Abstract
Aim
Adverse drug events lead to increased morbidity, mortality and health care costs. Pharmacogenetic testing that guides drug prescribing has the potential to reduced adverse drug events and increase drug effectiveness. Our aim was to quantify the clinical effectiveness of genotype-guided prescribing.
Methods
Three electronic databases were searched from January 1980 through December 2013. Studies were eligible if they were RCTs comparing genotype-guided prescribing with non-genetic informed prescribing, reported drug specific adverse drug events and clinical effectiveness outcomes. Two reviewers independently screened titles and abstracts, extracted data and assessed study quality. Meta-analyses of specific outcomes were conducted where data allowed.
Results
Fifteen studies, involving 5688 patients and 19 drugs, met the inclusion and exclusion criteria. Eight studies had statistically significant results for their primary outcome in favour of genotype-guided prescribing. Nine studies evaluated genotype-guided warfarin dosing. Analysis of percentage of time in therapeutic international normalized ratio range (1952 individuals) showed a statistically significant benefit in favour of genotype-guided warfarin dosing (mean difference = 6.67; 95% CI 1.34, 12.0, I2 = 80%). There was a statistically significant reduction in numbers of warfarin-related minor bleeding, major bleeding and thromboembolisms associated with genotype guided warfarin dosing, relative risk 0.57 (95% CI 0.33, 0.99; I2 = 60%). It was not possible to meta-analyze genotype-guided dosing for other drugs. Of the six non-warfarin genotype-guided trials, two demonstrated a statistically significant benefit for their primary outcome, odds ratio 0.03 (95% CI 0.00, 0.62, P < 0.001) for abacavir.
Conclusions
There is evidence of improved clinical effectiveness associated with genotype-guided warfarin dosing.
Keywords: adverse drug events, genotype-guided, pharmacogenetic, systematic review
Introduction
Many side effects or adverse reactions to medicines are predictable and are accepted risks of treatment. They can be avoided or minimized by careful medicine prescribing and use 1. Adverse drug events (ADE) are associated with increased morbidity and mortality 2,3, and elevated health care costs 2,4,5. It is thought that genetic testing could reduce the number of adverse drug events. The application of pharmacogenetic testing in routine clinical care to individualize drug selection, dose and treatment duration has been studied in the areas of cancer, antiretroviral and cardiovascular drug therapies 6–10. In response to this growing body of genetic and clinical evidence, the US Food and Drug Administration has issued over 150 drug label recommendations related to pharmacogenetic biomarker testing. The Clinical Pharmacogenetic Implementation Consortium has issued a series of guidelines on genotype-guided drug prescribing including for warfarin, clopidogrel, abacavir and tricyclic antidepressants 11–14. Despite the guidelines and experimental research there remains a lack of consensus concerning the clinical applicability of pharmacogenetic tests 15.
Genetic factors are known to make the largest contribution to inter-patient variability in warfarin dose requirements 16. Even though warfarin is the most commonly prescribed oral anticoagulant and a leading cause of ADEs 12,17, VKORC1 and/or CYP2C9 genotype-guided warfarin dosing fails to improve anticoagulation outcomes 18,19. However, previous evidence has been mixed. Some studies have demonstrated clinical utility such as improved time in target range with genotype-guided warfarin dosing 20–22. Recently, two large RCT reports that evaluated genotype-guided warfarin dosing have stimulated further debate, as they tested related hypotheses yet arrived at different results 6,23. These studies vary considerably in follow-up duration and dosing method, yet they are similar with respect to size and choice of primary outcome (time in therapeutic range). The emergence of new evidence and controversy regarding the clinical effectiveness of using genotype-guided warfarin dosing 16,24,25 indicates a need for a systematic review of genotype-guided dosing.
The reality of clinical practice is that many patients are on multiple medications and multi-morbidity is now the norm. The consequence is that in primary care and many other settings it is less useful to use a single drug/genetic tests but to use a broader set of tests for multiple drugs. No systematic review has been published that estimates the effectiveness of genotype-guided drug prescribing that is not restricted to the classic single drug/genetic tests approach. This study examines the current randomized controlled trial evidence for the prospective clinical use of pharmacogenetic information to improve effectiveness of drug prescribing as demonstrated by reduced harm and increased relative effectiveness.
Methods
Study design
This was a systematic review and meta-analysis of randomized control trials (RCTs) to answer the question: does genotype-guided prescribing reduce ADEs and improve drug treatment response?
Search strategy
Medline, Cochrane Central Register of Controlled Trials (CENTRAL) and pharmgkb.org databases were searched from January 1980 through December 2013. Pharmgkb.org is a pharmacogenomics knowledge resource that gathers, curates and distributes knowledge about the influence of human genetic variation on drug responses. The search strategy was developed by the authors with a librarian and piloted in Medline (Table 1). Reference lists from reviews and included articles were searched for relevant items by SW and RG. Abstracts were downloaded for articles considered to be potentially relevant and the inclusion criteria were then applied to these articles by two independent reviewers (RG, DD, SW). Disagreements were resolved through discussion.
Table 1.
