-L. [DOI] [PubMed] [Google Scholar]
- Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (2) two populations of patients with perforations. J Perinatol. 2006;26:185–8. doi: 10.1038/sj.jp.7211439. [DOI] [PubMed] [Google Scholar]
- Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J Perinatol. 2006;26:93–9. doi: 10.1038/sj.jp.7211429. [DOI] [PubMed] [Google Scholar]
- Baxter AL, Mallory MD, Spandorfer PR, Sharma S, Freilich SH, Cravero J. and Pediatric Sedation Research, C. Etomidate versus pentobarbital for computed tomography sedations: report from the Pediatric Sedation Research Consortium. Pediatr Emerg Care. 2007;23:690–5. doi: 10.1097/PEC.0b013e3181558d5c. [DOI] [PubMed] [Google Scholar]
- Black S, Shinefield H, Ray P, Lewis E, Hansen J, Schwalbe J, Coplan P, Sharrar R, Guess H. Postmarketing evaluation of the safety and effectiveness of varicella vaccine. Pediatr Infect Dis J. 1999;18:1041–46. doi: 10.1097/00006454-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Blumentals WA, Song X. The safety of oseltamivir in patients with influenza: Analysis of healthcare claims data from six influenza seasons. Med Gen Med Medscape General Medicine. 2007;9 :Arte. [PMC free article] [PubMed] [Google Scholar]
- Brunlof G, Tukukino C, Wallerstedt SM. Individual case safety reports in children in commonly used drug groups - signal detection. BMC Clin Pharmacol. 2008;8:1. doi: 10.1186/1472-6904-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casscells SW. Granger E, Kress AM, Linton A. The association between oseltamivir use and adverse neuropsychiatric outcomes among TRICARE beneficiaries, ages 1 through 21 years diagnosed with influenza. Int J Adolesc Med Health. 2009;21:79–89. doi: 10.1515/ijamh.2009.21.1.79. [DOI] [PubMed] [Google Scholar]
- Center KJ, Hansen JR, Lewis E, Fireman BH, Hilton B. Lack of association of Kawasaki disease after immunization in a cohort of infants followed for multiple autoimmune diagnoses in a large, phase-4 observational database safety study of 7-valent pneumococcal conjugate vaccine: Lack of association between Kawasaki disease and seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2009;28:438–40. doi: 10.1097/INF.0b013e318196934a. [DOI] [PubMed] [Google Scholar]
- Chaves SS, Haber P, Walton K, Wise RP, Izurieta HS, Schmid DS, Seward JF. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197:S170–7. doi: 10.1086/522161. [DOI] [PubMed] [Google Scholar]
- Chong E, Greenspan J, Kirkby S, Culhane J, Dysart K. Changing use of surfactant over 6 years and its relationship to chronic lung disease. Pediatrics. 2008;122:e917–21. doi: 10.1542/peds.2007-3193. [DOI] [PubMed] [Google Scholar]
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117:67–74. doi: 10.1542/peds.2005-0179. [DOI] [PubMed] [Google Scholar]
- Clarkson A, Choonara I, Marcovitch H. Surveillance for fatal suspected adverse drug reactions in the UK. Arch Dis Child. 2002;87:462–7. doi: 10.1136/adc.87.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Black S, Shinefield H, Mahoney L, Zavitkovsky A, Lewis E, Nikas A, Guess H, Coplan P. Post-marketing evaluation of the short term safety of COMVAX. Vaccine. 2004;22:536–43. doi: 10.1016/j.vaccine.2003.06.001. [DOI] [PubMed] [Google Scholar]
- De Vries TW, Langen-Wouterse JJ, van Puijenbroek E, Duiverman EJ, De Jong-Van DB. Reported adverse drug reactions during the use of inhaled steroids in children with asthma in the Netherlands. Eur J Clin Pharmacol. 2006;62:343–6. doi: 10.1007/s00228-006-0102-6. [DOI] [PubMed] [Google Scholar]
- De Vries TW, Langen-Wouterse JJ, De Jong-Van DB, Duiverman EJ. Hypertrichosis as a side effect of inhaled steroids in children. Pediatr Pulmonol. 2007;42:370–3. doi: 10.1002/ppul.20589. [DOI] [PubMed] [Google Scholar]