Medline search
| #1: (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab])OR drug therapy[sh] OR randomly[tiab] OR trial[tiab]) NOT (animals[mh] NOT humans[mh]) |
| #2: ‘Genotype’[Mesh] OR ‘Genotyping Techniques’[Mesh] OR ‘Genetic Association Studies’[Mesh] OR ‘Pharmacogenetics’[Mesh] OR ‘Genetics’[Mesh] OR ‘Reverse Genetics’[Mesh] OR ‘Genetics, Population’[Mesh] OR ‘Genetics, Medical’[Mesh] OR ‘Genetics, Behavioral’[Mesh] OR ‘Genetics, Microbial’[Mesh] OR ‘Physical Chromosome Mapping’[Mesh] OR ‘Dosage Compensation, Genetic’[Mesh] OR ‘Regulatory Sequences, Nucleic Acid’[Mesh] OR ‘Polymorphism, Genetic’[Mesh] OR ‘Polymorphism, Genetic’[Mesh] OR ‘Amplified Fragment Length Polymorphism Analysis’[Mesh] OR ‘Polymorphism, Single Nucleotide’[Mesh] OR ‘Polymorphism, Single-Stranded Conformational’[Mesh] OR ‘Polymorphism, Restriction Fragment Length’[Mesh] OR ‘DNA Copy Number Variations’[Mesh] |
| #3: abacavir OR ziagen OR acenocoumarol OR sintrom OR acepromazine OR acetophenazine OR allopurinol OR alloprin OR maloprim OR zyloprim OR amisulpride OR aripiprazole OR abilify OR azathioprine OR imuran OR azadan OR bupropion OR zyban OR wellbutrin OR capecitabine OR xeloda OR carbamazepine OR tegretol OR carbuterol OR epitol OR equetro OR chlorproguanil OR chlorpromazine OR chlorprothixene OR cisplatin OR citalopram OR celexa OR cladribine OR clofarabine OR clolar OR clozapine OR clozaril OR cytarabine OR cytosar OR dapsone OR droperidol OR erlotinib OR tarceva OR fludarabine OR fludara OR fluorouracil OR fluphenazine OR modecate OR fluspirilene OR gefitinib OR iressa OR gemcitabine OR gemzar OR haloperidol OR haldol OR ivacaftor OR kalydeco lithium OR carvolth OR duralit OR lithane OR lithman OR lithobid OR loxapine OR xyloc OR loxitane OR loxapac OR mercaptopurine OR purinethol OR mesoridazine OR methotrexate OR rheumatrex OR truxall OR methotrimeprazine OR methopromazine OR mepazine OR nozinan OR nelarabine OR adriance OR arranon OR olanzapine OR zyprexa OR paliperidone OR invega OR peginterferon alfa-2a OR pegasys OR sylatron OR peginterferon alfa-2b OR pegintron OR sylatron OR perazine OR perphenazine OR phenprocoumon OR pimozide OR orap OR pipothiazine OR piportil OR prochlorperazine OR comoro OR nu-prochlor OR promazine OR quetiapine OR seroquel OR remoxipride OR ribavirin OR virazole OR copegus OR rebetol OR ribasphere OR ribapak OR risperidone OR risperidal OR sertindole OR simvastatin OR zocor OR sulpiride OR tacrolimus OR advagraf OR prograf OR protopic OR ecori OR tegafur OR orzel OR thioguanine OR lanvis OR tabloid OR thioproperazine OR thioridazine OR thiothixene OR navane OR trifluoperazine OR terfluzine OR triflupromazine OR warfarin OR coumadin OR jantova OR ziprasidone OR zeldow OR geodon |
| #4: #1 AND #2 AND #3 |
Inclusion criteria
We included studies if physicians, in a clinical setting, were assigned randomly to use genetic information such as single nucleotide polymorphism (SNP) or copy number variation (CNV) to guide drug prescription (e.g. dose, choice of drug/no drug if no alternative) and measured clinical outcome or outcomes that determine benefit of using the genetic information. We excluded studies that retrospectively determined the association of genotype with drug response.
Data extraction
Independent double data extraction was performed using pre-designed and pilot-tested forms (RG, DD, SW). We contacted the authors of the included studies when reported outcome data were inadequate for meta-analysis. We extracted data on study design, clinical and safety outcomes. Any disagreements between the reviewers were resolved by discussion. For the purposes of this review, minor bleeding is defined as a bleeding event that required no additional testing and treatment, major bleeding is categorized as fatal bleeding, symptomatic bleeding in a critical area or organ, or a fall of haemoglobin requiring hospitalization or blood transfusion and thromboembolism is defined as a deep venous thrombosis, pulmonary embolism, or embolic stroke and the percentage of time in the therapeutic international normalized ratio (INR) range was defined as between 2.0 and 3.0, except by Anderson et al. 18 (1.8 to 3.2), Burmester et al. 36 (2.0 to 3.5), Hilman et al. 19 (1.9 to 3.0) and Huang et al. 37 (1.8 to 3.0).
Assessment of risk of bias and analysis
Two review authors independently assessed the risk of bias in each included study according to Cochrane Collaboration’s tool for assessing risk of bias 26. Any disagreements between the reviewers were resolved by discussion.
Data synthesis was performed using Review Manager version 5.2 27. Where the interventions were the same, or similar enough, and if there was no important clinical heterogeneity, we synthesized results in a meta-analysis. For measures of effect we used risk ratios (RR) with 95% confidence intervals (CI) for binary outcomes and mean differences (MD) with 95% CI for continuous outcomes. Due to significant statistical heterogeneity, we synthesized the data using a random effects analysis. All analyses included all participants in the treatment groups to which they were allocated (intention-to-treat analyses) as far as possible. Meta-analyses based on the random effects model were performed for warfarin dosing studies for percentage time in therapeutic INR, and for warfarin related minor, major and thromboembolism ADEs. Heterogeneity was assessed using I2 statistics, which is the proportion of total variance observed between the trials attributed to the differences between trials rather than to sampling error. I2 < 25% was considered as low in heterogeneity and I2 > 75% was of high heterogeneity 28.
Results
Study characteristics
Fifteen of 6686 identified studies satisfied the inclusion criteria (Figure 1) and evaluated clinical outcomes of genotype-guided interventions for 19 different drugs (Table 2). Studies analyzed a total of 5688 patients, varying in size, ranging from 26 to 1650 participants in the analysis of the primary outcome. Demographic characteristics of participants varied between studies. Of the 13 studies reporting ethnicity, one was 100% Caucasian participants and two studies were carried out with a 100% Chinese population. Studies were carried out in hospital settings in various countries, with the largest study being an international study involving 19 countries.
Figure 1.
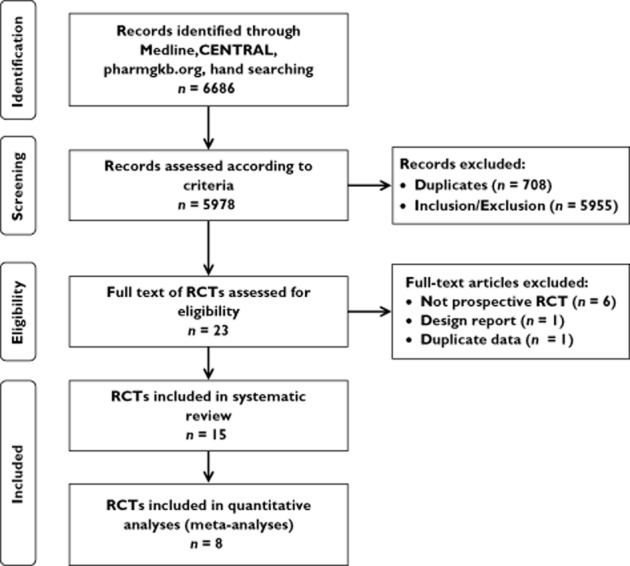
PRISMA flow diagram of study selection
Table 2.
Characteristics of studies
| Study | Country of study | Population Total number in trial (Intervention/Control) % Male Mean age Ethnicity | Drug prescribed | Genotype(s) used | Primary outcome(s) | Primary outcome result |
|---|---|---|---|---|---|---|
| Anderson et al. [18] | USA | 200 (101/99) | Warfarin | CYP2C9 VKORC1 | % out-of-range INRs | Relative % reduction = 7.3, P = 0.47 |
| 53% | ||||||
| 61 years | ||||||
| 94% Caucasian | ||||||
| Borgman et al. [35] | USA | 26 (13/13) | Warfarin | CYP2C9 VKORC1 | % time within therapeutic range | Experimental = 70.3 ± 17.9 |
| 54% | Control = 77.7 ± 11.3 | |||||
| 52 years | P = 0.441 | |||||
| 92% Caucasian | ||||||
| Burmester et al. [36] | USA | 225 (112/113) | Warfarin | CYP2C9 VKORC1 CYP4F2 | 1. Absolute prediction error relative to therapeutic dose | 1. Median difference = 0.39 mg day−1 (95% CI 0.26, 0.57), favours genotype model |
| 59% | ||||||
| 68 years (median) | 2. Time in therapeutic target range for 1st 14 days | 2. Median for both arms = 28.6%, P = 0.564 | ||||
| 100% Caucasian/Hispanic | ||||||
| Caraco et al. [21] | Israel | 191 (95/96) | Warfarin | CYP2C9 | 1. Time to reach therapeutic INR range | 1. Adjusted HR 3.95 (95% CI 2.77, 5.65), favours genotype model |
| 52% | ||||||
| 59 years (median) | 2. Time to reach stable anticoagulation | 2. HR 4.23 (95% CI 2.95, 6.07), favours genotype model | ||||
| Not stated | ||||||
| Hillman et al. [19] | USA | 38 (18/20) | Warfarin | CYP2C9 VKORC1 | Feasibility | Application of a CYP2C9 gene-based multivariate warfarin dosage calculator is feasible |
| 45% | ||||||
| 70 years | ||||||
| 100% Caucasian | ||||||
| Huang et al. [37] | China | 121 (61/60) | Warfarin | CYP2C9 VKORC1 | Time to reach stable warfarin dose | HR 1.93 (95% CI 1.26, 2.97), favours genotype model |
| 31% | ||||||
| 42 years | ||||||
| 100% Chinese | ||||||
| Kimmel et al. [23] | USA | 955 (514/501) | Warfarin | CYP2C9 VKORC1 | % time within therapeutic range | Adjusted mean difference: −8.3%, P = 0.01, favours control |
| 51% | ||||||
| 58 years (median) | ||||||
| 27% Black, 73% Non-Black | ||||||
| Mallal et al. [29] | 19 Countries | 1650 (803/847) | Abacavir | HLA-B*5701 | Reduced incidence of hypersensitivity reaction | OR 0.03 (95% CI 0.00, 0.62), favours genotype model |
| 73% | ||||||
| 42 years | ||||||
| 83% Caucasian | ||||||