- Deeks SL, Clark M, Scheifele DW, Law BJ, Dawar M, Ahmadipour N, Walop W, Ellis CE, King A. Serious adverse events associated with bacille Calmette-Guerin vaccine in Canada. Pediatr Infect Dis J. 2005;24:538–41. doi: 10.1097/01.inf.0000164769.22033.2c. [DOI] [PubMed] [Google Scholar]
- Dharnidharka VR, Ho PL, Stablein DM, Harmon WE, Tejani AH. Mycophenolate, tacrolimus and posttransplant lymphoproliferative disorder: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant. 2002;6:396–9. doi: 10.1034/j.1399-3046.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- Donahue JG, Kieke BA, Yih WK, Berger NR, McCauley JS, Baggs J, Zangwill KM, Baxter R, Eriksen EM, Glanz JM, Hambidge SJ, Klein NP, Lewis EM, Marcy SM, Naleway AL, Nordin JD, Ray P, Belongia EA. Varicella vaccination and ischemic stroke in children: Is there an association? Pediatrics. 2009;123:e228–34. doi: 10.1542/peds.2008-2384. [DOI] [PubMed] [Google Scholar]
- France EK, Glanz JM, Xu S, Davis RL, Black SB, Shinefield HR, Zangwill KM, Marcy SM, Mullooly JP, Jackson LA, Chen R. Safety of the trivalent inactivated influenza vaccine among children: A population-based study. Arch Pediatr Adolesc Med. 2004;158:1031–6. doi: 10.1001/archpedi.158.11.1031. [DOI] [PubMed] [Google Scholar]
- France EK, Glanz J, Xu S, Hambidge S, Yamasaki K, Black SB, Marcy M, Mullooly JP, Jackson LA, Nordin J, Belongia EA, Hohman K, Chen RT, Davis R. Risk of immune thrombocytopenic purpura after measles-mumps-rubella immunization in children. Pediatrics. 2008;121:e687–92. doi: 10.1542/peds.2007-1578. [DOI] [PubMed] [Google Scholar]
- Geier DA, King PG, Sykes LK, Geier MR. RotaTeq vaccine adverse events and policy considerations. Med Sci Monit. 2008;14:H9–16. [PubMed] [Google Scholar]
- Gold MS, Noonan S, Osbourn M, Precepa S, Kempe AE. Local reactions after the fourth dose of acellular pertussis vaccine in South Australia. Med J Aust. 2003;179:191–4. doi: 10.5694/j.1326-5377.2003.tb05497.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Heydrich J, Raches D, Wilens TE, Leichtner A, Mezzacappa E. Retrospective study of hepatic enzyme elevations in children treated with olanzapine, divalproex, and their combination. J Am Acad Child Adolesc Psychiatry. 2003;42:1227–33. doi: 10.1097/00004583-200310000-00014. [DOI] [PubMed] [Google Scholar]
- Haber P, Patel M, Izurieta HS, Baggs J, Gargiullo P, Weintraub E, Cortese M, Braun MM, Belongia EA, Miller E, Ball R, Iskander J, Parashar UD. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006 to September 25, 2007. Pediatrics. 2008;121:1206–12. doi: 10.1542/peds.2007-3793. [DOI] [PubMed] [Google Scholar]
- Hambidge SJ, Glanz JM, France EK, McClure D, Xu S, Yamasaki K, Jackson L, Mullooly JP, Zangwill KM, Marcy SM, Black SB, Lewis EM, Shinefield HR, Belongia E, Nordin J, Chen RT, Shay DK, Davis RL, DeStefano F. Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA. 2006;296:1990–7. doi: 10.1001/jama.296.16.1990. [DOI] [PubMed] [Google Scholar]
- Haukka J, Arffman M, Partonen T, Sihvo S, Elovainio M, Tiihonen J, Lonnqvist J, Keskimaki I. Antidepressant use and mortality in Finland: a register-linkage study from a nationwide cohort. Eur J Clin Pharmacol. 2009;65:715–20. doi: 10.1007/s00228-009-0616-9. [DOI] [PubMed] [Google Scholar]
- Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Association between thimerosal-containing vaccine and autism. J Am Med Assoc. 2003;290:1763–6. doi: 10.1001/jama.290.13.1763. [DOI] [PubMed] [Google Scholar]
- Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Childhood Vaccination and Type 1 Diabetes. N Engl J Med. 2004;350:1398–404. doi: 10.1056/NEJMoa032665. [DOI] [PubMed] [Google Scholar]
- Hviid A, Svanstrom H. Antibiotic use and type 1 diabetes in childhood. Am J Epidemiol. 2009;169:1079–84. doi: 10.1093/aje/kwp038. [DOI] [PubMed] [Google Scholar]
- Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973-2002. Int J Cancer. 2007;121:2233–40. doi: 10.1002/ijc.22827. [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Ackerson BK, Sy LS, Tran TN, Jones TL, Yao JF, Xie F, Craig Cheetham T, Saddier P. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine. 2009;27:4656–61. doi: 10.1016/j.vaccine.2009.05.056. [DOI] [PubMed] [Google Scholar]
- Jerrell JM, McIntyre RS. Adverse events in children and adolescents treated with antipsychotic medications. Hum Psychopharmacol. 2008;23:283–90. doi: 10.1002/hup.932. [DOI] [PubMed] [Google Scholar]
- Jerrell JM, Hwang TL, Livingston TS. Neurological adverse events associated with antipsychotic treatment in children and adolescents. J Child Neurol. 2008;23:1392–9. doi: 10.1177/0883073808319070. [DOI] [PubMed] [Google Scholar]
- Jerrell JM, McIntyre RS. Cardiovascular and neurological adverse events associated with antidepressant treatment in children and adolescents. J Child Neurol. 2009;24:297–304. doi: 10.1177/0883073808323523. [DOI] [PubMed] [Google Scholar]
- Karian VE, Burrows PE, Zurakowski D, Connor L, Mason KP. Sedation for pediatric radiological procedures: analysis of potential causes of sedation failure and paradoxical reactions. Pediatr Radiol. 1999;29:869–73. doi: 10.1007/s002470050715. [DOI] [PubMed] [Google Scholar]
- Kemp SF, Kuntze J, Attie KM, Maneatis T, Butler S, Frane J, Lippe B. Efficacy and safety results of long-term growth hormone treatment of idiopathic short stature. J Clin Endocrinol Metab. 2005;90:5247–53. doi: 10.1210/jc.2004-2513. [DOI] [PubMed] [Google Scholar]
- Kimland E, Rane A, Ufer M, Panagiotidis G. Paediatric adverse drug reactions reported in Sweden from 1987 to 2001. Pharmacoepidemiology Drug Saf. 2005;14:493–9. doi: 10.1002/pds.1121. [DOI] [PubMed] [Google Scholar]
- Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest. 2007;131:1753–9. doi: 10.1378/chest.06-3008. [DOI] [PubMed] [Google Scholar]
- Kuno-Sakai H, Kimura M. Removal of gelatin from live vaccines and DTaP-an ultimate solution for vaccine-related gelatin allergy. Biologicals. 2003;31:245–9. doi: 10.1016/s1045-1056(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Lewis E, Shinefield HR, Woodruff BA, Black SB, DeStefano F, Chen RT, Ensor R. Datalink W. Pediatr Infect Dis J. 2001;20:1049–54. doi: 10.1097/00006454-200111000-00009. , Vaccine Safety. Safety of neonatal hepatitis B vaccine administration. [DOI] [PubMed] [Google Scholar]
- Makela A, Nuorti JP, Peltola H. Neurologic disorders after measles-mumps-rubella vaccination. Pediatrics. 2002;110:957–63. doi: 10.1542/peds.110.5.957. [DOI] [PubMed] [Google Scholar]
- Maltz LA, Gauvreau K, Connor JA, Jenkins KJ. Clopidogrel in a pediatric population: prescribing practice and outcomes from a single center. Pediatr Cardiol. 2009;30:99–105. doi: 10.1007/s00246-008-9289-x. [DOI] [PubMed] [Google Scholar]
- Mancini J, Thirion X, Masut A, Saillard C, Pradel V, Romain F, Pastor MJ, Coudert C, Micallef J. Anxiolytics, hypnotics, and antidepressants dispensed to adolescents in a French region in 2002. Pharmacoepidemiology Drug Saf. 2006;15:494–503. doi: 10.1002/pds.1258. [DOI] [PubMed] [Google Scholar]
- Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, Patrick DM, Bowie WR, Fitzgerald JM. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003–10. doi: 10.1542/peds.2008-1146. [DOI] [PubMed] [Google Scholar]
- Mason KP, Zurakowski D, Karian VE, Connor L, Fontaine PJ, Burrows PE. Sedatives used in pediatric imaging: comparison of IV pentobarbital with IV pentobarbital with midazolam added. AJR Am J Roentgenol. 2001;177:427–30. doi: 10.2214/ajr.177.2.1770427. [DOI] [PubMed] [Google Scholar]
- Mason KP, Sanborn P, Zurakowski D, Karian VE, Connor L, Fontaine PJ, Burrows PE. Superiority of pentobarbital versus chloral hydrate for sedation in infants during imaging. Radiology. 2004;230:537–42. doi: 10.1148/radiol.2302030107. [DOI] [PubMed] [Google Scholar]