| Meynard et al. [30] | France | 525 (187/186/152) | Antiretroviral agents (12) | HIV anti-retroviral resistance mutations | Proportion with plasma HIV-1 RNA | Phenotyping = 35% |
| 81% | <200 copies ml−1 at week 12 | Genotyping = 44% | ||||
| 41 years | ||||||
| unknown | Controls = 36%. No significant difference between arms. | |||||
| Newman et al. [31] | UK | 322 (163/159) | Azathioprine | TMPT | Stopping azathioprine due to adverse drug reaction | OR 1.1 (95% CI 0.66, 1.8) |
| 83% | ||||||
| 42 years | ||||||
| 91% Caucasian | ||||||
| Pirmohamed et al. [6] | UK | 427 (211/216) | Warfarin | CYP2C9 VKORC1 | % time within therapeutic range | Adjusted mean difference: |
| Sweden | 62% | 7% (95% CI 3.3, 10.6), favours genotype model. | ||||
| 68 years | ||||||
| 99% Caucasian | ||||||
| Roberts et al. [32] | Canada | 187 (91/96) | Clopidogrel/prasugrel | CYP2C19 | Proportion with P2Y12 reactivity unit >234 after 1 week dual therapy treatment. | Experimental = 9 (10%) |
| 78% | Control = 16 (17%) | |||||
| 60 years | Adjusted P = 0.07 | |||||
| 95% Caucasian | ||||||
| Thervet et al. [33] | France | 236 (116/120) | Tacrolimus | CYP3A5 | Proportion within targeted therapeutic trough concentration after six doses. | Experimental = 43.2% (95% CI 36, 51.2) |
| 67% | ||||||
| 47 years | Control = 29.1% (95% CI 22.8, 35.5) | |||||
| 90% Caucasian | P = 0.03 | |||||
| Verhoef et al. [34] | Greece | 484 (239/245) | Acenocoumarol/phenprocoumon | CYP2C9 VKORC1 | % time within therapeutic range. | Experimental = 61.6 ± 23.3 |
| Netherlands | 60% | Control = 60.2 ± 23.2 | ||||
| 68 years | Difference: 1.4 (95% CI −2.8, 5.5) | |||||
| 97% Caucasian | P = 0.52 | |||||
| Wang et al. [38] | China | 101 (50/51) | Warfarin | CYP2C9 VKORC1 | Time to reach stable warfarin dose | HR 1.57 (95% CI 1.10, 3.28), favours genotype model. |
| 31% | ||||||
| 42 years | ||||||
| 100% Chinese | ||||||
Six RCTs evaluated genotype-guided prescribing for drugs other than warfarin (Table 2): abacavir selection as HIV antiretroviral therapy (HLA-B*5701), azathioprine dosing as inflammatory therapy (TMPT), clopidogrel vs. prasugrel selection as antiplatelet therapy prior to angioplasty (CYP2C19), tacrolimus dosing as an immunosuppressant post-transplantation (CYP3A5), acenocoumarol/phenprocoumon dosing as vitamin K antagonist therapy for atrial fibrillation or venous thrombosis (CYP2C9 and VKORC1) and antiretroviral selection as second line HIV therapy (various HIV resistance mutations) 29–34. Follow-up times for these studies ranged from 7 days to 4 months.
We identified nine RCTs evaluating genotype-guided warfarin dosing as vitamin K antagonist therapy for various indications 6,18,19,21,23,35–38. Seven of nine studies involved a combination of indications including atrial fibrillation, atrial flutter, deep venous thrombosis and pulmonary embolism, two studies included prosthetic valve and joint patients, one included pre-operative orthopaedic patients and two studies initiated warfarin prior to heart valve replacement. All nine studies reported on drug specific clinical effectiveness outcomes, with eight evaluating warfarin related ADEs and time within therapeutic INR, and five evaluating outcomes of adverse drug events. Seven studies used different dosing models for their genotype-guided and control dosing arms, whereas Huang et al. and Wang et al. used the same dosing algorithms for both genotype-guided and control and Kimmel et al. and Pirmohamed et al. used the same pharmacogenetic but different control algorithms 6,23,37,38. For the genotype-guided arm, two studies used dosing models that accounted only for CYP2C9 variants, while all other studies incorporated both CYP2C9 and VKORC1 variants and one study incorporated CYP2C9, VKORC1 and CYP4F2 variants. Follow-up times for our outcomes of interest (warfarin related ADEs and time within therapeutic range) ranged from 14 days to 12 weeks.
Risk of bias for all studies
Four studies were of very high methodological quality with all items categorized as low risk of bias (Figure 2A) and a further three were of high methodological quality with all items categorized as low risk of bias except one that was uncertain/unclear risk of bias. The greatest source of bias was observed in performance bias, the blinding of participants and personnel (Figure 2B).
Figure 2.
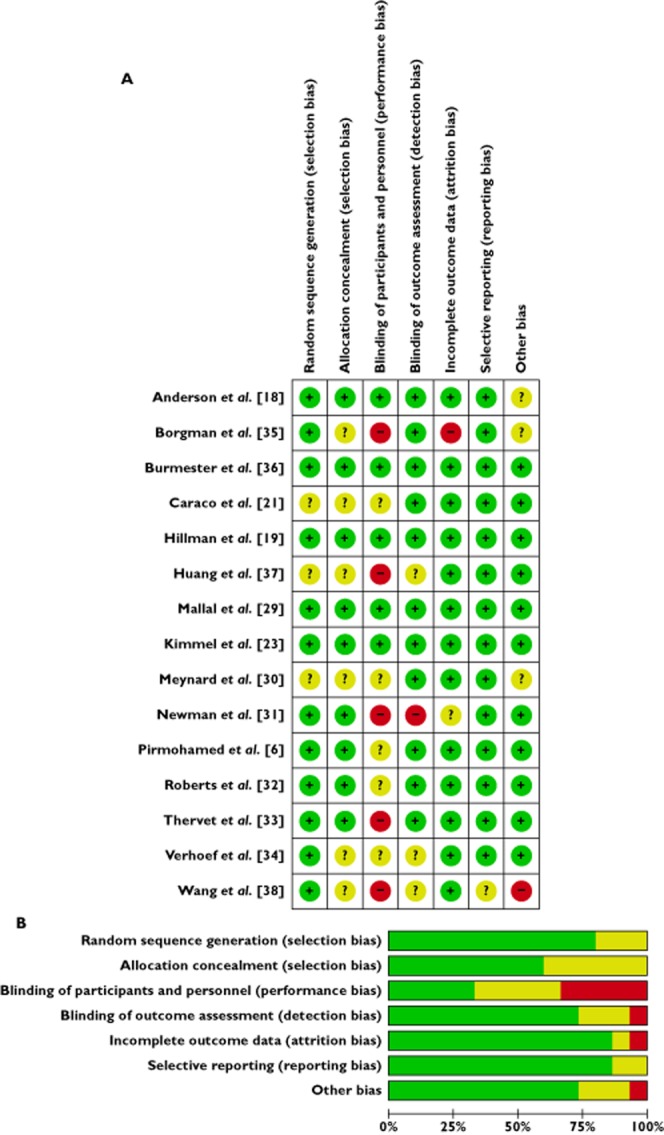
Risk of bias. (A) Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. (B) Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.  , low risk of bias;
, low risk of bias;  , unclear risk of bias;
, unclear risk of bias;  , high risk of bias
, high risk of bias
Non-warfarin trials
Of the six non-warfarin genotype-guided trials, two demonstrated a statistically significant benefit for their primary outcome. In renal transplant patients receiving tacrolimus either according to CYP3A5 genotype or according to the standard regime the proportion within the targeted therapeutic trough concentration (C0) after six doses was 43.2% (95% CI 36, 51.2) vs. 29.1% (95% CI 22.8, 35.5), respectively, P = 0.03 33. In patients infected with immunodeficiency virus type 1 excluding HLA-B*5701-positive patients, in the experimental arm, abacavir treatment resulted in a reduction in the incidence of hypersensitivity reactions, OR 0.03 (95% CI 0.00, 0.62, P < 0.001) 29. The other four non-warfarin trials did not show statistically significant improvements in the primary outcome that they defined. It was not possible to perform a meta-analysis on these studies due to clinical heterogeneity.
Genotype-guided warfarin dosing
Time within therapeutic INR range
Data were available for meta-analysis from eight studies, the study by Burmester et al. 36 was not included as data were available for only the first 14 days, when the estimate of the median times to stable therapeutic dose were 31 days (95% CI, 23, 36). A total of 1952 patients from seven studies are included in the meta-analysis (Figure 3) 6,18,19,21,23,35,37. The statistically significant mean difference is 6.67% (95% CI 1.34, 12.0) time within therapeutic international normalized ratio range, in favour of genotype-guided warfarin dosing. There is considerable heterogeneity in this analysis, I2 = 80%.
Figure 3.
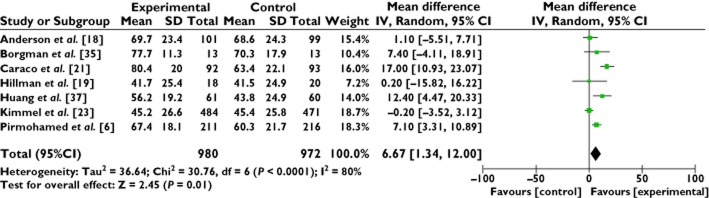
Forest plot: meta-analysis of genotype-guided prescribing to improve warfarin dosing; time within therapeutic international normalized ratio range (%), 14 to 60 days. Size of square reflects the study statistical weight, the horizontal lines indicate 95% confidence intervals (CI) and the diamond indicates summary mean difference estimate with the corresponding 95% CI
Risk of adverse haemorrhagic and thromboembolic events
Data were available for 2211 patients from seven studies for the meta-analysis of the risk of haemorrhagic and thromboembolic events (Figure 4) 6,18,19,21,23,35–37. Unpublished data from one study was used in this analysis. There was a total of 251 events observed, 107 in the genotype-guided group and 144 in the control group. The RR was significant, RR = 0.57 (95% CI 0.33, 0.99), with moderate heterogeneity, I2 = 60%.