- Mason KP, Zurakowski D, Connor L, Karian VE, Fontaine PJ, Sanborn PA, Burrows PE. Infant sedation for MR imaging and CT: oral versus intravenous pentobarbital. Radiology. 2004;233:723–28. doi: 10.1148/radiol.2333031872. [DOI] [PubMed] [Google Scholar]
- Mason KP, Zurakowski D, Zgleszewski SE, Robson CD, Carrier M, Hickey PR, DiNardo JA. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008v;18:403–11. doi: 10.1111/j.1460-9592.2008.02468.x. [DOI] [PubMed] [Google Scholar]
- Mason KP, Padua H, Fontaine PJ, Zurakowski D. Radiologist-supervised ketamine sedation for solid organ biopsies in children and adolescents. AJR Am J Roentgenol. 2009;192:1261–5. doi: 10.2214/AJR.08.1743. [DOI] [PubMed] [Google Scholar]
- McDonald KL, Huq SI, Lix LM, Becker AB, Kozyrskyj AL. Delay in diphtheria, pertussis, tetanus vaccination is associated with a reduced risk of childhood asthma. J Allergy Clin Immunology. 2008;121:626–31. doi: 10.1016/j.jaci.2007.11.034. [DOI] [PubMed] [Google Scholar]
- McMahon AW, Iskander JK, Haber P, Braun MM, Ball R. Inactivated influenza vaccine (IIV) in children <2 years of age: examination of selected adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) after thimerosal-free or thimerosal-containing vaccine. Vaccine. 2008;26:427–9. doi: 10.1016/j.vaccine.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Moore TJ, Weiss SR, Kaplan S, Blaisdell CJ. Reported adverse drug events in infants and children under 2 years of age. Pediatrics. 2002;110:e53. doi: 10.1542/peds.110.5.e53. [DOI] [PubMed] [Google Scholar]
- Niu MT, Erwin DE, Braun MM. Data mining in the US Vaccine Adverse Event Reporting System (VAERS): early detection of intussusception and other events after rotavirus vaccination. Vaccine. 2001;19:4627–34. doi: 10.1016/s0264-410x(01)00237-7. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults: A case-control study. Arch Gen Psychiatry. 2006;63:865–72. doi: 10.1001/archpsyc.63.8.865. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC. A case-control study of antidepressants and attempted suicide during early phase treatment of major depressive episodes. J Clin Psychiatry. 2008;69:425–32. doi: 10.4088/jcp.v69n0313. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Zito JM, Gardner JF. Pemoline hepatotoxicity and postmarketing surveillance. J Am Acad Child Adolesc Psychiatry. 2001;40:622–9. doi: 10.1097/00004583-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Schirm E, Tobi H, van Puijenbroek EP, Monster-Simons MH, De Jong-Van DenBerg. Reported adverse drug reactions and their determinants in Dutch children outside the hospital. Pharmacoepidemiology & Drug Saf. 2004;13:159–65. doi: 10.1002/pds.843. [DOI] [PubMed] [Google Scholar]
- Schlienger RG, Jick SS, Meier CR. Inhaled corticosteroids and the risk of fractures in children and adolescents. Pediatrics. 2004;114:469–73. doi: 10.1542/peds.114.2.469. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on RotaShield and intussusception: the role of age at the time of vaccination. J Infect Dis. 2005;192:S36–43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- Sondergard L, Kvist K, Andersen PK, Kessing LV. Do antidepressants precipitate youth suicide?: a nationwide pharmacoepidemiological study. Eur Child Adolesc Psychiatry. 2006;15:232–40. doi: 10.1007/s00787-006-0527-6. [DOI] [PubMed] [Google Scholar]
- Szarfman A, Tonning JM, Levine JG, Doraiswamy PM. Atypical antipsychotics and pituitary tumors: a pharmacovigilance study. Pharmacotherapy. 2006;26:748–58. doi: 10.1592/phco.26.6.748. [DOI] [PubMed] [Google Scholar]
- van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Min Res. 2003;18:913–8. doi: 10.1359/jbmr.2003.18.5.913. [DOI] [PubMed] [Google Scholar]