Figure 4.
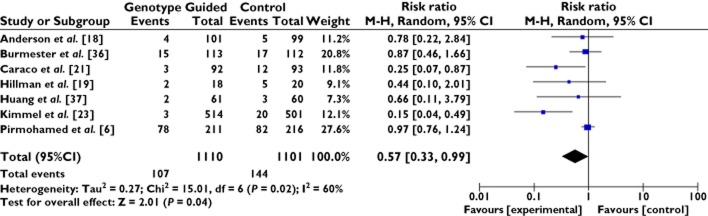
Forest plot of comparison: meta-analysis of genetically-guided prescribing to improve warfarin dosing; risk of adverse haemorrhagic and thromboembolic events. Size of square reflects the study statistical weight, the horizontal lines indicate 95% confidence intervals (CI) and the diamond indicates summary risk ratio estimate with the corresponding 95% CI
Discussion
The aim of this systematic review was to examine the evidence for the prospective clinical use of genotype information to improve the effectiveness of drug prescribing as demonstrated by reduced harm and increased relative effectiveness. Previous reviews have focussed on the use of genotype-guided prescribing for a single drug and we aimed to use a broader approach. We identified a reasonable size of literature relevant to our aim, but it was only possible to meta-analyze the studies of warfarin dosing. The limited literature outside warfarin dosing may reflect that warfarin is a commonly prescribed drug with a narrow therapeutic index and a wide variation in the dose required to reach therapeutic range. While there is an increase in RCTs that go beyond using genotyping to evaluate warfarin dosing, high level evidence is lacking regarding the clinical utility of testing for genetic variations associated with drug response. This is the first systematic review to incorporate data from the two most recent warfarin genotype-guided dosing RCTs, demonstrating that the use of genotype-guided dosing increases time within therapeutic international normalized ratio range, mean difference 6.67% (95% CI 1.34, 12.0). This is not in accordance with a 2012 systematic review that states ‘there is little evidence to support the use of genotyping, which conflicts with the US Food and Drug Administration (FDA) statement. … Our overall findings are in accordance with an older systemic review that did not find sufficient evidence to support the use of pharmacogenetics to guide warfarin therapy (Kangelaris, 2009). In addition, an editorial by Ansell, 2009 notes, “most problematic is that the intervention arm of each trial is considerably different”. Therefore, current use of genotyping is not underpinned by the evidence and should be discouraged.’ 39. The differences of opinion are partially due to the studies used in the systematic review. They included Anderson et al. 18, Burmester et al. 36 Caraco et al. 21 and Hillman et al. 19. Borgman et al. 35, Kimmel et al. 23, Pirmohamed et al. 6 and Wang et al. 38 were not published at the time of their review, an added 1509 patients. However there is still significant variability in terms of design quality, medical indication, length of follow-up and intervention design, indicating that our meta-analysis of time within therapeutic range should be interpreted with caution.
For the warfarin studies there were differences in study design related to the experimental vs. control algorithms employed to determine loading dose and in some cases dose revision and/or maintenance. For example, whereas the pharmacogenetic experimental loading dose and dose adjustment protocols were similar in the two most recent RCTs, the control dosing protocols were very different. Kimmel et al. 23 used CYP2C9 + VKORC1 genotype and the Gage clinical variable algorithm vs. the Gage clinical variable algorithm, Pirmohamed et al. 6 used CYP2C9 + VKORC1 genotype and the Avery clinical variable algorithm vs. 10 mg on day 1 and 5 mg on day 2. Kimmel et al. 23 saw no difference in time within therapeutic INR range, whereas Pirmohamed et al. 6 saw a modest difference in time within therapeutic INR range. The benefits of the genetic components of the pharmacogenetic algorithm in the study by Pirmohamed et al. 6 are hard to separate from the benefits of the clinical algorithm. It has been suggested that it was not surprising that differences were not seen between the Kimmel et al. 23 trial arms as they were comparing two multivariate models 16. The contribution of genetic variables to the success of warfarin dosing could have been masked by the fact that using a clinical only multivariate model for dose prediction and adjustment that requires rigorous INR testing and management is highly likely to be substantially better than real world settings that have standard local practice.
There are six genotype-guided warfarin dosing trials registered in clinicaltrials.gov that are currently actively recruiting or completed and awaiting study results. One of these is a large RCT of an estimated 1600 patients (the GIFT trial), which will compare therapeutic warfarin dosing using genotype and clinical information with warfarin dose requirements using clinical information only. This trial is powered for ADEs as a primary outcome measure (http://clinicaltrials.gov/ct2/show/NCT01006733?term=NCT01006733%26).
Our results are not definitive because of the statistical heterogeneity between trials. Although the overall quality of the included studies was high there was evidence of performance bias in many of the studies. This was mitigated by use of a ‘hard’ outcome measure, of ‘time within therapeutic INR range’.
In summary, this study has examined the evidence for the prospective clinical use of genotype-guided prescribing to improve effectiveness of drug prescribing and the evidence supports the use of genotype-guided prescribing for warfarin, tacrolimus and abacavir. RCTs of the more pragmatic clinical approach of using a multidrug/SNP process to inform prescribing need to be undertaken.