- Verstraeten T, Baughman AL, Cadwell B, Zanardi L, Haber P, Chen RT. Vaccine adverse event reporting system. Enhancing vaccine safety surveillance: a capture-recapture analysis of intussusception after rotavirus vaccination. Am J Epidemiol. 2001;154:1006–12. doi: 10.1093/aje/154.11.1006. [DOI] [PubMed] [Google Scholar]
- Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004;292:351–7. doi: 10.1001/jama.292.3.351. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Yao Q, Horbar JD, Carpenter JH, Lee SK, Ohlsson A. Changes in the use of postnatal steroids for bronchopulmonary dysplasia in 3 large neonatal networks. Pediatrics. 2006;118:e1328–35. doi: 10.1542/peds.2006-0359. [DOI] [PubMed] [Google Scholar]
- Wattigney WA, Mootrey GT, Braun MM, Chen RT. Surveillance for poliovirus vaccine adverse events, 1991 to 1998: impact of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Pediatrics. 2001;107:E83. doi: 10.1542/peds.107.5.e83. [DOI] [PubMed] [Google Scholar]
- Winterstein AG, Gerhard T, Shuster J, Johnson M, Zito JM, Saidi A. Cardiac safety of central nervous system stimulants in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2007;120:e1494–501. doi: 10.1542/peds.2007-0675. [DOI] [PubMed] [Google Scholar]
- Woods SW, Martin A, Spector SG, McGlashan TH. Effects of development on olanzapine-associated adverse events. J Am Acad Child Adolesc Psychiatry. 2002;41:1439–46. doi: 10.1097/00004583-200212000-00015. [DOI] [PubMed] [Google Scholar]
- Young HA, Geier DA, Geier MR. Thimerosal exposure in infants and neurodevelopmental disorders: an assessment of computerized medical records in the Vaccine Safety Datalink. J Neurol Sci. 2008;271:110–18. doi: 10.1016/j.jns.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Matsumura Y, Teratani T, Yoshimoto S, Mineno T, Nakagawa K, Nagahama M, Kuwata S, Takeda H. The application of an institutional clinical data warehouse to the assessment of adverse drug reactions (ADRs). Evaluation of aminoglycoside and cephalosporin associated nephrotoxicity. Methods Inf Med. 2007;46:516–22. [PubMed] [Google Scholar]
- WHO. 2006. The safety of medicines in public health programmes: Pharmacovigilance: an essential tool. Geneva, Switzerland: WHO Press, World Health Organization, WHO Essential Medicines and Pharmaceutical Policies Department.
- Luo X, Cappelleri JC, Frush K. A systematic review on the application of pharmacoepidemiology in assessing prescription drug-related adverse events in pediatrics. Curr Med Res Opin. 2007;23:1015–24. doi: 10.1185/030079907x182211. [DOI] [PubMed] [Google Scholar]
- CONSORT. 2012. The CONSORT Statement. Consolidated Standards of Reporting Trials. 2012. 1-2-
- Michel PS. weaknesses of available methods for assessing the nature and scale of harm caused by the health system: literature review. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- Dally A. Thalidomide: was the tragedy preventable? The Lancet. 1998;351:1197–9. doi: 10.1016/S0140-6736(97)09038-7. [DOI] [PubMed] [Google Scholar]
- Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. The Lancet. 2004;364:2021–29. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- Curtis LH, Weiner MG, Boudreau DM, Cooper WO, Daniel GW, Nair VP, Raebel MA, Beaulieu NU, Rosofsky R, Woodworth TS, Brown JS. Design considerations, architecture, and use of the Mini-Sentinel distributed data system. Pharmacoepidemiol Drug Saf. 2012;21:23–31. doi: 10.1002/pds.2336. [DOI] [PubMed] [Google Scholar]
- Coloma PM, Schuemie MJ, Trifiro G, Gini R, Herings R, Hippisley-Cox J, Mazzaglia G, Giaquinto C, Corrao G, Pedersen L, van der LJ, Sturkenboom M. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011;20:1–11. doi: 10.1002/pds.2053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item
Supporting info item