Competing Interests
All authors have completed the Unified Competing Interest form at and declare no support from any organization for the submitted work. MD reports grants from Rx&D and pharmaceutical companies, outside the submitted work in the previous 3 years. There are no other relationships or activities that could appear to have influenced the submitted work.
We thank Jessica Belle and Jaeyun Yoo, University of British Columbia for their work on designing and performing literature searches.
References
- Smith J. Building a Safer NHS for Patients: Improving Medication Safety. A Report by the Chief Pharmaceutical Officer. London: Department of Health; 2004. [Google Scholar]
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301–306. [PubMed] [Google Scholar]
- Phillips DP, Christenfeld N, Glynn LM. Increase in US medication-error deaths between 1983 and 1993. Lancet. 1998;351:643–644. doi: 10.1016/S0140-6736(98)24009-8. [DOI] [PubMed] [Google Scholar]
- Aspden P. Preventing Medication Errors. Washington, DC: National Academies Press; 2007. [Google Scholar]
- Bates DW, Spell N, Cullen DJ, Burdick E, Laird N, Petersen LA, Small SD, Sweitzer BJ, Leape LL. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277:307–311. [PubMed] [Google Scholar]
- Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, Kesteven P, Christersson C, Wahlstrom B, Stafberg C, Zhang JE, Leathart JB, Kohnke H, Maitland-van der Zee AH, Williamson PR, Daly AK, Avery P, Kamali F, Wadelius M Eu-Pact Group. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369:2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Libertone R, Liuzzi G. HIV pharmacogenetics in clinical practice: recent achievements and future challenges. Curr HIV Res. 2008;6:544–554. doi: 10.2174/157016208786501535. [DOI] [PubMed] [Google Scholar]
- Turner RM, Pirmohamed M. Cardiovascular pharmacogenomics: expectations and practical benefits. Clin Pharmacol Ther. 2014;95:281–293. doi: 10.1038/clpt.2013.234. [DOI] [PubMed] [Google Scholar]
- Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011;364:1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler HE, Maitland ML, Dolan ME, Cox NJ, Ratain MJ. Cancer pharmacogenomics: strategies and challenges. Nat Rev Genet. 2013;14:23–34. doi: 10.1038/nrg3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JK, Swen JJ, Thorn CF, Sangkuhl K, Kharasch ED, Ellingrod VL, Skaar TC, Muller DJ, Gaedigk A, Stingl JC Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther. 2013;93:402–408. doi: 10.1038/clpt.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, Stein CM, Anderson JL, Kimmel SE, Lee MT, Pirmohamed M, Wadelius M, Klein TE, Altman RB Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90:625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MA, Klein TE, Dong BJ, Pirmohamed M, Haas DW, Kroetz DL Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for HLA-B genotype and abacavir dosing. Clin Pharmacol Ther. 2012;91:734–738. doi: 10.1038/clpt.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, Roden DM, Klein TE, Shuldiner AR Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90:328–332. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704–2714. doi: 10.1001/jama.2011.1880. [DOI] [PubMed] [Google Scholar]
- Zineh I, Pacanowski M, Woodcock J. Pharmacogenetics and coumarin dosing – recalibrating expectations. N Engl J Med. 2013;369:2273–2275. doi: 10.1056/NEJMp1314529. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Cavallari LH. Pharmacogenetics and cardiovascular disease – implications for personalized medicine. Pharmacol Rev. 2013;65:987–1009. doi: 10.1124/pr.112.007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, Kahn SF, May HT, Samuelson KM, Muhlestein JB, Carlquist JF Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- Hillman MA, Wilke RA, Yale SH, Vidaillet HJ, Caldwell MD, Glurich I, Berg RL, Schmelzer J, Burmester JK. A prospective, randomized pilot trial of model-based warfarin dose initiation using CYP2C9 genotype and clinical data. Clin Med Res. 2005;3:137–145. doi: 10.3121/cmr.3.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Horne BD, Stevens SM, Woller SC, Samuelson KM, Mansfield JW, Robinson M, Barton S, Brunisholz K, Mower CP, Huntinghouse JA, Rollo JS, Siler D, Bair TL, Knight S, Muhlestein JB, Carlquist JF. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II) Circulation. 2012;125:1997–2005. doi: 10.1161/CIRCULATIONAHA.111.070920. [DOI] [PubMed] [Google Scholar]
- Caraco Y, Blotnick S, Muszkat M. CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clin Pharmacol Ther. 2008;83:460–470. doi: 10.1038/sj.clpt.6100316. [DOI] [PubMed] [Google Scholar]
- Epstein RS, Moyer TP, Aubert RE, O Kane DJ, Xia F, Verbrugge RR, Gage BF, Teagarden JR. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study) J Am Coll Cardiol. 2010;55:2804–2812. doi: 10.1016/j.jacc.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF, Rosenberg YD, Eby CS, Madigan RA, McBane RB, Abdel-Rahman SZ, Stevens SM, Yale S, Mohler ER, Fang MC, Shah V, Horenstein RB, Limdi NA, Muldowney JA, Gujral J, Delafontaine P, Desnick RJ, Ortel TL, Billett HH, Pendleton RC, Geller NL, Halperin JL, Goldhaber SZ, Caldwell MD, Califf RM, Ellenberg JH Coag Investigators. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369:2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie B. Do pharmacogenetics have a role in the dosing of vitamin K antagonists? N Engl J Med. 2013;369:2345–2346. doi: 10.1056/NEJMe1313682. [DOI] [PubMed] [Google Scholar]
- Roberts A. Anticoagulation therapy: genotype-guided anticoagulation therapy-the jury is still out. Nat Rev Cardiol. 2014;11:1. doi: 10.1038/nrcardio.2013.187. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England; Hoboken, NJ: Wiley Blackwell; 2008. [Google Scholar]
- The Cochrane Collaboration. Review Manager (RevMan) 5.2 edn. Copenhagen: The Nordic Cochrane Centre; 2012. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jagel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A Predict- Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- Meynard JL, Vray M, Morand-Joubert L, Race E, Descamps D, Peytavin G, Matheron S, Lamotte C, Guiramand S, Costagliola D, Brun-Vezinet F, Clavel F, Girard PM Narval Trial Group. Phenotypic or genotypic resistance testing for choosing antiretroviral therapy after treatment failure: a randomized trial. AIDS. 2002;16:727–736. doi: 10.1097/00002030-200203290-00008. [DOI] [PubMed] [Google Scholar]
- Newman WG, Payne K, Tricker K, Roberts SA, Fargher E, Pushpakom S, Alder JE, Sidgwick GP, Payne D, Elliott RA, Heise M, Elles R, Ramsden SC, Andrews J, Houston JB, Qasim F, Shaffer J, Griffiths CE, Ray DW, Bruce I, Ollier WE TARGET Study Recruitment Team. A pragmatic randomized controlled trial of thiopurine methyltransferase genotyping prior to azathioprine treatment: the TARGET study. Pharmacogenomics. 2011;12:815–826. doi: 10.2217/pgs.11.32. [DOI] [PubMed] [Google Scholar]
- Roberts JD, Wells GA, Le May MR, Labinaz M, Glover C, Froeschl M, Dick A, Marquis JF, O’Brien E, Goncalves S, Druce I, Stewart A, Gollob MH, So DY. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012;379:1705–1711. doi: 10.1016/S0140-6736(12)60161-5. [DOI] [PubMed] [Google Scholar]
- Thervet E, Loriot MA, Barbier S, Buchler M, Ficheux M, Choukroun G, Toupance O, Touchard G, Alberti C, Le Pogamp P, Moulin B, Le Meur Y, Heng AE, Subra JF, Beaune P, Legendre C. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87:721–726. doi: 10.1038/clpt.2010.17. [DOI] [PubMed] [Google Scholar]
- Verhoef TI, Ragia G, de Boer A, Barallon R, Kolovou G, Kolovou V, Konstantinides S, Le Cessie S, Maltezos E, van der Meer FJ, Redekop WK, Remkes M, Rosendaal FR, van Schie RM, Tavridou A, Tziakas D, Wadelius M, Manolopoulos VG, Maitland-van der Zee AH Eu-Pact Group. A randomized trial of genotype-guided dosing of acenocoumarol and phenprocoumon. N Engl J Med. 2013;369:2304–2312. doi: 10.1056/NEJMoa1311388. [DOI] [PubMed] [Google Scholar]
- Borgman MP, Pendleton RC, McMillin GA, Reynolds KK, Vazquez S, Freeman A, Wilson A, Valdes R, Jr, Linder MW. Prospective pilot trial of PerMIT versus standard anticoagulation service management of patients initiating oral anticoagulation. Thromb Haemost. 2012;108:561–569. doi: 10.1160/TH12-03-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester JK, Berg RL, Yale SH, Rottscheit CM, Glurich IE, Schmelzer JR, Caldwell MD. A randomized controlled trial of genotype-based Coumadin initiation. Genet Med. 2011;13:509–518. doi: 10.1097/GIM.0b013e31820ad77d. [DOI] [PubMed] [Google Scholar]
- Huang SW, Chen HS, Wang XQ, Huang L, Xu DL, Hu XJ, Huang ZH, He Y, Chen KM, Xiang DK, Zou XM, Li Q, Ma LQ, Wang HF, Chen BL, Li L, Jia YK, Xu XM. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenet Genomics. 2009;19:226–234. doi: 10.1097/FPC.0b013e328326e0c7. [DOI] [PubMed] [Google Scholar]
- Wang M, Lang X, Cui S, Fei K, Zou L, Cao J, Wang L, Zhang S, Wu X, Wang Y, Ji Q. Clinical application of pharmacogenetic-based warfarin-dosing algorithm in patients of Han nationality after rheumatic valve replacement: a randomized and controlled trial. Int J Med Sci. 2012;9:472–479. doi: 10.7150/ijms.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtani KR, Heneghan CJ, Nunan D, Bankhead C, Keeling D, Ward AM, Harrison SE, Roberts NW, Hobbs FD, Perera R. Optimal loading dose of warfarin for the initiation of oral anticoagulation. Cochrane Database Syst Rev. 2012;(12) doi: 10.1002/14651858.CD008685.pub2. CD008685. [DOI] [PMC free article] [PubMed] [Google Scholar]


